Abstract
Introduction
The hypothesis of this study is night shift work exposure can increase the risk of female breast cancer. To validate this hypothesis, the authors conducted a two-stage dose-response meta-analysis with improved quality on this topic.
Methods
The medical librarian searched PubMed, EMBASE, and the Cochrane Library on December 30th, 2022. The eight inclusion criteria were determined and strictly applied to the selection process. A reliable dose-response meta-analysis methodology was applied.
Results
Reliable 10 cohort (total cases: 15,953, and total person-years: 6,812,138) and 11 case-control reports (total cases: 9196, and total controls:12,210) were included in the final analysis. The pooled risk ratio (RR) of female breast cancer (from cohort studies) for 1, 10, 20, and 30 years of night shift work exposure was 1.0042 (95% CI 1.0014–1.0070), 1.0425 (95% CI 1.0138–1.0719), 1.0867 (95% CI 1.0278–1.1490), and 1.1328 (95% CI 1.0419–1.2317), respectively. The pooled odds ratio (OR) of female breast cancer (from case-control studies) for 1, 10, 20, and 30 years of night shift work exposure was 1.0213 (95% CI 1.0108–1.0319), 1.2346 (95% CI 1.1129–1.3695), 1.5242 (95% CI 1.2386–1.8756), and 1.8817 (95% CI 1.3784–2.5687), respectively.
Discussion
This study has several strengths from the perspective of a dose-response meta-analysis: Strictly applied eight inclusion criteria, separately synthesized RRs from cohort studies and ORs from case-control studies, clearly defined exposure dose, years of night shift work for each risk estimate, a reliable dose-response meta-analysis methodology, and careful considering of selection, exposure, and outcome biases and confounder adjustment for each study. This careful consideration of potential biases and confounding led to the exclusion of unreliable two cohort and five case-control studies.
Similar content being viewed by others
Introduction
The relationship between night shift work and breast cancer has been discussed in many previous studies. However, a definite conclusion was not made. In a systematic review and meta-analysis study by Van et al., the pooled risk ratio (RR) from cohort and nested case-control studies were 0.98 (95% CI 0.93–1.03) and 1.14 (95% CI 0.89–1.46), respectively, without statistical significance [1]. Only the pooled odds ratio (OR) from case-control studies was 1.34 (95% CI 1.17–1.53) with statistical significance. However, this study extracted only one representative risk estimate for night shift work versus day work from each study, and these risk estimates were synthesized to calculate pooled risk estimates. In a systematic review and meta-analysis study by Manouchehri et al., the pooled RR for the subjects with < 10 years of night shift work exposure was 1.13 (95% CI 1.03–1.24) with statistical significance. However, the pooled OR for the subjects with ≥ 10 years of night shift work exposure was 1.08 (95% CI 0.99–1.17) with statistical insignificance [2]. However, this study classified the exposure dose (years of night shift work) into only two categories, < 10 years and ≥ 10 years, and did not consider a dose-response relationship.
Even though the conclusions of individual articles and meta-analyses are divergent, the biological background for the association between night shift work and breast, prostate, and colorectal cancer is rather stable. The International Agency for Research on Cancer (IARC) classified night shift work as Group 2 A (probably carcinogenic) carcinogen for these three cancers. This indicates that night shift work has limited evidence of carcinogenicity in humans, sufficient evidence of carcinogenicity in experimental animals, and strong evidence that night shift work exhibits key characteristics of carcinogens [3]. National Toxicology Program (NTP) concluded that there is sufficient evidence of carcinogenicity between night shift work exposure and breast cancer, based on the collective body of cancer epidemiology and mechanistic studies in humans [4]. There are many studies supporting the biological background of this association [5]. Detailed potential biological mechanistic connections between night shift work and breast cancer are provided in Supplementary texts A.
Furthermore, many previous studies on the association between light exposure at night and breast cancer support a positive association between night shift work and breast cancer because the biological background mechanisms for these two topics are similar [6,7,8]. A detailed explanation for the rationale is provided in Supplementary texts B.
For the association between night shift work and breast cancer, several studies even reported an effect modification by the hormone receptor status of breast cancer. Detailed explanation is provided in Supplementary texts C.
The hypothesis of this study is that night shift work exposure can increase the risk of female breast cancer. To validate this hypothesis, the authors conducted a two-stage dose-response meta-analysis on this topic. Because of several methodologic flaws observed in previous meta-analysis studies, the authors strictly determined and applied the inclusion criteria and applied strict statistical principles to each process of the meta-analysis. Most importantly, the exposure dose for each risk estimate was clearly defined based on the texts of individual original articles. Finally, the authors applied a reliable two-stage dose-response meta-analysis methodology [9, 10]. Based on these efforts to improve the quality of this dose-response meta-analysis, this study could add to the existing body of evidence.
Methods
Literature search
A literature search was conducted by a medical librarian in the medical library of Inha University, Incheon, South Korea (information specialist Minji Kim commented on the acknowledgment section). The medical librarian searched PubMed, EMBASE, and the Cochrane Library on December 30th, 2022.
Inclusion criteria and selection of articles
The inclusion criteria were as follows: (i) the article should deal with the relationship between night shift work and breast cancer. (ii) The exposure of interest (night shift work) should be stated clearly in the text. Articles dealing with working experience in jobs that could be associated with night shift work as exposure of interest without a clear statement of night shift work were excluded because these jobs could not include night shift work in some cases. Articles dealing with light exposure at night, disturbance of the sleep-wake cycle, or the level of melatonin hormone as the exposure of interest were all excluded. Night shift work should be dealt with as occupational exposure, whether it was dealt with as a categorical or continuous variable. Variations in night shift work definition in each study were separately summarized in a table and considered in the evidence synthesis. (iii) Quantitative analysis should be included. The results should be provided as an RR or OR in cohort or case-control studies, respectively. Hazard ratio (HR) in survival analyses can be interpreted as RR based on the following previous studies [11,12,13]. (iv) Literature written only in English was included. (v) Articles with only human subjects (not animal subjects) were included. (vi) For article type, only the original article was included. However, if all other criteria were met, a letter to the editor was also included after a careful examination. The abstract was excluded because research abstracts are usually published as full articles in academic journals after an academic conference. Including an abstract could cause a duplication of the original research data. (vii) The articles dealing with male breast cancer were excluded. Only articles dealing with female breast cancer were included. (viii) Several articles were included additionally after the screening and review of the bibliographies of essential articles.
The first and corresponding author, JM, and the third author, YM, conducted the selection process separately. After this process, two selection results were compared with each other, and these two authors discussed them. Under this discussion, a final selection result was decided.
Exposure, outcome, and confounding aspect of each study
The authors carefully examined each study from the perspective of selection, exposure, and outcome biases and confounding. The results of this examination were summarized in separate tables. Based on these results, the overall reliability of a study was assessed.
Data extraction
Risk estimates from each study were extracted to construct the dose-response meta-analysis dataset. For the construction of a dose-response relationship, each risk estimate for each dose category of night shift work exposure was extracted. The dose of interest in this study was the years of night shift work.
Examination of publication bias
The existence of publication bias was examined using Begg’s funnel plot and Egger’s regression test. If Begg’s funnel plot shows an asymmetric shape, the existence of a publication bias was suspected. Egger’s regression test uses the precision and the standardized effect size of the effect estimate from a study as the independent variable and dependent variable, respectively [14]. If Egger’s regression test result shows a statistically significant result, the existence of a publication bias could be suspected. The statistically significant p-value for publication bias was set at 0.05.
For Egger’s regression test, only one representative effect estimate (for example, RR for cohort studies or OR for case-control studies) is needed for each study. Therefore, the authors applied the same two-stage dose-response meta-analysis method used in this study (will be explained in subsection 2.6) to each study separately [9, 10]. Then, the authors calculated a representative RR or OR of breast cancer for one year increase in night shift work from each study. These RRs or ORs and calculated variance were used to conduct Egger’s regression test.
Dose-response meta-analyses
For the investigation of the dose-response relationship between the years of night shift work (exposure dose) and the incidence of breast cancer (response), a two-stage dose-response meta-analysis was applied [9, 10].
First, the authors calculated a point dose for each dose range of exposure (years of night shift work). For a finite range with a lower and upper limit, we applied the median value for the range. For the highest dose range category, we added the half value of the interval for other dose ranges to the lower limit of the highest category. For example, if the dose ranges are comprised of 0, 0–10, 10–20, 20–30, and > 30, we assigned 0, 5, 15, 25, and 35 (30+(10/2)) as the point doses.
Second, we applied the two-stage dose-response meta-analysis methodology [10]. This process is composed of two stages. The first stage is to estimate the dose-response association between the adjusted log risk ratios and the levels of a specific exposure (point dose) in a particular study. The linear regression model is.
where the dependent variable y is an n×1 vector of log relative risks (not including the reference one), and X is a n×p matrix containing the non-referent values of the dose and/or some transforms of it (e.g., splines, polynomials). The variance-covariance matrix COV(ε) is equal to the following symmetric matrix.
where the covariance among (log) risk ratios implies the non-diagonal elements of S are unlikely to be equal to zero. For the covariance approximation, the authors applied a method devised by Greenland and Longnecker. The second stage is to combine study-specific estimates for the estimation of the trend. Each study included in the dose-response meta-analysis can be expressed as.
where \(\:{\widehat{V}}_{j}+\psi\:\) = \(\:{\varSigma\:}_{j}\). The marginal model defined in Eq. 3 has independent within-study and between-study components. In the between-study components, \(\:{\beta\:}_{j}\) is assumed to be sampled from \(\:{N}_{p}(\beta\:,\psi\:)\), where ψ is the unknown between-study covariance matrix. Here, β can be interpreted as the population-average outcome parameters, namely the coefficients defining the pooled dose-response trend. The prediction of interest in a dose-response analysis is the relative risk for the disease comparing two exposure values. Given a range of exposure x and a chosen reference value \(\:{x}_{ref}\), the predicted pooled dose-response association can be obtained as follows
where X and \(\:{X}_{ref}\) are the design matrices evaluated, respectively, in x and \(\:{x}_{ref}\). A (1-α/2) % confidence interval for the predicted pooled dose-response curve is given by
where \(\:\widehat{V}\left(\widehat{\beta\:}\right)\) is the estimated covariance matrix of \(\:\widehat{\beta\:}.\)
A dose-response meta-analysis was conducted two times, using all studies and only reliable studies, respectively.
Statistical software
For all statistical analyses, R software version 4.2.2 was used. For a dose-response meta-analysis, the R package ‘dosresmeta’ was used [10].
Results
Literature search and screening
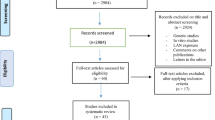
Application form for systematic search is provided in Supplementary material B. Search terms and used syntax are provided in Supplementary material C. Search results in each of 3 databases are provided in Supplementary material D. The PRISMA flow diagram with grey literature is provided in Fig. 1. In the PubMed, EMBASE, and Cochrane Library, 211, 329, and 85 articles were searched, respectively. Among a total of these 625 articles, 152 articles were duplicated articles. Finally, 473 articles remained.
Researchers examined the title and abstract of 473 remaining articles. Among these remaining 473 articles, 276 articles were excluded (252 and 21 due to distant topics and animal subjects, respectively), and 197 candidate articles remained. For these 197 candidate articles, the original text was attached and examined (a brief full-text review). Through this step, 109 articles were excluded (82, 13, 7, and 7, due to inclusion criterion (i), (ii), (iii), and (vi), respectively), and 88 articles remained. For these remaining 88 articles, the authors conducted a thorough full-text review and strictly applied the pre-defined eight inclusion criteria for final selection. Through this step, 67 articles were excluded (49, 10, 3, 1, and 4 due to inclusion criteria (i), (ii), (ii), (iv), and (vi), respectively), and 21 articles remained.
Through a careful search of bibliographies of essential articles, the authors could find additional 23 candidate articles. For these 23 candidate articles, the original text was attached and examined (a brief full-text review). Through this step, 13 articles were excluded (9, 3, and 1 due to inclusion criteria (i), (ii), and (iii), respectively), and the other 10 articles remained. For these remaining 10 articles, researchers conducted a thorough full-text review and strictly applied the pre-defined eight inclusion criteria for final selection. Through this step, 5 articles were excluded (4 and 1 due to inclusion criteria (i) and (ii), respectively), and the other 5 articles remained.
Finally, 10 cohort study articles and 16 case-control study articles were included. For these 26 included articles, 12 cohort, and 16 case-control reports were included.
PRISMA flow diagram with grey literature [15]
Characteristics of included studies
Table 1 provides the characteristics of 10 cohort studies that were included (12 reports). The total number of cases and person-years were 18,086 cases and 7,987,557 person-years, respectively. The study year for cohort construction starts in 1959, and the specific data extraction and analysis were conducted from 1992 to 2014. Three study reports were from the UK and the US, respectively, and another two study reports were from Sweden. One report was from the Netherlands, Canada, China, and Finland, respectively. One report applied dichotomous exposure classification, shift with night versus day shifts [18]. The longest range for years of night shift work was from 0 to 45 years, and the shortest range for years of night shift work was from 0 to > 10 years. The longest range for point dose (years of night shift work) was from 0 to 37.5 years, and the shortest range for point dose was from 0 to 9. The highest RR reported was 1.79 (95% CI 1.06–3.01) for the category of > 20 years compared to no night shift work in Schernhammer et al. (2006) [25]. The lowest RR reported was 0.69 (95% CI 0.20–2.41) for the category of 5–9 years compared to no night shift work in Harma et al. (2022) [24].
Table 2 provides the characteristics of the included 16 case-control studies (16 reports). The total number of cases, controls, and total participants was 17,805, 21,184, and 38,989, respectively. The study year ranged from 1960 to 1982 to 2015–2019. Two studies did not report the study year [27, 31]. Three studies were from Denmark. Two studies were from the US and China, respectively. One study was reported from Mexico, Australia, Canada, France, Spain, Germany, South Korea, Norway, and Poland, respectively. Four studies applied dichotomous exposure classification, night shift work versus no night shift work [27, 31, 37, 38]. The longest range for years of night shift work was from 0 to 30–39 years, and the shortest range for years of night shift work was from 0 to > 4.5 years. The longest range for point dose (years of night shift work) was from 0 to 37.5 years, and the shortest range for point dose was from 0 to 5.4 years. The highest OR reported was 8.58 (95% CI 2.19–33.78) for the category of ‘night shift work’ compared to ‘no night shift work’ in Bustamante et al. (2019) [27]. The lowest OR reported was 0.32 (95% CI 0.12–0.83) for the category of ≥ 8 years compared to no night shift work in O’Leary et al. (2006) [33].
Supplementary material E provides point dose estimates (years of night shift work) for studies with dichotomous exposure classification, night shift work versus no night shift work. The rationale for these calculations is co-provided (main texts and calculation).
Exposure, outcome, and confounding aspects of each study
Supplementary material F summarized the selection, exposure, outcome, and confounding aspects of each study, which were extracted from the main texts of each study. Table 3 and 4 provide a summary table of special features for each study from the perspective of exposure, outcome, and confounding. In addition, the overall reliability of each study is provided in the rightmost column of these tables. Table 3 and 4 are for cohort and case-control studies, respectively. For selection bias, all studies did not have any special features related to this aspect. Therefore, we omitted the column for selection bias in Table 3 and 4.
For cohort studies, two studies were rated unreliable. Jones et al. (2019) was rated unreliable because of incomplete exposure assessment for night shift work that ended before the last 10-year period from the start of the study [17]. Knutsson et al. (2013) was rated unreliable because of only dichotomous categorization for night shift work: ‘shift with night versus day [18]. The total number of cases and person-years for reliable studies were 15953 cases and 6812138 person-years, respectively.
Five case-control studies were rated unreliable. Bustamante et al. (2019) was rated unreliable because of only dichotomous exposure categorization: night shift work versus no night shift work [27]. Fritschi et al. (2013) was rated unreliable because the exposure assessment for night shift work was rather simple. The main exposure of interest was sleep patterns and not night shift work [29]. Hansen et al. (2001) was rated unreliable because of only dichotomous categorization: all night work combined versus daytime work [31]. Wang et al. (2015) was rated unreliable because of only dichotomous categorization of night shift work exposure: ever versus never night shift worker [37]. Yang et al. (2019) was rated unreliable because of only dichotomous exposure categorization: ever versus never night shift worker [38]. The total number of cases, controls, and total participants for reliable studies was 9196, 12,210, and 21,406, respectively.
Publication bias
Figure 2 provides each Begg’s funnel plot for cohort studies and case-control studies, respectively. The points in each funnel plot showed a relatively symmetric distribution (low possibility of publication bias). Supplementary material G provided the representative RRs and ORs calculated from each study using the same dose-response meta-analysis method used in this study. The Egger’s regression test result for cohort studies and case-control studies showed a p-value of 0.1437 and 0.1430, respectively. Based on these results, we concluded that the possibility of publication would be low.
Dose-response meta-analysis
Table 5 Provides the results of the dose-response meta-analyses for only reliable studies. First, the dose-response meta-analysis model for cohort studies and case-control studies was statistically significant with a p-value of 0.0035 and 0.0001, respectively. The pooled RR of female breast cancer (from cohort studies) for 1, 10, 20, and 30 years of night shift work exposure was 1.0042 (95% CI 1.0014–1.0070), 1.0425 (95% CI 1.0138–1.0719), 1.0867 (95% CI 1.0278–1.1490), and 1.1328 (95% CI 1.0419–1.2317), respectively. The pooled OR of female breast cancer (from case-control studies) for 1, 10, 20, and 30 years of night shift work exposure was 1.0213 (95% CI 1.0108–1.0319), 1.2346 (95% CI 1.1129–1.3695), 1.5242 (95% CI 1.2386–1.8756), and 1.8817 (95% CI 1.3784–2.5687), respectively. Figure 3 provides each dose-response meta-analysis plot for cohort studies and case-control studies, respectively, using only reliable studies. Supplementary material H provides the results of the dose-response meta-analyses for all studies and the dose-response meta-analysis plot for all cohort and case-control studies.
Dose-response meta-analysis plot for only reliable cohort (upper figure) and case-control studies (lower figure). The blue line is a continuous line of risk estimates along the increasing exposure (years of night shift work). Dashed lines are upper and lower bounds of 95% confidence intervals for risk estimates. Model: two-stage dose-response meta-analysis [9, 10]
Discussion
In this dose-response meta-analysis study, reliable 10 cohort and 11 case-control reports were included. Egger’s regression test result indicated that the possibility of publication bias is low. The risk of breast cancer increased by 0.42, 4.25, 8.67, and 13.28% after 1, 10, 20, and 30 years of night shift work exposure, respectively, according to cohort studies. The risk of breast cancer increased by 2.13, 23.46, 52.42, and 88.17% after 1, 10, 20, and 30 years of night shift work exposure, respectively, according to case-control studies.
Review of previous meta-analyses
The following studies are recent systematic review and meta-analysis studies on the relationship between night shift work. Recent works are essential because they tried to synthesize all available evidence from individual articles published until the most recent days.
Van et al. concluded that night shift work is not associated with a risk of breast cancer [1]. In this study, only the case-control study group showed a statistically significant increased risk of breast cancer for ever night shift work versus never night shift work (pooled OR of 1.34, 95% CI 1.17–1.53). The pooled risk estimate for the nested case-control study group (pooled OR of 1.14, 95% CI 0.89–1.46) and that for the cohort study group (pooled RR of 0.98, 95% CI 0.93–1.03) were statistically insignificant for ever night shift work versus never night shift work. However, this study treated OR and RR as the same risk estimate and combined these two different types of risk estimates without any valid conversion. As seen in our results, the ORs from case-control studies tend to be higher than the RRs from cohort studies. This difference is also distinct in this study. Strictly speaking, OR can be converted into RR if the non-exposed prevalence for each OR is given [43]. In addition, this study did not consider a dose-response relationship between the years of night shift work and the risk of breast cancer. Only one risk estimate was extracted from each study, and this single estimate was used to represent the individual study. However, in occupational health and environmental health, a correct exposure assessment and consideration of dose-response relationship are essential keys for correct risk definition. Contrary to this study, the authors applied a reliable two-stage dose-response meta-analysis methodology to utilize multiple exposure doses and corresponding risk estimates reported in each individual article.
Manouchehri et al. concluded the pooled RR and 95% CI of breast cancer for < 10 years of night shift work was 1.13 (95% CI 1.03–1.24), and that for ≥ 10 years of night shift work was 1.08 (95% CI 0.99–1.17) [2]. In particular, studies with high quality and those adjusted for reproductive factors and family history of breast cancer showed an increased risk of breast cancer for night shift workers with statistical significance. This study classified the period of night shift work exposure into two categories: <10 years and ≥ 10 years of night shift work. However, the ≥ 10 years group showed a statistically insignificant increased risk, and < 10 years group showed a statistically significant increased risk. The statistically insignificant result for ≥ 10 years of night shift work could be due to a healthy worker survivor effect.
Dun et al. also reported that night shift work is not associated with the risk of breast cancer (pooled OR of 1.009, 95% CI 0.984–1.033) in a meta-analysis study [44].
Examination of publication bias in a two-stage dose-response meta-analysis
In this study, the authors conducted Egger’s regression test using the representative RRs or ORs calculated by applying the same dose-response meta-analysis method to the effect estimates from each individual study separately. This method was devised by the authors, and further discussion is needed on this methodology. The authors will publish a methodology paper on this methodology with various examples, including the one from this study.
Strengths and limitations of this study
Until today, the papers and meta-analysis papers indicated somewhat incompatible results on the association between night shift work exposure and female breast cancer incidence. This was because of (i) non-strict and non-transparent application of inclusion criteria for meta-analysis, (ii) misunderstanding of some essential differences between effect estimates obtained from different types of study design (for example, RRs and ORs), (iii) inaccurate exposure assessment in original studies or the application of inaccurate exposure dose in the meta-analysis, and (iv) the primitive evidence synthesis methods applied for the investigation of a dose-response relationship. On the contrary, this study (i) strictly applied eight transparent inclusion criteria, and (ii) thoroughly separated RRs from cohort studies and ORs from case-control studies and synthesized each type of effect estimates separately. In addition, (iii) exposure dose (years of night shift work) was clearly defined in evidence synthesis based on the years of night shift work reported in individual studies. Finally, (iv) the authors applied a reliable two-stage dose-response meta-analysis method reported in recent literature [9, 10].
Another strength of this study is a thorough review of previous studies regarding selection, exposure, outcome, and confounding aspects. Based on this thorough review process, the authors carefully excluded unreliable two cohort and five case-control studies from the initially included studies. This process enhanced the reliability of the results.
On the contrary, this study also has several limitations. First, this study separated cohort studies from case-control studies. However, under the rare disease assumption, the ORs acquired from case-control studies can be regarded as RRs acquired from cohort studies for rare diseases such as breast cancer [43]. The decision to separate these two types of studies was based on relatively lower risk estimates acquired from cohort studies than those from case-control studies. In future studies, the reason why the risk estimates from case-control studies are generally higher than those from cohort studies should be investigated further, and the risk estimates from case-control studies should be incorporated to those from cohort studies. Second, even though the authors analyzed the selection, exposure, outcome, and confounding aspects of each study, there would have been a remaining inter-study variation in the dose-response meta-analysis. In the future, if a cohort study with a far larger number of participants was conducted, a more conclusive result could be ascertained. Third, there is a possibility of survivorship bias. For example, women with 30 years of night shift work history should have survived at least 30 years after the start of night shift work to be included in this study as a 30-year night shift worker. Because the studies included in this meta-analysis defined the outcome as the incidence of or admission due to breast cancer, other causes of death except for breast cancer could have introduced this bias if the incidence rate of breast cancer had been different between dead night shift workers due to other causes and study participants. In future studies, this possibility should be considered. Fourth, environmental factors such as exposure to environmental estrogens or comorbidities such as type 2 diabetes mellitus and obesity may have contributed to the overall risk of breast cancer. Even though each included study adjusted for various potential confounders, it might have been difficult to consider confounders such as environmental estrogens or the use of personal care products (containing hormone disruptors) in these previous studies. These potential confounders should be considered in future studies.
Conclusion
The risk of breast cancer increased by 0.42, 4.25, 8.67, and 13.28% after 1, 10, 20, and 30 years of night shift work exposure, respectively, according to a dose-response meta-analysis of 10 reliable cohort studies. The risk of breast cancer increased by 2.13, 23.46, 52.42, and 88.17% after 1, 10, 20, and 30 years of night shift work exposure, respectively, according to a dose-response meta-analysis of 11 reliable case-control studies. This study has several strengths from the perspective of a dose-response meta-analysis: Strictly applied eight inclusion criteria, separately synthesized RRs from cohort studies and ORs from case-control studies, clearly defined exposure dose, years of night shift work for each risk estimate, a reliable dose-response meta-analysis methodology, and careful considering of selection, exposure, and outcome biases and confounder adjustment for each study. This careful consideration of potential biases and confounding led to the exclusion of unreliable two cohort and five case-control studies.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Van NTH, Hoang T, Myung SK. Night shift work and breast cancer risk: a meta-analysis of observational epidemiological studies. Carcinogenesis. 2021;42(10):1260–9.
Manouchehri E, Taghipour A, Ghavami V, Ebadi A, Homaei F, Latifnejad Roudsari R. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Womens Health. 2021;21(1):89.
IARC_Working_Group_on_the_Identification_of_Carcinogenic_Hazards_to_Humans. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. In: Lyon FR (ed.) Night shift work edn. International Agency for Research on Cancer © International Agency for Research on Cancer, 2020. For more information contact publications@iarc.fr.; 2020.
National_Toxicology_Program. NTP cancer hazard assessment report on night shift work and light at night. edn. Research Triangle Park (NC): National Toxicology Program; 2021.
Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273–84.
Urbano T, Vinceti M, Wise LA, Filippini T. Light at night and risk of breast cancer: a systematic review and dose-response meta-analysis. Int J Health Geogr. 2021;20(1):44.
Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. Total visual blindness is protective against breast cancer. Cancer Causes Control. 2009;20(9):1753–6.
Pukkala E, Ojamo M, Rudanko S-L, Stevens RG, Verkasalo PK. Does incidence of breast Cancer and prostate Cancer decrease with increasing degree of visual impairment. Cancer Causes Control. 2006;17(4):573–6.
Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28.
Crippa A, Orsini N. Multivariate Dose-Response Meta-Analysis: the dosresmeta R Package. J Stat Softw Code Snippets. 2016;72(1):1–15.
Diaz-Quijano FA. A simple method for estimating relative risk using logistic regression. BMC Med Res Methodol. 2012;12(1):14.
Lee J, Chia KS. Estimation of prevalence rate ratios for cross sectional data: an example in occupational epidemiology. Occup Environ Med. 1993;50(9):861–2.
Nijem K, Kristensen P, Al-Khatib A, Bjertness E. Application of different statistical methods to estimate relative risk for self-reported health complaints among shoe factory workers exposed to organic solvents and plastic compounds. Norsk Epidemiologi. 2009;15(1).
Sterne JAC, Becker BJ, Egger M. The Funnel Plot. In: Publication bias in meta-analysis edn.; 2005, pp. 73–98.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Akerstedt T, Knutsson A, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and breast cancer in women: a Swedish cohort study. BMJ Open. 2015;5(4):e008127–008127.
Jones ME, Schoemaker MJ, McFadden EC, Wright LB, Johns LE, Swerdlow AJ. Night shift work and risk of breast cancer in women: the generations Study cohort. Br J Cancer. 2019;121(2):172–9.
Knutsson A, Alfredsson L, Karlsson B, Åkerstedt T, Fransson EI, Westerholm P, Westerlund H. Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scand J Work Environ Health. 2013;39(2):170–7.
Koppes LLJ, Geuskens GA, Pronk A, Vermeulen RCH, De Vroome EMM. Night work and breast cancer risk in a general population prospective cohort study in the Netherlands. Eur J Epidemiol. 2014;29(8):577–84.
McNeil J, Heer E, Willemsen RF, Friedenreich CM, Brenner DR. The effects of shift work and sleep duration on cancer incidence in Alberta`s Tomorrow Project cohort. Cancer Epidemiol. 2020;67:101729.
Pronk A, Ji BT, Shu XO, Xue S, Yang G, Li HL, Rothman N, Gao YT, Zheng W, Chow WH. Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol. 2010;171(9):953–9.
Sweeney MR, Sandler DP, Niehoff NM, White AJ. Shift work and working at night in relation to breast cancer incidence. Cancer Epidemiol Biomarkers Prev. 2020;29(3):687–9.
Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang X-S, Roddam AW, Gathani T, Peto R, Green J, et al. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst. 2016;108(12):djw169.
Harma M, Ojajarvi A, Koskinen A, Lie JA, Hansen J. Shift work with and without night shifts and breast cancer risk in a cohort study from Finland. Occupational Environ Med. 2022.
Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–11.
Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93(20):1563–8.
Bustamante-Montes LP, Flores-Meza B, Hernández-Valero MA, Cárdenas-López A, Dolores-Velázquez R, Borja-Bustamante P, Borja-Aburto VH. Night shift work and risk of breast Cancer in women. Arch Med Res. 2019;50(6):393–9.
Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. JNCI: J Natl Cancer Inst. 2001;93(20):1557–62.
Fritschi L, Erren TC, Glass DC, Girschik J, Thomson AK, Saunders C, Boyle T, El-Zaemey S, Rogers P, Peters S, et al. The association between different night shiftwork factors and breast cancer: a case–control study. Br J Cancer. 2013;109(9):2472–80.
Grundy A, Richardson H, Burstyn I, Lohrisch C, SenGupta SK, Lai AS, Lee D, Spinelli JJ, Aronson KJ. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70(12):831.
Hansen J. Increased breast Cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1).
Menegaux F, Truong T, Anger A, Cordina-Duverger E, Lamkarkach F, Arveux P, Kerbrat P, Févotte J, Guénel P. Night work and breast cancer: a population-based case-control study in France (the CECILE study). Int J Cancer. 2013;132(4):924–31.
O’Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, Gammon MD, Leske MC. Fields ftE, Group BCoLIS: Shift work, light at night, and breast cancer on long island, New York. Am J Epidemiol. 2006;164(4):358–66.
Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Ardanaz E, Altzibar JM, Sanchez VM, Gómez-Acebo I, Llorca J, et al. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur J Epidemiol. 2016;31(9):867–78.
Pesch B, Harth V, Rabstein S, Baisch C, Schiffermann M, Pallapies D, Bonberg N, Heinze E, Spickenheuer A, Justenhoven C et al. Night work and breast cancer – results from the German GENICA study. Scand J Work Environ Health 2010(2):134–41.
Pham T-T, Hwang M, Lee E-S, Kong S-Y, Jung S-Y, Lee S, Kim J, Ha M, Kim S-Y, Park B. Night-shift work and risk of breast cancer in Korean women. Clin Epidemiol. 2019;11:743–51.
Wang P, Ren F-M, Lin Y, Su F-X, Jia W-H, Su X-F, Tang L-Y, Ren Z-F. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16(4):462–8.
Yang W, Shi Y, Ke X, Sun H, Guo J, Wang X. Long-term sleep habits and the risk of breast cancer among Chinese women: a case–control study. Eur J Cancer Prev. 2019;28(4).
Hansen J, Lassen CF. Nested case–control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–6.
Lie J-AS, Roessink J, Kjærheim K. Breast Cancer and night work among Norwegian nurses. Cancer Causes Control. 2006;17(1):39–44.
Szkiela M, Kusidel E, Makowiec-Dabrowska T, Kaleta D. How the intensity of night shift work affects breast cancer risk. Int J Environ Res Public Health. 2021;18(9):4570.
Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012;48(11):1722–9.
Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1.
Dun A, Zhao X, Jin X, Wei T, Gao X, Wang Y, Hou H. Association between Night-Shift Work and Cancer Risk: updated systematic review and meta-analysis. Front Oncol. 2020;10:1006.
Acknowledgements
The authors appreciate the efforts of Minji Kim, an information specialist in the medical library of Inha University, Incheon, South Korea.
ORCID ID: 0000-0002-1951-0583.
NAME: Minji Kim.
Address: Medical Library, Inha University Hospital, Inhang-ro 27, Jung-gu, Incheon, 22332 Republic of Korea.
Telephone: +82-32-890-2643.
Email: mjkim0311@inhauh.com.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Authors contributionJinyoung Moon: Conceptualization, Resources, Data Curation, Software, Writing – Original Draft, Visualization, Supervision, Project administrationAtsuko Ikeda-Araki: Writing – Review & Editing, Methodology, ValidationYongseok Mun: Investigation, Formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that all experiments were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Authors open researcher and Contributor ID (ORCID)
Jinyoung Moon: https://orcid.org/0000-0001-5948-823X.
Atusko Ikeda-Araki: https://orcid.org/0000-0002-3065-262X.
Yongseok Mun: https://orcid.org/0000-0003-0123-0361.
Registration information for meta-analysis
This dose-response meta-analysis was not registered. However, the authors can provide any information regarding the process of evidence synthesis upon request.
Protocol information
The protocol for this dose-response meta-analysis was not prepared. However, the authors can provide any information regarding the process of evidence synthesis upon request.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moon, J., Ikeda-Araki, A. & Mun, Y. Night shift work and female breast cancer: a two-stage dose-response meta-analysis for the correct risk definition. BMC Public Health 24, 2065 (2024). https://doi.org/10.1186/s12889-024-19518-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19518-2