Abstract
Background
In 2016, we conducted a systematic review to assess the feasibility of treatment monitoring for people living with HIV (PLHIV) receiving antiretroviral therapy (ART) in low and middle-income countries (LMICs), in line with the 90-90-90 treatment target. By 2020, global estimates suggest the 90-90-90 target, particularly the last 90, remains unattainable in many LMICs. This study aims to review the progress and identify needs for public health interventions to improve viral load monitoring and viral suppression for PLHIV in LMICs.
Methods
A literature search was conducted using an update of the initial search strategy developed for the 2016 review. Electronic databases (Medline and PubMed) were searched to identify relevant literature published in English between Dec 2015 and August 2021. The primary outcome was initial viral load (VL) monitoring (the proportion of PLHIV on ART and eligible for VL monitoring who received a VL test). Secondary outcomes included follow-up VL monitoring (the proportion of PLHIV who received a follow-up VL after an initial elevated VL test), confirmation of treatment failure (the proportion of PLHIV who had two consecutive elevated VL results) and switching treatment regimen rates (the proportion of PLHIV who switched treatment regimen after confirmation of treatment failure).
Results
The search strategy identified 1984 non-duplicate records, of which 34 studies were included in the review. Marked variations in initial VL monitoring coverage were reported across study settings/countries (range: 12–93% median: 74% IQR: 46–82%) and study populations (adults (range: 25–96%, median: 67% IQR: 50–84%), children, adolescents/young people (range: 2–94%, median: 72% IQR: 47–85%), and pregnant women (range: 32–82%, median: 57% IQR: 43–71%)). Community-based models reported higher VL monitoring (median: 85%, IQR: 82-88%) compared to decentralised care at primary health facility (median: 64%, IRQ: 48-82%). Suboptimal uptake of follow-up VL monitoring and low regimen switching rates were observed.
Conclusions
Substantial gaps in VL coverage across study settings and study populations were evident, with limited data availability outside of sub-Saharan Africa. Further research is needed to fill the data gaps. Development and implementation of innovative, community-based interventions are required to improve VL monitoring and address the “failure cascade” in PLHIV on ART who fail to achieve viral suppression.
Similar content being viewed by others
Background
In 2014, UNAIDS launched the 90-90-90 treatment target: by 2020, 90% of all people living with HIV (PLHIV) know their status, 90% of all people diagnosed with HIV infections receive antiretroviral therapy (ART) and 90% of all people on ART have suppressed viral load [1]. Evidence from a large body of research demonstrates that ART can improve the health of PLHIV and stop onward transmission [2,3,4]. Modelling data suggests achieving the 90-90-90 target, which means having 73% of all PLHIV virally suppressed, coupled with scaling up other prevention measures, could reduce new HIV infection and HIV-related death worldwide by 90% between 2010 and 2030 [5]. On the basis of this evidence, universal HIV testing and treatment in a broader health system approach has been identified as a key strategy for ending the epidemic by 2030, even in the absence of an effective vaccine [6].
In low and middle-income countries (LMICs), there has been an intense focus on scale-up of ART programs to increase treatment coverage in PLHIV through decentralisation of HIV services [7,8,9]. Global estimates indicate a significant increase in the number of PLHIV with access to ART over the past decade: from 6.4 (95% uncertainty intervals (UIs) 5.9–6.4) million in 2009 to 25.4 (95% UIs 24.5–25.6) million by the end of 2019, with 95% of people taking ART residing in LMICs [10, 11]. Decentralised HIV care, with services delivered at primary care level, is feasible and can improve patient access and adherence to HIV treatment whilst maintaining high quality of care in various settings [12,13,14]. However, there is legitimate concern about the sustainability of ART programs in LMICs without substantial financial and technical support from international donors and implementing partners [15, 16]. An increase in ART coverage means a greater need for treatment monitoring to ensure program effectiveness and efficiency. Treatment monitoring, however, requires substantial infrastructure, supply chain management system, financial and human resources, which are already scarce in many decentralised settings [17].
In 2016, we conducted a systematic review to assess the feasibility of ART monitoring for PLHIV in the context of decentralised HIV treatment and care in LMICs [18]. The conclusions were as follows: (1) There were limited published data on the coverage of treatment monitoring, particularly viral load (VL) monitoring for PLHIV on ART in real-world settings; (2) There were potential gaps in the coverage and quality of treatment monitoring services across countries and regions; (3) There was an urgent need to strengthen treatment monitoring (particularly VL monitoring) to improve the HIV continuum of care in LMICs; and (4) Point-of-care (POC) diagnostics could play an important role in scaling up and improving the quality of treatment monitoring for PLHIV on ART in decentralised settings.
Despite continuous gains in access to testing and treatment since 2015, the ambitious 90-90-90 treatment target, particularly the last 90, remains unattainable in many LMICs [19]. Reaching the new 95-95-95 target in 2030 will be an uphill task for these countries. To make meaningful progress towards this treatment goal will require much greater use of VL monitoring – the gold standard approach for assessment of HIV treatment at individual, program and population levels. The objectives of this study were to: (1) outline the current state of research and progress on VL monitoring for PLHIV on ART in decentralised settings in LMICs, and (2) identify gaps in research and the needs for public health interventions to improve VL monitoring coverage and quality of HIV treatment programs for PLHIV in LMICs.
Methods
The study was designed and reported in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) statement [20]. The initial search strategy developed for the 2016 review was used to search MEDLINE for articles published from 1 to 2015 (end date of prior search) to 31 May 2020. The search strategy is available online [18]. In addition, a PubMed search was conducted using following search terms: “HIV”, “routine viral load”, and “viral load monitoring” for the same period (1 December 2015–31 May 2020) with an updated search conducted in August 2021. Bibliographies of all articles included for full-text review were searched manually to identify relevant studies.
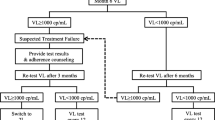
Prior to July 2021, WHO recommended that patients with elevated VL (≥ 1000 copies/ml) should be given enhanced adherence consultation (EAC) followed by a repeat VL test within 3–6 months of the initial VL. If the repeat VL remains greater than 1000 copies/ml, treatment failure is confirmed and patients should be switched to second-line ART [21]. For the purpose of this review, the following outcomes of interest were pre-defined: Primary outcome: Initial VL monitoring (the proportion of PLHIV active on first-line ART (the main treatment option available at decentralised settings in LMICs) and eligible for VL monitoring who receive a VL test for treatment monitoring purposes). Secondary outcomes include: (i) Follow-up VL monitoring (the proportion of PLHIV on ART who received a follow-up VL test following an initial elevated VL as defined by included studies); (ii) Confirmation of treatment failure (the proportion of PLHIV on ART with two consecutive elevated VL results among those who received a follow-up VL test) and; (iii) Switching treatment regimen (the proportion of PLHIV on ART switching treatment regimen among those with confirmed treatment failure as defined in included studies).
To be included in this review, studies must meet the following inclusion criteria: (1) was conducted in LMICs; (2) involved PLHIV receiving first-line ART and eligible for VL monitoring; (3) involved decentralised HIV treatment and care, defined as having treatment and treatment follow-up provided in non-hospital settings – primary health care facilities or community-based health care services; and (4) reported the primary outcome of interest. Only studies published in English were included.
Data were extracted electronically using a pre-defined data extraction form. The following information was extracted: study details (first author and year of publication, study design and data source, study population, study sites/locations, study period and study objective); VL testing model; external funding and/or technical supports received and outcome of interests. Information on treatment regimen and VL threshold for definition of elevated VL and treatment failure were also extracted, if reported. Data on outcomes of interest are presented by time point or follow-up period as per the included studies. Outcomes by model of care (community-based vs. primary health care) and subgroups of study population (e.g., children, adolescent, pregnant women, etc.) are extracted (if reported) and presented to enable data comparison. Descriptive statistics were applied to describe the outcomes of interest across countries and study settings, study populations and models of care.
Results
The search strategy yielded 1984 records after removal of duplicates. Screening titles and abstracts identified 66 studies for full-text examination, of which 32 studies met the inclusion criteria and were included. Bibliography review identified two additional studies, making a total of 34 studies included in the review (Fig. 1).
Twenty-nine of the 34 included studies were conducted in and/or had data collected from sub-Saharan Africa (SSA) countries, including South Africa [22,23,24,25,26,27,28,29,30,31,32], Zimbabwe [33,34,35,36], Rwanda [37, 38], Lesotho [39], Swaziland [40], Senegal [41], Malawi [42], Democratic Republic of Congo [43], Kenya [44], Mozambique [45] and Uganda [46,47,48]. Twenty studies were designed to assess VL monitoring or VL cascade in PLHIV on ART with long-term follow-up of > = 12 months and large sample sizes (> 500). Most (28/34) studies used routinely collected program data in real-world settings, with seven studies focusing on children, adolescent and young people aged 0–24 years, four studies on pregnant women. One study reported data from 18 LMICs in Asia and Africa over 12 year-period (2006–2018) [49] and one study was conducted among HIV infected incarcerated people [50] at three correctional complexes in South Africa and Zambia (Table 1).
Wide ranges of VL monitoring coverage in program settings were observed. Studies of adults/patients of all ages, children, adolescents (aged 0–19 years) and young people (aged < 25) years), and pregnant women reported VL coverage of 25–96% (median: 67% interquartile range IQR: 50-84%), 2–94% (median: 72%, IQR: 47-85%) and 32–82% (median: 57%, IQR: 43-71%), respectively. Studies include community-based care models reported higher initial VL monitoring coverage (median: 85%, IQR: 82-88%) compared to decentralised care at primary health facility (median: 64%, IRQ: 48-82%) (Fig. 2). Reported proportions of patients on first-line ART who received a first VL monitoring test within 6–12 months of ART initiation ranged from 12% [49] to 94% [31] (median: 74%, IQR: 46-82%). Similarly, reported annual uptake of VL monitoring varied across countries, from 25% in Zimbabwe [36] to 94% in Kenya [44] (median: 66%, IQR: 47-87%) (Table 2).
Of 11 studies from South Africa, four were conducted in Cape Town, reporting relatively high VL coverage of 72–89% [23, 25,26,27]. Studies with interventions to improve quality of ART program had a VL coverage of > 90% whilst other studies reported a VL range of 25-81%. Of the 17 studies conducted in other SSA countries, 10 studies (59%) reported funding and/or technical support from international donors/implementing partners for implementation of ART and/or VL monitoring programs. Two of four studies from Zimbabwe with donors’ support reported VL coverage at 63% [35] and 91% [33], whilst the others (with no external funding/technical support) reported VL coverage of 32% [34] and 25% [36]. Two studies reported coverage by sites, enabling a comparison between VL monitoring in decentralised care versus centralized/hospital care. A study conducted in Malawi reported slightly higher VL coverage at decentralised clinics (with direct support from Médecins Sans Frontières – MSF) than district hospitals (86% vs. 82%) [42]. In contrast, a study at public health facilities in Zimbabwe reported a significantly lower uptake of VL testing at rural health clinics (26.5%) than hospital settings (44.6%) [34].
Of five studies in Asia, three were conducted in Myanmar using national HIV program data and reported a range of VL coverage from 34% [53] to 57% [52, 55]. Two other studies were conducted in research contexts where VL testing was historically not available: one reported the outcomes of an intervention project to improve the cascade of care among key populations in Indonesia [54], and the other was a trial designed to assess the feasibility of dried blood spot (DBS) use for VL monitoring in remote regions in Vietnam [51]. Free VL testing was provided to study participants, and coverage was 71–73% at 6 months after ART initiation.
Thirteen studies provided data on the proportion of patients on ART receiving a follow-up VL test after an initial elevated VL (follow-up VL monitoring). Overall, the coverage for follow-up VL monitoring was lower than that of initial VL monitoring with nine out of 13 studies reporting gaps of 2–48% points (Table 2). The reported coverage also varies across study settings and countries, from 25 to 88% (median 65.5%, IQR: 38–77%). Eleven studies reported the outcomes of follow-up VL monitoring, and the proportions of patients with two consecutives elevated VL measurements (confirmation of treatment failure) varied from 26 to 83%, with a median of 62% (IQR: 49.5–74.5%). The reported proportion of patients switching to second-line ART after confirmation of treatment failure also varied, ranging from 18 to 85% (median 45%, IQR: 36–71%).
Discussion
The findings of this review demonstrate an increasing interest in research on VL monitoring and VL cascades for PLHIV receiving treatment at decentralised settings in LMICs over the past six years. Nearly 60% (20/34) of the included studies were specifically designed to evaluate the implementation and/or reported the outcomes of VL monitoring program for PLHIV on ART. Most of these studies were published in 2019–2021. This represents a substantial increase in the number of studies focused on VL monitoring compared to our previous review [18], with only two of 21 included studies assessing the coverage of treatment/VL monitoring services for PLHIV on ART. Recent global estimates showed that whilst the number of PLHIV who know their HIV status and the number of PLHIV who are on treatment increased steadily between 2015 and 2018, the proportion of PLHIV with viral suppression has remained stable with substantial variations across regions and many LMICs lagging behind[19]. Insufficient access to VL testing, lack of appropriate action on VL results and lack of access to second and third ART regimens were key barriers in translating significant gains in knowledge of HIV status and treatment coverage to viral suppression among PLHIV in low-resource settings [56].
Consistent with findings from previous studies, the results of this review show that there are still significant gaps in VL monitoring across countries and regions. Studies that reported high VL monitoring coverage and use of VL results for patients’ management were conducted in settings with one or more of the following: (1) support from international donors and implementing partners; (2) innovative interventions and models of service delivery for decentralisation of HIV treatment and care, such as “hub and spoke”, “differentiated care” or “adherence club”; and (3) research clinics with free HIV services, including VL testing, for participants. From programmatic perspectives, whilst studies conducted in research or program contexts with external funding and technical supports may have limited generalizability, the finding that community-based models of care, implemented by local government or health authorities, can deliver high VL coverage is encouraging, and supports the continued scale-up of decentralisation of HIV treatment and care in LMICs.
LMICs face immense financial and implementation challenges in provision of HIV treatment and routine VL monitoring for PLHIV on ART. External financial and technical supports with a multi-sector approach are crucial for the set-up and delivery of such services, particularly in decentralised settings where VL monitoring was historically unavailable [57]. However, as HIV treatment (and VL monitoring) programs mature, such interventions and supports must be integrated within the local healthcare system to ensure sustainability and long-term gains. Evidence from this review shows that even within a program, clinical sites with more direct support outperformed others in term of service delivery (VL monitoring coverage) and patients’ outcomes (VL suppression) [42]. Therefore, it is critical to strengthen local health system capacity to ensure efficient use of available resources and maintain desirable outcomes when direct external support declines. Operational research is needed to identify cost-effective interventions and best practices to improve VL outcomes for PLHIV in LMICs.
There was also evidence of a narrow geographical focus for research on HIV treatment and VL monitoring programs with most of the data come from SSA countries. Even within SSA, almost all studies included in this review were conducted in Eastern and Southern Africa – the region with the second-highest point estimate of the proportion of people who are virally suppressed among all PLHIV (58%, 95%UIs: 50–66%), behind only high-income countries with well-developed health care systems in Western and Central Europe and North America (64%, 95%UIs: 54–74%) [19]. This raises a concern about the dearth of data from countries and regions with lower level of VL coverage and viral suppression rate. Our study findings call for a renewed focus of financial resources and international research efforts in settings with limited data available and likely lack of progress in improving VL monitoring and viral suppression among PLHIV on ART.
Our findings suggest that research and targeted interventions are needed to improve VL monitoring coverage in vulnerable populations, including children, adolescents and young people, pregnant women and key populations (KPs) living with HIV.
As HIV treatment programs expand in many LMICs, children and adolescents are a priority population for the scale-up of VL monitoring to improve quality of care and treatment outcomes [58]. The startling variation (2–94%) in VL testing coverage among children and adolescents identified herein suggests that in some low-resource settings in LMICs, the health care system is unprepared and unable to address the needs of this vulnerable population. This review found only four studies that report VL monitoring in pregnant women receiving care at decentralised care settings. The findings are in line with those of previous studies [59, 60], suggesting suboptimal VL monitoring in pregnant women living with HIV on ART, with higher coverage in hospital settings [34] and/or programs funded by international donor/implementing partners [40]. Further research is needed to determine an optimal VL monitoring schedule in this population, develop and implement targeted interventions supporting those who receive PMTCT and HIV services at decentralised settings in LMICs. Ad hoc VL testing (e.g., using POC tests) should also be available for targeted VL monitoring as medically indicated or in case turnaround time for laboratory test is too long for meaningful clinical interventions.
Our review highlights the lack of data on VL monitoring for KPs in real-world settings. Only two studies [46, 54] reported the proportion of PLHIV on ART receiving VL monitoring among members of KPs with high coverage. These data, however, have poor generalizability, because levels of dedicated staff and resources would not be sustainable outside the research context. Many LMICs have concentrated HIV epidemics among KPs. Monitoring VL and level of viral suppression in PLHIV who are members of KPs and engage in high-risk behaviours is crucial to determine the trajectory of the epidemic. If those who do not achieve VL suppression and engage in high-risk behaviours are the driving force of HIV transmission in the community [61], then the ultimate goal of reducing population-level HIV incidence and ending the HIV epidemic will not be achieved [62].
Routine VL monitoring for PLHIV on ART is only meaningful if accompanied by the use of VL results for appropriate and timely clinical action. The findings of this review indicate suboptimal uptake of follow-up VL among patients with initial elevated VL (median 66%, IQR: 38–77%); high proportion of confirmed treatment failure among those patients who had a follow-up VL (median 62%, IQR: 50–75%) and a low switching rate among those with confirmed treatment failure (median 45%, IQR: 36–71%). These findings are of particular concern, because they could indicate inadequate EAC and/or a high level of treatment failure due to drug resistance that requires switching the ART regimen. It is known that prolonged treatment regimen failure significantly increases the risk of multiple drug-resistant mutations and compromises the efficacy of second-line ART in HIV-infected children [63], adults [64], and pregnant women [65]. PLHIV on ART who fail to achieve viral suppression enter a “failure cascade” that can jeopardise individual health and the overall effectiveness of HIV programs [66]. Operations research and intervention strategies are needed to address treatment adherence and the failure cascade to preserve the efficacy of first/second-line ART, thereby avoiding exhausting treatment options in LMICs.
This review contributes to a growing body of literature highlighting the importance of VL monitoring and VL cascade analysis for planning, implementation and evaluation of HIV treatment programs in the era of “Test and Treat” [67,68,69]. In decentralised settings in LMICs, technological and health system challenges occur in each step of the cascade. VL measurement is mostly done with laboratory assays, and fresh plasma is the preferred sample type. However, the cold chain system for blood sample transportation and 24-hour time window requirements preclude the use of this sample type in rural and remote areas. Dried blood spot (DBS) is an alternative sampling method, but may reduce the accuracy of VL measurement due to the detection of proviral DNA and intracellular RNA in whole blood samples, leading to over-quantification of viral load result [70]. It is noted that in the newly updated WHO guideline [71], a VL cut-off of 50 copies/ml was added on the treatment monitoring algorithm to identify PLHIV with low-level viremia (50-1000 copies/ml). This addition is likely to make the use of DBS for routine VL monitoring even more challenging as the limit of detection of 50 copies/ml, using this sampling method, is unlikely to be achieved with current VL testing platforms [72].
New sampling technologies [73, 74] have emerged, enabling accurate VL measurement by removing blood cells from the plasma component without the need for centrifugation. These are promising alternatives to the DBS as they could allow the detection of low-level viremia. However, further research is needed to determine the limit of detection, feasibility, and cost-effectiveness of these new devices/methods in real-world settings. New POC VL technologies have also become available and have the potential to decentralise VL testing. The use of these technologies, especially when VL results are more time-sensitive (such as VL results for breast feeding women and their children, people with an initial elevated VL) would be important to enable timely clinical decision and improve treatment outcomes. As HIV treatment programs mature, the number of PLHIV on ART will continue to increase, as well as the need for VL monitoring, second-line [75] and third-line ART [76], even in settings with lack of access to drug resistance testing. These challenges need to be addressed simultaneously and systematically to ensure the long-term effectiveness and efficacy of HIV treatment in LMICs.
Limitation
This review had several limitations. Only peer-reviewed articles published in English were considered, meaning grey literature (unpublished and/or non-peer-reviewed and/or non-English national/program reports) with data on the outcomes of interest (e.g., annual PEPFAR monitoring and evaluation reports) was overlooked. The lack of data from geographic regions outside of SSA also raises a concern about bias and the generalizability of the findings. Another limitation is that some of the recently published studies report data collected 10–15 years ago which may not necessarily represent the recent uptake of VL monitoring. However, given the nature of the data reported from included studies (e.g., a wide range of VL coverage across study settings); these limitations are unlikely to affect the results and their interpretation substantially. Few studies reported a complete viral load cascade (number/proportion of patients with routine VL tests, unsuppressed VL, enhanced adherence counselling, follow-up VL and confirmed treatment failure). Researchers are encouraged to report on the viral load cascade of patients failing first-line ART and patients on second-line ART to enable understanding of the magnitude of the problem and identify intervention strategies and resources required to meet the needs of these vulnerable patients.
Conclusions
Since 2016, there has been marked growth in the peer-reviewed literature on VL monitoring and VL cascade in PLHIV in LMICs. Most studies were conducted in SSA with significant financial and technical support from international/bilateral donors and implementing partners. Available data suggest significant gaps in VL monitoring services across countries and geographic regions, highlighting the needs to strengthen health system capacity for effective and sustainable implementation of routine VL monitoring for PLHIV who receive ART at decentralised care settings in LMICs. Particular attention is needed to rectify the failure cascade to ensure that VL monitoring and follow-up clinical actions are taken for individuals on ART who fail to achieve viral suppression. To advance the global agenda towards ending the HIV epidemic by 2030, we must fill the data gaps outside SSA; support the development and implementation of targeted interventions to improve VL monitoring and the VL cascade among populations at high risk of unsuppressed VL but with poor access to HIV treatment and care.
Availability of data and materials
All data generated or analysed during this study are included in the manuscript.
Abbreviations
- ART:
-
antiretroviral therapy
- DBS:
-
Dried blood spot
- EAC:
-
Enhanced adherence counselling
- KPs:
-
Key populations
- LMICs:
-
Low and middle-income countries
- PLHIV:
-
People living with HIV
- POC:
-
Point of care
- PMTCT:
-
Prevention of mother to child transmission
- SSA:
-
sub-Saharan Africa
- VL:
-
Viral load
References
UNAIDS, 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014, Joint United Nations Progamme on HIV/AIDS: Geneva, Switzeland. Available at https://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed 14 July 2020.
Quinn TC, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9.
Grinsztejn B, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90.
Nachega JB, et al. Achieving Viral Suppression in 90% of People Living With Human Immunodeficiency Virus on Antiretroviral Therapy in Low- and Middle-Income Countries: Progress, Challenges, and Opportunities. Clin Infect Dis. 2018;66(10):1487–91.
Stover J, et al. What Is Required to End the AIDS Epidemic as a Public Health Threat by 2030? The Cost and Impact of the Fast-Track Approach. PLoS One. 2016;11(5):e0154893.
Bekker LG, et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society-Lancet Commission. Lancet. 2018;392(10144):312–58.
Charles B, Lalthanmawia R. Providing HIV treatment closer to patient’s homes compared to more centralised treatment. Clin Epidemiol Global Health. 2013;1(2):94–5.
Kredo T, et al. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev. 2013;(6):Cd009987.
Suthar AB, et al. Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS. 2014;28(Suppl 2):S175-85.
UNAIDS, Global HIV & AIDS statistics – 2020 Fact Sheet 2020, Joint United Nations Programme on HIV and AIDS: Geneva, Swizerland. Available at https://www.unaids.org/en/resources/fact-sheet. Accessed 10 July 2020.
UNAIDS, How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. 2015, Joint United Nations Programme on HIV and AIDS Geneva. Switzerland. Available at https://www.unaids.org/en/resources/documents/2015/MDG6_15years-15lessonsfromtheAIDSresponse. Accessed 20 Dec 2019.
Mutevedzi PC, et al. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010;88(8):593–600.
Boyer S, et al. Performance of HIV care decentralization from the patient’s perspective: health-related quality of life and perceived quality of services in Cameroon. Health Policy Plan. 2012;27(4):301–15.
Abongomera G, et al. Patient-level benefits associated with decentralization of antiretroviral therapy services to primary health facilities in Malawi and Uganda. Int Health. 2018;10(1):8–19.
Haakenstad A, et al. Potential for additional government spending on HIV/AIDS in 137 low-income and middle-income countries: an economic modelling study. Lancet HIV. 2019;6(6):e382–95.
Resch S, Ryckman T, Hecht R. Funding AIDS programmes in the era of shared responsibility: an analysis of domestic spending in 12 low-income and middle-income countries. Lancet Glob Health. 2015;3(1):e52-61.
Roberts T, et al. Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clin Infect Dis. 2016;62(8):1043–8.
Pham MD, et al. Feasibility of antiretroviral treatment monitoring in the era of decentralized HIV care: a systematic review. AIDS Res Ther. 2017;14(1):3.
Marsh K, et al. Global, regional and country-level 90-90-90 estimates for 2018: assessing progress towards the 2020 target. AIDS. 2019;33(Suppl 3):S213–26.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendation for a public health approach - Second edition. 2016, The World Health Organization: Geneva, Switzerland. Available at https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 20 Sept 2021.
Iwuji CC, et al. Clinical outcomes after first-line HIV treatment failure in South Africa: the next cascade of care. HIV Med. 2020;21(7):457–62.
Kehoe K, et al. Long-term virologic responses to antiretroviral therapy among HIV-positive patients entering adherence clubs in Khayelitsha, Cape Town, South Africa: a longitudinal analysis. J Int AIDS Soc. 2020;23(5):e25476.
Le Roux KW, et al. A Case Study of an Effective and Sustainable Antiretroviral Therapy Program in Rural South Africa. AIDS Patient Care & Stds. 2019;33(11):466–472.
Euvrard J, et al. How accurately do routinely reported HIV viral load suppression proportions reflect progress towards the 90-90-90 target in the population on antiretroviral treatment in Khayelitsha, South Africa? South Afr Med J Suid-Afrikaanse Tydskrif Vir Geneeskunde. 2019;109(3):174–7.
Copelyn J, et al. Short-term outcomes of down-referral in provision of paediatric antiretroviral therapy at Red Cross War Memorial Children’s Hospital, Cape Town, South Africa: A retrospective cohort study. South Afr Med J Suid-Afrikaanse Tydskrif Vir Geneeskunde. 2018;108(5):432–8.
Tsondai PR, et al. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc. 2017;20(Suppl 4):21649.
Haghighat R, et al. The HIV care cascade for adolescents initiated on antiretroviral therapy in a health district of South Africa: a retrospective cohort study. BMC Infect Dis. 2021;21(1):60.
Woldesenbet SA, et al. Coverage of maternal viral load monitoring during pregnancy in South Africa: Results from the 2019 national Antenatal HIV Sentinel Survey. HIV Med. 2021;22(9):805–15. https://doi.org/10.1111/hiv.13126. Epub 2021 Jul 1.
Mshweshwe-Pakela N, et al. Feasibility of implementing same-day antiretroviral therapy initiation during routine care in Ekurhuleni District, South Africa: Retention and viral load suppression. South Afr J HIV Med. 2020;21(1):1085.
Sunpath H, et al. Targeting the third ‘90’: introducing the viral load champion. Public Health Action. 2018;8(4):225–31.
Moyo F, et al. Near-real-time tracking of gaps in prevention of mother-to-child transmission of HIV in three districts of KwaZulu-Natal Province, South Africa. S Afr Med J. 2018;108(4):319–24.
Tapera T, et al. Effects of a Peer-Led Intervention on HIV Care Continuum Outcomes Among Contacts of Children, Adolescents, and Young Adults Living With HIV in Zimbabwe. Glob Health Sci Pract. 2019;7(4):575–84.
Nyakura J, et al. Viral load testing among women on ‘option B+’ in Mazowe, Zimbabwe: How well are we doing? PLoS One. 2019;14(12):e0225476.
Nyagadza B, et al. Scaling up HIV viral load monitoring in Manicaland, Zimbabwe: challenges and opportunities from the field. Public Health Action. 2019;9(4):177–81.
Apollo T, et al. Provision of HIV viral load testing services in Zimbabwe: Secondary data analyses using data from health facilities using the electronic Patient Monitoring System. PLoS One. 2021;16(1):e0245720.
Ndagijimana Ntwali JD, et al. Viral load detection and management on first line ART in rural Rwanda. BMC Infect Dis. 2019;19(1):8.
Cyamatare Rwabukwisi F, et al. Five-year Outcomes Among Children Receiving Antiretroviral Therapy in a Community-based Accompaniment Program in Rural Rwanda. Pediatr Infect Dis J. 2016;35(11):1222–4.
Amzel A, et al. Community-Based Interventions to Reach 95-95-95 for Children and Adolescents: An Exploratory Programmatic Review From Lesotho. J Acquired Immune Deficiency Syndromes. 2018;78(Suppl 2):S81–7.
Etoori D, et al. Challenges and successes in the implementation of option B + to prevent mother-to-child transmission of HIV in southern Swaziland. BMC Public Health. 2018;18(1):374.
Cisse AM, et al. High level of treatment failure and drug resistance to first-line antiretroviral therapies among HIV-infected children receiving decentralized care in Senegal. BMC Pediatr. 2019;19(1):47.
Nicholas S, et al. Point-of-care viral load monitoring: outcomes from a decentralized HIV programme in Malawi. J Int AIDS Soc. 2019;22(8):e25387.
Moudachirou R, et al. Retention and sustained viral suppression in HIV patients transferred to community refill centres in Kinshasa, DRC. Public Health Action. 2020;10(1):33–7.
Kadima J, et al. Adoption of routine virologic testing and predictors of virologic failure among HIV-infected children on antiretroviral treatment in western Kenya. PLoS One. 2018;13(11):e0200242.
Swannet S, et al. Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health. 2017;9(4):206–14.
Namale G, et al. Sustained virological response and drug resistance among female sex workers living with HIV on antiretroviral therapy in Kampala, Uganda: a cross-sectional study. Sex Transm Infect. 2019;95(6):405–11.
Nakalega R, et al. Non-uptake of viral load testing among people receiving HIV treatment in Gomba district, rural Uganda. BMC Infect Dis. 2020;20(1):727.
Opito R, et al. Treatment outcome of the implementation of HIV test and treat policy at The AIDs Support Organization (TASO) Tororo clinic, Eastern Uganda: A retrospective cohort study. PLoS One. 2020;15(9):e0239087.
Brazier E, et al. Effects of national adoption of Treat-All guidelines on pre-ART CD4 testing and viral load monitoring after ART initiation: A regression discontinuity analysis. Clin Infect Dis. 2021;73(6):e1273–e1281. https://doi.org/10.1093/cid/ciab222.
Herce ME, et al. Universal test-and-treat in Zambian and South African correctional facilities: a multisite prospective cohort study. Lancet HIV. 2020;7(12):e807–16.
Kyaw NT, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005–2015. PLoS One. 2017;12(2):e0171780.
Thinn KK, et al. Uptake of routine viral load testing among people living with HIV and its implementation challenges in Yangon region of Myanmar: a mixed-methods study. BMJ Open. 2019;9(12):e032678.
Ya SST, et al. Performance and Outcomes of Routine Viral Load Testing in People Living with HIV Newly Initiating ART in the Integrated HIV Care Program in Myanmar between January 2016 and December 2017. Trop Med Infect Dis. 2020;5(3):140. https://doi.org/10.3390/tropicalmed5030140.
Januraga PP, et al. The cascade of HIV care among key populations in Indonesia: a prospective cohort study. Lancet HIV. 2018;5(10):e560–8.
Nguyen TA, et al. Feasibility of dried blood spots for HIV viral load monitoring in decentralized area in North Vietnam in a test-and-treat era, the MOVIDA project. PLoS One. 2020;15(4):e0230968.
Ehrenkranz PD, et al. The missed potential of CD4 and viral load testing to improve clinical outcomes for people living with HIV in lower-resource settings. PLoS Med. 2019;16(5):e1002820.
Carmona S, Peter T, Berrie L. HIV viral load scale-up: multiple interventions to meet the HIV treatment cascade. Curr Opin HIV AIDS. 2017;12(2):157–64.
Han WM, et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV. 2021;8(12):e766–75.
Sandbulte M, et al. Maternal viral load monitoring: Coverage and clinical action at 4 Kenyan hospitals. PLoS One. 2020;15(5):e0232358.
Yotebieng M, et al. HIV viral suppression among pregnant and breastfeeding women in routine care in the Kinshasa province: a baseline evaluation of participants in CQI-PMTCT study. J Int AIDS Soc. 2019;22(9):e25376.
Cote AM, et al. Transactional sex is the driving force in the dynamics of HIV in Accra, Ghana. AIDS. 2004;18(6):917–25.
Huerga H, et al. Higher risk sexual behaviour is associated with unawareness of HIV-positivity and lack of viral suppression - implications for Treatment as Prevention. Sci Rep. 2017;7(1):16117.
Vaz P, et al. Compromise of Second-Line Antiretroviral Therapy Due to High Rates of Human Immunodeficiency Virus Drug Resistance in Mozambican Treatment-Experienced Children With Virologic Failure. J Pediatric Infect Dis Soc. 2020;9(1):6–13.
Kantor R, et al. HIV-1 second-line failure and drug resistance at high-level and low-level viremia in Western Kenya. AIDS. 2018;32(17):2485–96.
Pennings PS. HIV Drug Resistance: Problems and Perspectives. Infect Dis Rep. 2013;5(Suppl 1):e5.
Labhardt ND, et al. When patients fail UNAIDS’ last 90 - the “failure cascade” beyond 90-90-90 in rural Lesotho, Southern Africa: a prospective cohort study. J Int AIDS Soc. 2017;20(1):21803.
Etoori D, et al. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second-line switches in Swaziland. J Int AIDS Soc. 2018;21(10):e25194.
Glass TR, et al. The viral load monitoring cascade in a resource-limited setting: A prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PLoS One. 2019;14(8):e0220337.
Ford N, et al. HIV viral resuppression following an elevated viral load: a systematic review and meta-analysis. J Int AIDS Soc. 2019;22(11):e25415.
Smit PW, et al. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS One. 2014;9(3):e86461.
WHO, Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: Recommendation for a public health approach. 2021, The World Health Organization: Geneva, Switzerland. Available at https://www.who.int/publications/i/item/9789240031593. Accessed 30 Mar 2022.
WHO, HIV molecular diagnostics toolkit to improve access to viral load testing and infant diagnosis. 2019, The World Health Organization: Geneva, Switzerland. Available at https://www.who.int/publications/i/item/9789241516211. Accessed 30 Mar 2022.
Pham MD, et al. Performance of a Novel Low-Cost, Instrument-Free Plasma Separation Device for HIV Viral Load Quantification and Determination of Treatment Failure in People Living with HIV in Malaysia: a Diagnostic Accuracy Study. J Clin Microbiol. 2019;57(4):e01683–18. https://doi.org/10.1128/JCM.01683-18. Print 2019 Apr.
Carmona S, et al. Separation of Plasma from Whole Blood by Use of the cobas Plasma Separation Card: a Compelling Alternative to Dried Blood Spots for Quantification of HIV-1 Viral Load. J Clin Microbiol. 2019;57(4):e01336–18. https://doi.org/10.1128/JCM.01336-18. Print 2019 Apr.
Estill J, et al. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV. 2016;3(3):e132-9.
Eholie SP, et al. Implementation of an intensive adherence intervention in patients with second-line antiretroviral therapy failure in four west African countries with little access to genotypic resistance testing: a prospective cohort study. Lancet HIV. 2019;6(11):e750–9.
Acknowledgements
The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program received by the Burnet Institute. We thank Campbell Aitken at the Burnet Institute for his valuable comments on the draft of the manuscript.
Funding
The authors receive no funding for this work.
Author information
Authors and Affiliations
Contributions
MDP, SL, SC conceptualise the study. MDP, HVN performed literature search, data extraction and data analysis. MDP wrote the main manuscript. HVN and MDP prepared the figure and tables. DA, SC, SL reviewed and commented on the first and final drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pham, M.D., Nguyen, H.V., Anderson, D. et al. Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead – a systematic review. BMC Public Health 22, 1203 (2022). https://doi.org/10.1186/s12889-022-13504-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-13504-2