Abstract
Background
Using Zonulin and Copeptin as potential obesity markers in children, hasn’t yet been focused.
Aim
To evaluate the association between serum levels of both Zonulin and Copeptin with the obesity markers, and to assess their role as metabolic disturbance predictors in obese children.
Methods
A case-control study comprised 111 Egyptian children (45 males and 66 females); aged 6–10 years to avoid the effect of puberty (prepubertal). They were classified according to their body mass index (BMI) percentiles into: 72 obese (BMI ≥ 95th ), and 39 control ones (BMI > 15th - <85th ), based on the Egyptian Growth Charts for children and adolescents. Anthropometric parameters and blood pressure were measured, and body composition analysis, lipid profile, Zonulin, and Copeptin levels were assessed.
Results
The obese group showed a significantly higher value of Copeptin and a lower value of Zonulin than the control one Also, the obese group showed significant negative correlations between Zonulin and both anthropometric obesity markers and body composition, whereas Copeptin showed significant positive ones. Moreover, significant positive correlations were found between Copeptin and both body weight and fat distribution. Insignificant correlations were observed between both serum Zonulin and Copeptin levels and blood pressure and lipid profile.
Conclusion
Zonulin and Copeptin cannot be used as metabolic disturbance predictors, among Egyptian children, as they were insignificantly correlated with lipid profile or blood pressure.
Similar content being viewed by others
Introduction
Overweight and obesity are regarded as essential causes of morbidity and mortality worldwide. Their prevalence has dramatically increased and has become a global pandemic issue, among both children and adolescents [1]. This global problem imposes the discovery of novel obesity biomarkers, as well as, its associated metabolic disorders [2]. In Egypt, Hassan et al. [3]; in 2018; reported that the estimated prevalence of overweight and obese Egyptian children aged 6 to 18 years in Giza Governorate, was 11.0% and 19.5%, respectively based on the Standardized Egyptian Growth Curves for children and adolescents [4]. Whereas it was 13.3% and 10.5%, respectively among Upper Egypt primary schools’ children, according to the Centers for Disease Control “CDC” growth chart, as reported by Abd El-Aty et al.; in 2020 [5].
Obesity can be attributed to several genetic, environmental and behavioral factors [6]. Tuncay et al. [7], suggested that the pro-inflammatory peptides released due to intestinal barrier dysfunction, in obesity, may play an important role. Moreover, serum zonulin level may be regarded as a valuable marker of intestinal permeability [8].
Zonulin, is a protein that acts via modulating the intracellular tight junctions, thus increasing the intestinal permeability in the small intestine. The increased exposure to pathogens and allergens, due to weakening of the intestinal barrier leads to obesity and metabolic abnormalities [9]. The intestinal epithelium plays a major role in nutrient digestion, solutes and electrolytes absorption in addition to acting as a barrier; tightly controlling antigen leakage from the intestinal lumen to the sub-mucosa. In turn, this leakage disturbs the balance between immune response and tolerance, and hence causes inflammation. Secondary to increased Zonulin, the only known physiological modulator of intercellular tight junctions is lost, and hence allows uncontrolled attack of microbial and dietary antigens [10]. Little information is available about the relationship between Zonulin and obesity, and the possibility of its use as valuable marker for obesity and metabolic disorders in children and adolescents [11].
Copeptin, the Arginine vasopressin (AVP) surrogate, is a peptide co-synthesized together with the AVP hormone in the endothelial cells and pituitary gland. It is characterized by its vasoactive, immune modulating, and metabolic properties, short half-life, higher stability, being easier to measure than the active hormone AVP and requiring fewer pre-analytical procedures. More recently, Copeptin has been used as a reliable marker of serum AVP concentration; as it is secreted in equimolar amounts with AVP [12,13,14].
Although, several studies conducted on adult populations have assessed the efficacy of Copeptin in diagnosing pathways of AVP-related diseases [14, 15], but in children no studies were found except that of Tuli et al. [2] who studied the plasma level of Copeptin and its use as a significant diagnostic tool for AVP-related diseases in children .
Reviewing the literature, no studies were found to assess the relationship between Zonulin and Copeptin as potential markers for obesity and their associated disorders in both children and adolescents. Hence, the aim of the current study was to assess the association between both Zonulin and Copeptin serum levels and the obesity and metabolic markers, in addition to their roles as potential predictor markers for metabolic disturbances in obese children.
Subjects and methods
Subjects
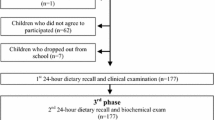
This case-control study comprised 111 children (45 ♂ and 66 ♀); whose ages ranged between 6 and 10 years (to avoid the effect of puberty) i.e. pre-pubertal. The exclusion criteria (by full History taking and clinical examination) were the presence of any sign of puberty according to Tanner stage, presence of identified causes of obesity (genetic syndromes, chromosomal or endocrinal disorders), chronic diseases (cardiovascular, gastrointestinal, and respiratory), or drug use like steroids; that would interfere with the type of obesity and affect the normal growth of the children. Also, any child with a BMI between 85th to 95th percentiles (overweight) was excluded from the study. All participating obese children were suffering from exogenous simple obesity. Based on Egyptian Growth Curves for children and adolescents, BMI ≥ 95th percentile was considered obese, for age and sex [4]. According to their BMI percentiles, participants were classified into 2 groups: group (1) comprising 72 obese children (BMI ≥95th percentile), and group (2) comprising 39 control ones (BMI >15th - <85th percentiles). The study was conducted; during the period between December 2018 and February 2021; on children who attending the “Management of Visceral Obesity and Growth Disturbances clinic”, in the “MRCE” (the Medical Research Centre of Excellence) of the “NRC” the National Research Centre, Giza, Egypt.
Ethical consent
Approvals from Ethics Committees of both Faculty of Postgraduate Childhood Studies and the “National Research Centre” were obtained (Approval No. 17/123). After explaining the promising benefits of the study in ascertaining the impact of obesity on health, informed written consent was obtained from either parent.
Methods
For each child the following was done: blood pressure assessment, anthropometric measurements, and body composition and laboratory investigations.
Blood pressure assessment
A standardized mercury sphygmomanometer was used to measure both systolic and diastolic blood pressures; with the subject in sitting position and with an appropriate cuff covering at least 2/3 of the left upper arm length, and not surpassing on the antecubital space. Three consecutive readings were taken, then if the error was acceptable, the mean was recorded [16].
Anthropometric measurements
The anthropometric parameters measured included: weight (Wt), height (Ht), waist circumference (WC), hip circumference (HC) and the skin folds thicknesses of: triceps, biceps, sub scapular, suprailiac and abdominal, following the “IBP” recommendations [17].
Body weight was recorded using a standing digital SECA scale balance (Model 707), to the nearest 0.1 kg. Average body height was recorded to the nearest 0.1 cm, after being measured three successive times, using a wall mounted Holtain Stadiometer. Waist circumference (WC) was measured using simple non-stretchable plastic measuring tape, to the nearest 0.1 cm, at a level midway between the lower rib margin and iliac crest, all around the body in horizontal position. Hip circumference (HC) was measured using simple non-stretchable plastic measuring tape, measuring the largest diameter at the maximum extension of the buttocks above the symphisis pubis overlapping the apex of the buttocks, to the nearest 0.1 cm. Skin fold thickness was measured to the nearest 0.1 mm., at the left side of the body; with the Holtain Skin fold Calipers [Holtain ltd., UK, Wales, Cross well, no. 646,420].
From the previous measurements, the following ratios and indices were calculated: body mass index (BMI); using the formula: BMI = Weight (Kg) / [Height (m2)] [18]; waist/hip ratio in cm (WHR), waist/height ratio in cm. (WHTR), peripheral obesity index (= triceps + biceps skin fold thickness) in mm. and central obesity index (= Sub scapular + Suprailiac + abdominal skin fold thicknesses) in mm.
After calculating the BMI for every child, it was plotted, according to his age, on the Egyptian Sex-Specific Growth Chart [4], as a result they were classified as 72 obese and 39 normal weight (control) groups.
Body composition analysis
Body Composition Analysis was measured using computerized Holtain’s BCA (Holtain Body Composition Analyzer (Holtain ltd., UK, Wales, Crosswell No. 646512). Impedance measurement was done 2 or 3 hours after eating and within 30 minutes of voiding urine. Being in supine position, the child laid with the arms at the side of his body, but not touching it. Following the manual instructions, four self-adhesive disposable electrodes were applied; 2 to the back of the right wrist joint and 2 to the front of the right ankle joint. A constant current of 800 µA at 50 kHz was applied across the body. The impedance of the body under test, according to Ohm’s Law, was given by the corresponding voltage drop. The subject’s sex, age, weight and height; approximated to the nearest unit; together with the measured value of the impedance were fed into specially programmed ‘organizer’ which then displays the BF% (the fraction of the total body fat mass), Fat Mass (FM); the fraction of the total body weight that is adipose tissue), Fat-Free Mass (FFM); the fraction of the total body weight that is not adipose tissue), body water content (liter), Basal Metabolic Rate (BMR) in kilo calories at rest at rest: the rate at which the body uses energy, to maintain vital functions.
Laboratory investigations
A 12 h of fasting, a 5 ml venous blood sample of was obtained from every child. Samples were left to clot, then centrifuged and sera were separated to be kept at -80 °C for further assessment of lipid profile, zonulin and Copeptin levels.
Quantitative enzymatic colorimetric determination of serum triglycerides was assessed using test kit code no: SU033, SU034, SU035 (CHEMELEX, S.A., Barcelona). Assessment of total cholesterol was done using, CHOD-POD enzymatic colorimetric method, serum test kit Ref: 101–0440/101–0526 (CHRONOLAB SYSTEMS, Barcelona). HDL was assessed using kit code no: SU014 (CHEMELEX, S.A., Barcelona). Serum triglycerides, total cholesterol and HDL were assessed according to the method of Tietz [19]. While LDL was assessed using kit REF: 99 06 10 (QUIMICA CLINICA APLICADA S.A., Spain) according to Polvinyl Sulphate method of Demacker et al. [20].
Both serum Zonulin and Copeptin were assessed using BRAHMSCT-proAVP LIA Kit (B.R.A.H.M.S. GmbH, Hennigsdorf Germany). Enzyme Linked Immunosorbent Assay (ELISA) was used to assess Serum Zonulin, based on the principle of competitive enzyme immunoassay. In this assay, the micro plate in the kit is pre-coated with antibody specific to the Zonulin. The standard is reconstituted and prepared by serial dilution with sample diluents. The serum samples are diluted 1:2000 for zonulin. Standards and samples are loaded into the appropriate micro titer plate wells and any Zonulin present is bound by immobilized antibody. A standard curve of known concentration of analyte is established and the concentration of Zonulin in the samples is calculated accordingly. The sensitivity of ELISA assays for Zonulin is 0.156 ng/mL; with a detection range of 0.6–10.8 ng/ mL. Serum Copeptin samples were collected and then assessed; in tubes containing (EDTA); in parallel to serum osmolality, by an immune-luminometric assay, with detection limit < 0.4 pmol/L and functional assay sensitivity < 1 pmol/L, a detection range 15–270 pg/ L.
Statistical analysis
The gathered data were statistically analyzed using the SPSS computer program version 16 (Statistical Package for Social Science). Data Normality test was done using the Kolmogorov-Smirnov. Descriptive statistics (mean ± SD) were calculated for the anthropometric and body composition parameters and laboratory investigations. Quantitative parametric comparison of the 2 groups was done using the Mann-Whitney test, to find group differences. To evaluate the correlations between both Zonulin and Copeptin and all the studied variables, Spearman´s correlation was used. In all statistical analyses, the value of P < 0.01 was regarded as highly significant, while P < 0.05 was considered statistically significant.
Results
Non-parametric tests were carried out since the studied variables were not normally distributed (i.e. BMI, WC, HC, all skin fold thicknesses, body composition and laboratory investigations; particularly Zonulin and Copeptin). Moreover, insignificant sex differences were reported among all the studied variables (Table 1), hence the analyses were done without sex differentiation.
Table 2 revealed highly significant differences between obese and control groups (blood pressure, anthropometric and body composition parameters), where the obese group had higher values; except BMR where the control group had higher values. Concerning the laboratory investigations, the obese group had higher significant values of total cholesterol (p = 0.030) and lower highly significant values of HDL (p = 0.000). Levels of both LDL and TG showed insignificant differences between the obese and control group. The obese group had a higher significant value of Copeptin (p = 0.031) and a lower significant value of Zonulin (p = 0.029).
Table 3 presented Spearman’s correlation analysis, between both Zonulin and Copeptin and the other studied parameters, among the obese children. Serum Zonulin showed significant negative correlation, whereas serum Copeptin showed significant positive correlations with the obesity markers: [BMI (p = 0.001, p = 0.000), WC (p = 0.006, p = 0.001), HC (p = 0.000), WHTR (p = 0.006, p = 0.002), SCSF (p = 0.002, p = 0.006)] and body composition [FM (p = 0.035, p = 0.009), FFM (p = 0.004, p = 0.000), TBW (p = 0.011, p = 0.001), and BMR (p = 0.006, p = 0.001)]. Moreover, serum Copeptin had significant positive correlation with Weight (p = 0.017), and fat distribution [TSF (p = 0.032), ABSF (p = 0.004), and Central obesity index (p = 0.007)]. Moreover, serum Zonulin and Copeptin had significant negative correlation with each other (P = 0.000). Results of the present study revealed insignificant correlations between both Zonulin and Copeptin and all of the studied lipid profile and blood pressure.
Table 4 showed Spearman’s correlation analysis between both Zonulin and Copeptin and the other studied variables among control group. It revealed that serum Zonulin had significant negative correlation, while serum Copeptin had significant positive correlations with Weight (p = 0.000), Height (p = 0.000), HC (p = 0.031, p = 0.007), and TG (p = 0.029, p = 0.011). Whereas, Serum Zonulin had significant positive correlation with WHTR (p = 0.001, p = 0.000), and HDL (p = 0.012).While, serum Copeptin had significant negative correlation with some obesity markers [WHR (p = 0.035), WHTR (p = 0.000)], fat distribution [most of the investigated skinfold thickness: BSF (p = 0.004), TSF (p = 0.018), SCSF (p = 0.021), ABSF (p = 0.004) and peripheral obesity index (p = 0.004)]. Although, the findings of the present study revealed highly significant negative correlations between Zonulin and Copeptin (P = 0.000), yet both Zonulin and Copeptin showed insignificant correlations with blood pressure (SBP and DBP), cholesterol and LDL.
Discussion
Obese children are more likely to become obese adults who are more likely to have chronic diseases. Moreover, they may develop early symptoms of chronic disease without being conscious of the problem, and consequently worsen the probable disease complications [21].
Insignificant sex differences were recorded in the current study; particularly in serum Zonulin and Copeptin. This came in agreement with previous studies which revealed insignificant sex difference among obese children and adolescents in the serum Zonulin level [11, 22, 23], and serum Copeptin [2, 24, 25] .
The present study found that obese children had significantly higher values than normal-weight healthy children, in all the studied obesity markers (anthropometric measurements), fat distribution, and body composition parameters; except BMR; and total cholesterol, and significantly lower value of HDL and BMR, but there were insignificant differences between obese and control children regarding LDL and TG.
In agreement with the current results, the increased values of the anthropometric parameters (BMI, WC, HC, WHR and WHTR) and body composition (FM and BF %) in obese children more than the normal weight ones were reported in different studies [11, 23, 26, 27]. Coinciding with current laboratory findings, significantly higher total cholesterol and significantly lower HDL-C were reported by Tuncay et al. [7], Song et al. [28], and Martin et al. [29] in obese children and adolescents.
In contrary to current results, Tuncay et al. [7], Liudmyla et al. [10] and Martin et al. [29], recorded significant higher triglycerides and LDL-C; in obese children and adolescents. While similar HDL-C levels in both obese and normal weight children was recorded by Yin et al. [25]. This might be attributed to differences of age group, ethnicity or effect of puberty.
The current results revealed that; the obese group showed significantly higher value of Copeptin and a lower value of Zonulin than the control one. There was significant negative correlation between serum Zonulin and Copeptin among both obese and control children. Among the obese group, levels of serum Zonulin revealed significant negative correlations, while Copeptin showed significant positive correlations with some obesity markers (BMI, WC, HC, WHTR, SCSF) and body composition (TBW, FFM and FM and BMR), and insignificant correlations with blood pressure and lipid profile. In addition, Copeptin had significant positive correlations with fat distribution (TSF, ABSF and central obesity index).
Similar with the results of the current study, higher Copeptin level was reported by Gerdi et al. [2], among obese children than in non-obese ones, and by Tenderenda-Banasiuk et al. [30] and Rothermel et al. [31] among both obese children and adolescents. Hossam et al. [13] reported significant correlation between serum Copeptin level and BMI. Saleem et al. [32] and Enhörning et al. [33]. attributed the elevated serum Copeptin level in overweight children and adolescents to the role played by Copeptin, as one of the mediators associated with chronic stress like obesity and metabolic syndrome, i.e. activation of the hypothalamic-pituitary-adrenal axis by AVP.
In disagreement to current findings, Schiel et al. [34] found that serum Copeptin level, in diabetic obese children, was lower than that of the healthy control group. Lewandowski et al. [35] also found lower serum Copeptin levels in obese adults compared to normal-weight ones. Gohar et al. [36] observed an insignificant association between the levels of serum Copeptin and both body weight and waist circumference “WC”, when adjusting their results for age and BMI. Moreover, a significant higher level of Zonulin level was reported by Tuncay et al. [7], Kim et al. [37], Liudmyla et al. [10]. , and Strashok et al. [11], in obese children and adolescents.
Moreover, Tuncay et al. [7] found positive correlation between serum Zonulin levels and BMI, BF% and LDL-C., whereas in obese and normal weight/control male groups it was negatively correlated with HDL-C. Lusikelelwe et al. [9] found that; among overweight/obese females; Zonulin was positively associated with BMI. This may be explained by the sample sex and age difference; as that of Lusikelelwe et al. comprised South African adult females only, but that of the current study included Egyptian children of both sexes.
Strashok et al. [11] found positive correlations between Zonulin level, body weight and abdominal fat distribution. Liudmyla et al. [10] also found positive correlations between Zonulin level and body weight in adolescents. This may be explained by the different age group of the current study and the effect of puberty.
It is worth mentioning that Zonulin in the current study correlated negatively with the obesity markers (most of the anthropometric measurements) and body composition. It may be related to the fact that nobody has a clear understanding of the physiological significance of the structural differences between the children and adult Zonulin. Hence, these differences could further support the possibility of multiple functions of Zonulin in children [9,10,11]. Lusikelelwe et al. [9] investigated the association between the metabolic imbalance with obesity and intestinal permeability, and suggested that gut permeability could be the cause, the consequence, or both of obesity and its metabolic disorders. In addition, they hypothesized that Zonulin level represents more interstitial injury than inflammation caused by the injury, thus reflecting instead the extent (area) rather than severity of intestinal injury [38]. This could explain the findings of the present study, although this necessitates further studies.
Reviewing the literature, the association between the serum levels of both Zonulin and Copeptin, in children, hasn’t yet been investigated. Nevertheless, few studies have discussed such relations in adult individuals [39, 40].
In summary, among obese children, serum Zonulin and Copeptin had reverse (negative and positive respectively) significant correlations with the obesity markers and body composition parameters; and insignificant correlations with either lipid profile or blood pressure. Moreover serum Copeptin had positive significant correlations with the fat distribution.
Conclusion
Among Egyptian children, Zonulin and Copeptin had significant negative relation with each other, and Copeptin is related more to obesity than Zonulin. However they cannot be used as predictors of metabolic disturbance, as they had insignificant correlations with lipid profile or blood pressure.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request, after taking the permission of our institute “National Research Centre”.
References
Sedky A, Gaber M, Magdy N, El Safoury S. American University in Cairo ―AUC‖ Knowledge Fountain.The Public Policy HUB. Combating the high prevalence of obesity among Egyptian households: a pilot study: Port-Said households. The School of Global Affairs and Public Policy (GAPP); 2021.
Tuli G, Munarin J, Tessaris D, Einaudi S, Matarazzo P, de Sanctis L. Distribution of plasma copeptin levels and influence of obesity in children and adolescents. Eur J Pediatr. Jan; 2021;180(1):119–26. https://doi.org/10.1007/s00431-020-03777-3. Epub 2020 Aug 18. PMID: 32809080; PMCID: PMC7782451.
Hassan NE, El-Masry SA, El Batrawy SR, Khalil A, Ali MM, Al Tohamy M, Abo Hashish M. Relationship between breast feeding duration and risk of overweight/obesity among Egyptian children. Egypt Pediatr Association Gaz. 2018;66(1):9–14. https://doi.org/10.1016/j.epag.2018.01.001.
Ghalli I, Salah N, Hussien F, et al. Egyptian growth curves for infants, children and adolescents. In: Satorio A, Buckler JMH, Marazzi N, editors. Crecere nel mondo. Italy: Ferring; 2008.
Abd El-Aty NS, Osman SR, Ahmed ES, GadAllah MA. Overweight and obesity prevalence among Upper Egypt primary schools’ children using Egyptian and CDC growth charts. Appl Nurs Res Dec. 2020;56:151346. https://doi.org/10.1016/j.apnr.2020.151346.
Al-Jawaldeh A, Abul-Fadl A. Regional disparities in prevalence of obesity among school-aged children in Egypt: a country case study from the Eastern Mediterranean Region. Indian J Child Health. 2021;8(8):262–8.
Küme T, Acar S, Tuhan H, Çatlı G, Anık A, Gürsoy Çalan Ö, Böber E, Abacı A. (2017): The Relationship between Serum Zonulin Level and Clinical and Laboratory Parameters of Childhood Obesity. J Clin Res Pediatr Endocrinol. 2017;9(1):31–38.
Seethaler B, Basrai M, Neyrinck AM, Nazare JA, Walter J, Delzenne NM, Bischoff SC. (2021): Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol Gastrointest Liver Physiol. 2021; 321(1):G11-G17. https://doi.org/10.1152/ajpgi.00113.2021. Epub 2021 May 19. PMID: 34009040.
Mkumbuzi L, Mfengu MMO, Engwa GA, Sewani-Rusike CR. (2020): Insulin Resistance is Associated with Gut Permeability Without the Direct Influence of Obesity in Young Adults Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 13: 2997–3008.
Parkhomenko LK, Strashok LA, Khomenko MA. Role of zonulin in the devolpment of liver fibrosis in obese children. Wiadomości Lekarskie. 2021;74(1):77–82.
Strashok L, Turchyna S, Shevchenko N, Yeloyeva Z, Belousova O, Tsodikova O. Modern aspects of Hepatology: liver steatosis and fibrosis through the prism of Comorbidity in Pediatric Practice. ScienceRise: Med Sci. 2021;3(42):50–5. https://doi.org/10.15587/2519-4798.2021.
Ding C, Magkos F. Oxytocin and vasopressin systems in obesity and metabolic health: mechanisms and perspectives. Curr Obes Rep. 2019;8(3):301–16.
Mustafa HK, Gaballah AM, Fathalla MS. Assessment of serum copeptin in poorly controlled type 1 diabetes. Zagazig Univ Med J May. 2020;26(3):397–404. https://doi.org/10.21608/zumj.2019.14517.1322.
Fenske W, Refardt J, Chifu I, Schnyder I, Winzeler B, Drummond J, Ribeiro-Oliveira A Jr. Copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med. 2018;379(5):428–39.
Bichet DG. Regulation of thirst and vasopressin release. Annu Rev Physiol. 2019;81:359–73.
Kirkendall WM. Recommendations for human blood pressure determination by sphygmomanometers. Circulation. 1980;62:1146A–55A.
Hiernaux J, Tanner J. (1969): Growth and physical studies. In: Weiner JS and Lourie SA, editors Human.
Krebs N, Himes J, Jacohson D. (2007): Assessment of Child and Adolescent Overweight and Obesity. Pediatrics; 120: SI93-S228.
Tietz NW. (1995): Clinical guide to laboratory tests, Philadelphia.
Demacker PN, Hijmans AG, Brenninkmeijer BJ, et al. Five methods for determining low-density lipoprotein cholesterol compared. Clin Chem. 1984;30:1797–800.
Al – Tufaily Y, Ibrahim R, Hassnawi FS. Assessment of prevalence and risk factors of obesity in pediatric age group between (5–15) years. Journal of University of Babylon for pure and applied science (JUBPAS). May-August. 2021;29(2):119–36.
Pacifico L, Bonci E, Marandola L, Romaggioli S, et al. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(45):17107–14.
Pinheiro G, Mello J, Gaya A, Gaya AR. Blood pressure in children: Association with Anthropometric Indicators, body composition, Cardiorespiratory Fitness and Physical Activity. Arquivos brasileiros de cardiologia. 2021;116:950–6.
Rothermel J, Kulle A, Holterhus P, Toschke C, Lass N, Reinehr T. Copeptin in obese children and adolescents: relationships to body mass index, cortisol and gender. Clin Endocrinol. 2016;85:868–87.
Yin C, Liu W, Xu E, Zhang M, Lv W, Lu Q, Xiao Y. Copeptin and Nesfatin-1are interesting interrelated biomarkers playing a role in the pathogenesis of insulin resistance in Chinese obese children. Annals Nutr Metabolism. 2020;76(4):223–32. https://doi.org/10.1159/000508883. Epub 2020 Oct 7.
Lee EKP, Choi RCM, Liu L, Gao T, Yip BHK, Wong SYS. Preference of blood pressure measurement methods by primary care doctors in Hong Kong: a cross-sectional survey. BMC Fam Pract. 2020;21:1–9.
Leskinen T, Eloranta AM, Tompuri T, Saari A, Ollila H, Mäkelä J, Niinikoski H, Lagström H. Changes in body composition by age and obesity status in preschool-aged children: the STEPS study. Eur J Clin Nutr. Jan; 2021;75(1):57–65.
Song K, Park G, Choi Y, Oh JS, Choi HS, Suh J, Chae HW. Association of vitamin D status and physical activity with lipid profile in Korean children and adolescents: a population-based study. Children. 2020;7(11):241.
Darenskaya MA, Kolesnikova LI, Rychkova LV, Kravtsova OV, SemenovaNV, Kolesnikov SI. Relationship between lipid metabolism state, lipid peroxidation and antioxidant defense system in girls with constitutional obesity[J]. AIMS Mol Sci. 2021;8(2):117–26. https://doi.org/10.3934/molsci.2021009.
Tenderenda-Banasiuk E, Wasilewska A, Filonowicz R, et al. Serum copeptin levels in adolescents with primary hypertension. Pediatr Nephrol. 2014;29:423–9.
Rothermel J, Kulle A, Holterhus PM, Toschke C, Lass N, Reinehr T. Copeptin in obese children and adolescents: relationships to body mass index, cortisol and gender. Clin Endocrinol. 2016;85(6):868–73.
Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley THJ, Kullo IJ. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J Clin Endocrinol Metab. 2009;94:2558–64.
Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121:2102–8.
Schiel R, Perenthaler TJ, Steveling A, Stein G. Plasma copeptin in children and adolescents with type 1 diabetes mellitus in comparison to healthy controls. Diabetes Res Clin Pract. 2016;118:156–61.
Lewandowski KC, Lewinski A, Skowronska-Jozwiak E, et al. Copeptin under glucagon stimulation. Endocrine. 2016;52:344–51.
Sedaghat G, Montazerifar F, Keykhaie MA, Karajibani M, Shourestani S, Dashipour A. Effect of pre-meal water intake on the serum levels of Copeptin, glycemic control, lipid profile and anthropometric indices in patients with type 2 diabetes mellitus: a randomized, controlled trial. J Diabetes Metabolic Disorders. 2021;20:171–7.
Kim JH, Heo JS, Baek KS, Kim SY, Kim JH, Baek KH, Kim KE, Sheen YH. Zonulin level, a marker of intestinal permeability, is increased in association with liver enzymes in young adolescents. Clin Chim Acta. 2018;481:218–24.
Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. (2006): Zonulin up regulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006; 55(5):1443-9. https://doi.org/10.2337/db05-1593. PMID: 16644703.
Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. Endocrinol Invest. 2017;40:1–8.
Calgin MK, Cetinkol Y. Decreased levels of serum Zonulin and copeptin in chronic Hepatitis-B patients. Pak J Med Sci. 2019;35(3):842–6.
Acknowledgements
We would like to acknowledge our institute “National Research Centre’; Egypt”; without its fund this study could not be done. Authors are also grateful to everybody participated in this study; the children who were the participants of this study, the technicians who helped in the laboratory analysis and the doctors who participated in collection of the data. Without their help, this study couldn’t have been completed.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S A. El-Masry and R A. Mahmoud conceived and designed the study. S A. El-Masry: Analysis and interpretation of the data. Sh Hamdy responsible for laboratory investigations. N E Hassan and M M. Aly supervised data collection, H R Abdallah and D Y. Elalfy participated in the collection of the references. S Megahed collected the data. All authors contributed to the collection of references, drafting of the article and final approval of the version to be submitted. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of both Faculty of Postgraduate Childhood Studies, and the “National Research Centre; Egypt” (Approval No. 17/123). After explaining the promising benefits of the study in ascertaining the impact of obesity on health, informed written consents were obtained from either parent.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) the present work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Raufe El-Masry, S.A., Mahmoud, R.A., Hassan, N.E. et al. Zonulin and copeptin relation to some metabolic markers in school-aged obese children. BMC Pediatr 24, 140 (2024). https://doi.org/10.1186/s12887-024-04617-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04617-1