Abstract
Background
The sedative dexmedetomidine has been shown to reduce mortality in adult patients with severe sepsis, but it is not known whether children benefit. This study explored the effects of dexmedetomidine on the outcomes of children with severe sepsis with mechanical ventilation.
Methods
In this retrospective cohort study, children with severe sepsis requiring mechanical ventilation from 2016 to 2020 were categorized as dexmedetomidine and non-dexmedetomidine group. The propensity score matching was performed to match cases in both groups. The primary outcome was 28-day mortality, and the secondary outcomes were acute kidney injury, ventilator-free days, lengths of PICU and hospital stays. The Kaplan-Meier method and was the log-rank test used to estimate the 28-day mortality rate and assess between-group differences.
Results
In total, 250 patients were eligible patients: 138 in the dexmedetomidine group and 112 in the non-dexmedetomidine group. After 1:1 propensity score matching, 61 children in each group. dexmedetomidine group showed more lower 28-day mortality (9.84% vs. 26.23%, P = 0.008). During the 7-day observation period after PICU admission, the dexmedetomidine group showed significantly lower neurological and renal sub-scores at day 7 and serum creatinine level at day 3 and day 7. There were no statistical differences in the incidence of acute kidney injury, ventilator-free days, lengths of PICU and hospital stays between the two groups.
Conclusions
dexmedetomidine treatment in children with severe sepsis is associated with better outcomes and should therefore be considered for the sedation strategy.
Similar content being viewed by others
Introduction
Sepsis is a serious inflammatory condition that can develop after dysregulation of the host immune response to infection [1]. Proper care of patients with sepsis, especially those requiring mechanical ventilation, often requires sedation to reduce the stress and anxiety associated with invasive interventions [1,2,3,4,5]. Dexmedetomidine is a selective α2-adrenergic agonist that is administered as a sedative, [6] and has been shown to attenuate inflammatory reactions and organ damage in both animal and adult studies [7,8,9,10]. Notably, some studies have shown that the use of dexmedetomidine is associated with reduced mortality among adult patients with sepsis [9, 11].
The use of dexmedetomidine in infants and children in Europe and the United States has been increasing for several years [12]. In China, dexmedetomidine is recommended in the experts’ consensus on analgesia and sedation for children in pediatric intensive care unit (PICU) [13]. Due to the particularity of children, the pharmacokinetics of dexmedetomidine in children is different from that in adults [14]. The effects of dexmedetomidine on the mortality of pediatric patients with sepsis have not been reported, even though sepsis is among the leading causes of death among children worldwide [2]. Therefore, we conducted a retrospective cohort study to evaluate the effects of dexmedetomidine on the outcome of mechanically ventilated children with severe sepsis in Shandong Province, China.
Methods
Study design and patient selection
This multicenter retrospective cohort study was conducted in the PICUs of two tertiary care hospitals in Shandong Province, China: Shandong provincial Hospital Affiliated to Shandong First Medical University and Qingdao Women and Children Hospital. Data from children admitted to these centers with severe sepsis requiring mechanical ventilation between 1 and 2016 and 31 December 2020 were screened for inclusion in the study. The information of the children was obtained through the electronic medical record inquiry system. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and has been registered in the Chinese Clinical Trial Registration Center (ID: ChiCTR2100047250) and approved by the local ethics committee of each participating hospital (SWYX. NO: 2021 − 187). Guardian consent was waived for this type of study.
Researchers were trained before data collection. During the study, the coordinator and physicians in charge were responsible for validating the collected data and checking for any suspicious errors or missing values.
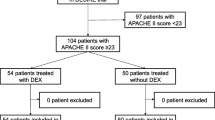
The criteria for inclusion were (i) an age between 28 days and 18 years, (ii) patients with severe sepsis diagnosed according to the International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics, [15] (iii) patients undergoing mechanical ventilation for at least 24 h. Patients were excluded from the study if they were discharged against medical advice, or if there was insufficient clinical information. Patients who met the inclusion criteria were divided into DEX group (treated with dexmedetomidine) and non-DEX group (treated without dexmedetomidine) on the basis of the sedation strategy during PICU hospitalization. (Fig. 1).
Comprehensive medical treatment
All patients were administered standardized treatment according to the International Pediatric Sepsis Consensus Conference (2005) and Surviving Sepsis Campaign International Guidelines (2012) [15, 16].
Sedative and analgesia strategy
All patients received midazolam for sedation and fentanyl for analgesia. The loading dose of midazolam 0.1–0.3 mg/kg body weight was intravenous infusion for 10 min, followed by a continuous intravenous infusion at 1–5 µg·kg− 1·min− 1, and fentanyl titrated at 1 ~ 4 µg·kg− 1·h− 1.The aim of sedation was to establish a score from − 2 to 0 on the Richmond agitation sedation scale (RASS). The goal of analgesia was Face Legs Activity Cry Consolability (FLACC) < 4. If the depth of sedation could not be reached, other sedative drugs (dexmedetomidine or phenobarbital sodium or chloral hydrate) will be combined. Sedation was maintained throughout the duration of mechanical ventilation or as needed. According to whether dexmedetomidine was used, patients were separated into two groups including the DEX group (received dexmedetomidine 0.3 ~ 0.6 µg·kg− 1·h− 1 intravenous infusion as combined sedation) and non-DEX group (received phenobarbital sodium or chloral hydrate as combined sedation) (Fig. 2).The amount and the duration of dexmedetomidine used see in Suppl Table 1.
Definitions
Sepsis-induced acute kidney injury (AKI) is defined as any of the following (not graded): increase in serum creatinine level (Scr) by ≥ 0.3 mg/dl (≥ 26.5 µmol/l) within 48 h; or increase in Scr to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume < 0.5ml/kg/hour for 6 h [17].
The pediatric Sequential Organ Failure Assessment (pSOFA) score included six sub-scores for six organ systems (respiratory, coagulation, hepatic, cardiovascular, neurologic, and renal). The worst value for every variable in each 24-hour period was used to calculate the sub-score for each of the 6 organ systems. Each sub-score ranges 0–4 points, and higher score indicate a worse outcome. Daily pSOFA score was the sum of the 6 sub-scores [18] (Suppl Table 2).
Outcomes
The primary outcomes were 28-day mortality. The secondary outcomes were the number of ventilator-free days in a period of 28 days, the lengths of PICU and hospital stays, the incidence of sepsis-induced AKI, and the trajectory of subscores of the pSOFA which were obtained at day 1, 3, and 7 of PICU admission.
Statistical analysis
For baseline characteristics, quantitative data (non-normally distributed) were expressed as medians and interquartile ranges (IQRs) and compared by the Mann-Whitney U test. Qualitative data were expressed as numbers and percentages and compared by the χ [2] test or Fisher’s exact test.
To balance baseline characteristics between groups and estimate the association between DEX administration and outcomes in children with severe sepsis, propensity score matching (PSM) was performed using 1:1 nearest neighbor matching (caliper value is 0.2) to match cases in both groups. Propensity scores were adjusted for age, weight, ratio of surgical patients, pSOFA-1, number of organ damage, proportion of patients with septic shock, comorbidities, and use of vasoactive drugs, blood purification, as well as fluid resuscitation estimating the probability by logistic regression. The standardized mean difference was calculated to evaluate the efficiency of propensity score matching in reducing the differences between the two groups. To account for missing values and correlations among repeated measurements, the generalized estimating equation(GEE)was adopted for comparisons of pSOFA and biological markers changes between the groups.
For comparison of the probability of patients’ 28-day survival for two groups, the Kaplan–Meier survival method was used to draw survival probability curves and the log–rank test was used for statistical assessment. Cox proportional-hazards analysis was used to assess the relationship between DEX administration and mortality, corrected for baseline variables. The effect of DEX use on the incidence of AKI was estimated using a logistic regression model. Linear regression was used to evaluate the association between DEX use and the ventilator-free days, the length of PICU and hospital stay.
All probability values were two-tailed, and statistical significance was defined as P < 0.05. Statistical analysis was performed using R 4.1.1 software for Windows.
Results
Patients’ characteristics
The two PICUs, located in Jinan and Qingdao, China, had a mean of 20 beds each, and these two units accepted both medical and surgical patients. A total of 425 children with severe sepsis requiring mechanical ventilation were initially enrolled, and finally 250 met the inclusion criteria: 138 in the DEX group and 112 in the non-DEX group (Fig. 1). The overall 28-day mortality of these 250 pediatric severe sepsis was 18.8%. There were no differences between the DEX group and non-DEX group in sex ratio, hospital acquired infections ratio, types of pathogens, proportion of patients receiving nephrotoxic drugs and glucocorticoid. Patients in non-DEX group were younger than those in the DEX group. The pSOFA-1 day was significantly higher in the DEX group (7.0 vs. 5.50, P = 0.007). The number of organs damaged in the DEX group was significantly more than in the non-DEX group (3 vs. 2, P = 0.013), and more patients in the DEX group complicated with septic shock (48.55% vs. 34.82%, P = 0.040). In addition, more patients in the DEX group received vasoactive drugs, fluid resuscitation and blood purification (P<0.001) (Table 1).
We performed 1:1 PSM to balance the baseline characteristics between the two groups, resulting in 61 cases in each group. After PSM, the baseline characteristics were balanced between the two groups, such as age, weight, ratio of surgical patients, pSOFA-1 day, number of organ damage, proportion of patients with septic shock, comorbidities, and use of vasoactive drugs, blood purification, as well as fluid resuscitation (Table 2).
Effects of dexmedetomidine on laboratory values and pSOFA scores over the 7-day observation period
Urine output volumes and Scr, bood urea nitrogen (BUN), Interleukin-6 (IL-6), procalcitonin (PCT) and lactic acid (Lac) levels on day 1, 3, and 7 after PICU admission are shown in Table 3. After PSM, compared the changes in the non-DEX group from baseline to day 7, the DEX group showed more favorable changes in Scr level at day 3 and day 7. However, there were no significant differences between the two group in the changes of IL-6, PCT, BUN, Lac and urine output volumes. (Table 3)
Compared with the non-DEX group, the DEX group had no substantial advantage in pSOFA score during the 7-day observation period. Additionally, we analyzed the trajectories of the six sub-scores. In contrast to the non-DEX group, the DEX group showed significantly lower neurological and renal sub-scores at day 7, though there were no statistical differences in respiratory, coagulation, hepatic and cardiovascular sub-scores between the two groups. (Table 3)
Effects of dexmedetomidine on outcomes
After PSM, the 28-day mortality rate in the DEX group was significantly lower than that in the non-DEX group (9.84% vs. 26.23%, P = 0.008). By plotting the 28-day Kaplan-Meier cure, the survival rate of the DEX group was significantly higher than that of the non-DEX group (P = 0.0096) (Fig. 3). Although the incidence of AKI was lower in the DEX group than that in the non-DEX group, the difference was not statistically significant (9.84% vs. 19.67%, P = 0.063). There were no significant differences in ventilator-free, the length of PICU and hospital stay between the two groups. (Table 4)
Discussion
This study included 250 pediatric cases with severe sepsis and received mechanical ventilation, in which the 28-day overall mortality rate was 18.8%. After 1:1 PSM, DEX group showed more lower 28-day mortality than non-DEX group (9.84% vs. 26.23%, P = 0.008). During the 7-day observation period after PICU admission, although there was no statistical difference in the incidence of AKI between the two groups, the DEX group showed a statistically significant reduction in Scr level at day 3 and day 7 compared with the non-DEX group. Furthermore, in contrast to the non-DEX group, the DEX group showed significantly lower neurological and renal sub-scores at day 7, though there were no statistical differences in respiratory, coagulation, hepatic and cardiovascular sub-scores between the two groups.
A retrospective cohort study in Japan involving 50,671 adult patients who received mechanical ventilation for sepsis showed that the use of dexmedetomidine was associated with reduced all-cause 28-day mortality and a shorter duration of mechanical ventilation [19]. Likewise, Kawazoe Y et al. found that the 28-day mortality of patients with sepsis treated with dexmedetomidine decreased by 8%, though the difference was not statistically significant [20]. Sequentially, Kawazoe Y and his colleagues conducted a subgroup randomized controlled trial and then found that dexmedetomidine significantly reduced the in-hospital mortality in septic patients with Acute Physiology and Chronic Health Evaluation II (APACHE II) scores ≥ 23 [9]. In line with these previous studies in adults, our study found that, for pediatric severe sepsis with mechanical ventilation, dexmedetomidine could decrease the 28-day mortality by 16.39% while the duration of mechanical ventilation did not change significantly.
Moreover, we compared the change of renal function between using or not using dexmedetomidine. We found that the renal sub-score of pSOFA was significantly improved and the decrease of Scr level (at day 3, day 7) was significantly greater in the DEX group than that in the non-DEX group, which indicated dexmedetomidine had protective effect on kidney. However, there was no significant difference between these two groups in the incidence of AKI, which may because the sample size is not enough.
Dexmedetomidine also was reported having neuroprotective effects in ischemia/reperfusion [21]. Pandharipande et al. found that patients with sepsis who received dexmedetomidine had more time without brain dysfunction than those who took lorazepam [11]. Mei B et al. showed that dexmedetomidine reduced systemic inflammation, neuroinflammation, injury of BBB and cognitive dysfunction in septic mice [22]. The study by Tain M et al. suggested that the neuroprotective effect of dexmedetomidine on septic mice was achieved by correcting peripheral Th1/Th2/Th17 shift and reducing proinflammatory cytokines in the hippocampus [23]. In the present study, we found that the use of dexmedetomidine reduced the neurological subscore of pSOFA in children with severe sepsis over the 7-day observational period, further supporting the protective effects of dexmedetomidine.
As far as the potential mechanism, several studies have shown that, unlike other sedatives such as midazolam and propofol, dexmedetomidine as an α2 agonist may potentially modify inflammatory and immune pathways by a number of ways under acute inflammatory conditions [24]. Firstly, dexmedetomidine could alleviate the inflammatory reaction [24,25,26,27,28]. Some studies in animals have shown that dexmedetomidine could prevent sepsis-induced AKI by reducing the levels of inflammatory cytokines, such as tumor necrosis factor-alpha, monocyte chemotactic protein-1 and interleukin-6, and ameliorate renal dysfunction [29, 30]. Secondly, dexmedetomidine was reported to be able to activate the cholinergic anti-inflammatory pathway and then reduce sepsis-related lung injury in mice [31]. Furthermore, dexmedetomidine can alleviate the oxidative stress reaction and reduces apoptosis in the pathogenesis of sepsis [32,33,34]. In addition, dexmedetomidine was also found to be able to reduce the level of norepinephrine in the blood, resulting in an increase in renal blood flow and urinary output [35]. In general, dexmedetomidine was speculated to improve organ functions by multiple mechanisms, thereby reducing the mortality of sepsis.
In our study, over the 7-day observation period, the decrease of IL-6 level in DEX group was slightly more than that of non-DEX group (no statistical difference) ,meanwhile, the level of PCT showed the same trend. All these may support dexmedetomidine could reduce the inflammatory reaction.
Several limitations need to be acknowledged. First, due to a retrospective cohort study, the differences in baseline characteristics between groups may affect the results of the study. The PSM analysis could balance the baseline characteristics between the two groups. Therefore, the influence of confounding factors should be small. But some confounding factors, such as the timing of antimicrobial initiation, are still hard to avoid in retrospective study. Second, we investigated the effect of dexmedetomidine on mortality and organ function in mechanically ventilated children with sepsis, but the mechanism remains unclear which merits further study. Third, we included only mechanically ventilated children with sepsis. It remains unclear whether dexmedetomidine is effective for children without mechanical ventilation. Further research is needed.
Conclusion
The results from this study indicate that the use of dexmedetomidine may improve the mortality of pediatric severe sepsis patients with mechanical ventilation, probably by a multi-organ protective effect. However, further studies are needed to verify the benefits and identify the specific mechanisms.
Data Availability
The datasets supporting the conclusions of this article are included within the article. The underlying datasets are available from the corresponding author on reasonable request.
References
Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. https://doi.org/10.1007/s00134-017-4683-6.
Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study [published correction appears in Am J Respir Crit Care Med. 2016;193(2):223-4]. Am J Respir Crit Care Med. 2015;191(10):1147–57. https://doi.org/10.1164/rccm.201412-2323OC.
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of Disease Study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. https://doi.org/10.1016/S0140-6736(10)60549-1.
Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–80. https://doi.org/10.1016/S0140-6736(09)62072-9.
Coursin DB, Coursin DB, Maccioli GA, Dexmedetomidine. Curr Opin Crit Care. 2001;7(4):221–6. https://doi.org/10.1097/00075198-200108000-00002.
Weatherby KE, Zwilling BS, Lafuse WP. Resistance of macrophages to Mycobacterium avium is induced by alpha2-adrenergic stimulation. Infect Immun. 2003;71(1):22–9. https://doi.org/10.1128/IAI.71.1.22-29.2003.
Gets J, Monroy FP. Effects of alpha- and beta-adrenergic agonists on Toxoplasma gondii infection in murine macrophages. J Parasitol. 2005;91(1):193–5. https://doi.org/10.1645/GE-3242RN.
Nakashima T, Miyamoto K, Shima N, et al. Dexmedetomidine improved renal function in patients with severe sepsis: an exploratory analysis of a randomized controlled trial. J Intensive Care. 2020;8:1. https://doi.org/10.1186/s40560-019-0415-z. Published 2020 Jan 2.
Fayed NA, Sayed EI, Saleh SM, Ehsan NA, Elfert AY. Effect of dexmedetomidine on hepatic ischemia-reperfusion injury in the setting of adult living donor liver transplantation. Clin Transpl. 2016;30(4):470–82. https://doi.org/10.1111/ctr.12713.
Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial [published correction appears in Crit Care. 2011;15(1):402]. Crit Care. 2010;14(2):R38. https://doi.org/10.1186/cc8916.
Plambech MZ, Afshari A. Dexmedetomidine in the pediatric population: a review. Minerva Anestesiol. 2015;81(3):320–32.
(Chinese references) Subspecialty Group of Emergency Medicine, the Society of Pediatrics, Chinese Medical Association. Zhonghua Er Ke Za Zhi (Chinese). 2019;57(5):324–30. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.05.002. Subspecialty Group of Pediatrics, the Society of Emergency Medicine, Chinese Medical Association; Society of Pediatric Critical Care, Chinese Medical Doctor Association. [Experts’ consensus on sedation and analgesia for children in pediatric intensive care unit of China (2018)].
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–52. https://doi.org/10.1002/jps.23574.
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. ;. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. https://doi.org/10.1097/CCM.0b013e31827e83af.
Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. ;. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. Published 2013 Feb 4. https://doi.org/10.1186/cc11454.
Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a Pediatric Sequential Organ failure Assessment score and evaluation of the Sepsis-3 definitions in critically Ill Children. JAMA Pediatr. 2017;171(10):e172352. https://doi.org/10.1001/jamapediatrics.2017.2352.
Aso S, Matsui H, Fushimi K, Yasunaga H. Dexmedetomidine and Mortality from Sepsis requiring mechanical ventilation: a japanese Nationwide Retrospective Cohort Study. J Intensive Care Med. 2021;36(9):1036–43. https://doi.org/10.1177/0885066620942154.
Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of Dexmedetomidine on Mortality and Ventilator-Free days in patients requiring mechanical ventilation with Sepsis: a Randomized Clinical Trial. JAMA. 2017;317(13):1321–8. https://doi.org/10.1001/jama.2017.2088.
Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–6. https://doi.org/10.1097/01.ccm.0000128579.84228.2a.
Mei B, Li J, Zuo Z. Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor. Brain Behav Immun. 2021;91:296–314. https://doi.org/10.1016/j.bbi.2020.10.008.
Tian M, Wang W, Wang K, et al. Dexmedetomidine alleviates cognitive impairment by reducing blood-brain barrier interruption and neuroinflammation via regulating Th1/Th2/Th17 polarization in an experimental sepsis model of mice. Int Immunopharmacol. 2021;101(Pt B):108332. https://doi.org/10.1016/j.intimp.2021.108332.
Flanders CA, Rocke AS, Edwardson SA, Baillie JK, Walsh TS. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: a systematic review of animal and human studies. Crit Care. 2019;23(1):402. https://doi.org/10.1186/s13054-019-2690-4. Published 2019 Dec 11.
Talke P, Bickler PE. Effects of dexmedetomidine on hypoxia-evoked glutamate release and glutamate receptor activity in hippocampal slices. Anesthesiology. 1996;85(3):551–7. https://doi.org/10.1097/00000542-199609000-00014.
Szelényi J, Kiss JP, Vizi ES. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-alpha production by alpha2- and beta-adrenoceptors in mice. J Neuroimmunol. 2000;103(1):34–40. https://doi.org/10.1016/s0165-5728(99)00234-9.
Wu GJ, Chen JT, Tsai HC, Chen TL, Liu SH, Chen RM. Protection of Dexmedetomidine Against Ischemia/Reperfusion-Induced apoptotic insults to neuronal cells occurs Via an intrinsic mitochondria-dependent pathway. J Cell Biochem. 2017;118(9):2635–44. https://doi.org/10.1002/jcb.25847.
Li B, Li Y, Tian S, et al. Anti-inflammatory Effects of Perioperative Dexmedetomidine administered as an Adjunct to General Anesthesia: a Meta-analysis. Sci Rep. 2015;5:12342. https://doi.org/10.1038/srep12342. Published 2015 Jul 21.
Hsing CH, Lin CF, So E, et al. α2-Adrenoceptor agonist dexmedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5. Am J Physiol Renal Physiol. 2012;303(10):F1443–53. https://doi.org/10.1152/ajprenal.00143.2012.
Kang K, Gao Y, Wang SC, et al. Dexmedetomidine protects against lipopolysaccharide-induced sepsis-associated acute kidney injury via an α7 nAChR-dependent pathway. Biomed Pharmacother. 2018;106:210–6. https://doi.org/10.1016/j.biopha.2018.06.059.
Liu Z, Wang Y, Wang Y, et al. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol. 2016;35:210–6. https://doi.org/10.1016/j.intimp.2016.04.003.
Chen JH, Yu GF, Jin SY, Zhang WH, Lei DX, Zhou SL, et al. Activation of α2 adrenoceptor attenuates lipopolysaccharide-induced hepatic injury. Int J Clin Exp Pathol. 2015;8(9):10752–9.
Qiao H, Sanders RD, Ma D, Wu X, Maze M. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care. 2009;13(4):R136. https://doi.org/10.1186/cc8012.
Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110(5):1077–85. https://doi.org/10.1097/ALN.0b013e31819daedd.
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–94. https://doi.org/10.1097/00000542-200008000-00016.
Acknowledgements
None.
Funding
This study was supported by the General Fund of Shandong Provincial Health Department (No. 2016WS0414).
Author information
Authors and Affiliations
Contributions
C.Z., Y.Y. and T.Z. conceptualized the study concept and design. C.Z., Y.Y. and Y. J conducted research; C.Z., Y.Y. and T.Z. analyzed the data; and C.Z. and Y.Y. drafted the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Ethics approval
This work has been carried out in accordance with the Declaration of Helsinki of the World Medical Association. The study protocol has been registered in the Chinese Clinical Trial Registration Center (ID: ChiCTR2200056383) and approved by the ethics committee of Shandong provincial Hospital Affiliated to Shandong First Medical University and Qingdao Women and Children Hospital. The need for informed consent was waived by the ethics committee of Shandong provincial Hospital Affiliated to Shandong First Medical University and Qingdao Women and Children Hospital, because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, C., Yin, Y., Zhang, T. et al. Dexmedetomidine improves the outcomes for pediatric severe sepsis with mechanical ventilation. BMC Pediatr 23, 406 (2023). https://doi.org/10.1186/s12887-023-04232-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04232-6