Abstract
Background
Dexmedetomidine is widely used in patients with sepsis. However, its effect on septic patients remains controversial. The objective of this study was to summarize all randomized controlled trials (RCTs) examining dexmedetomidine use in sepsis patients.
Methods
This systematic review and meta-analysis included RCTs comparing dexmedetomidine with other sedatives in adult sepsis patients. We generated pooled relative risks (RRs) and standardized mean differences and performed trial sequential analysis and a cumulative meta-analysis. The primary outcome was mortality, and the secondary outcomes were the length of the intensive care unit stay, duration of mechanical ventilation, number of ventilation-free days, incidence of total adverse event, incidence of delirium, and levels of interleukin 6, tumor necrosis factor alpha, and alanine aminotransferase.
Results
We included 19 RCTs that enrolled 1929 patients. Compared with other sedatives, dexmedetomidine decreased the all-cause mortality (RR 0.83; 95% confidence interval [CI] [0.69, 0.99]) and inflammatory response (interleukin 6 and tumor necrosis factor alpha levels at 24 h: standardized mean difference (SMD) − 2.15; 95% CI [− 3.25, − 1.05] and SMD − 1.07, 95% CI [− 1.92, − 0.22], respectively). Trial sequential analysis showed that it is not up to required information size. The overall risk adverse events was similar between dexmedetomidine and the other sedatives (RR 1.27, 95% CI [0.69, 2.36]), but dexmedetomidine increased the risk of arrhythmias (RR 1.43, 95% CI [0.59, 3.51]). Length of intensive care unit stay (SMD − 0.22; 95% CI [− 0.85, − 0.41]), duration of mechanical ventilation (SMD 0.12; 95% CI [− 1.10, 1.35]), incidence of delirium (RR 0.98; 95% CI [0.72, 1.33]), and levels of alanine aminotransferase and creatinine at 24 h were not significantly reduced.
Conclusions
Dexmedetomidine in sepsis patients could significantly reduce mortality compared with benzodiazepines but not with propofol. In addition, dexmedetomidine can significantly decrease inflammatory response in patients with sepsis compared with other sedatives. Dexmedetomidine might lead to an increased incidence of arrhythmias, but its safety profile did not show significant differences in the incidence of total adverse events. Future RCTs are needed to determine the sepsis patient population that would benefit most from dexmedetomidine and its optimal dosing regimen.
Similar content being viewed by others
Background
Sepsis is the systemic inflammatory response syndrome caused by infection. It affects millions of patients per year and has a high risk of mortality, which has become a major global health problem [1] [2] [3]. The Global Burden of Diseases Study showed that sepsis affects at least 49 million patients each year, causing 11 million deaths and accounting for 19.7% deaths worldwide [4] [5]. Epidemiological data showed that over 20% of the septic patients required mechanical ventilation [6], which is associated with enormous costs for health care systems worldwide. The main clinical goal of the 2021 Surviving Sepsis Campaign was to optimize sepsis treatment and improve patient outcomes.
Dexmedetomidine is frequently used for patient comfort and safety, which is an integral component of the therapy concept for mechanically ventilated patients to reduce their anxiety and the stress level associated with tracheal intubation and other invasive interventions [7] [8]. In addition, it can be used to alleviate the symptoms of sepsis-induced encephalopathy in non-ventilated patients [9].
Basic and translational studies showed that among the recommended sedatives, dexmedetomidine (alpha2 receptor agonist) has anti-inflammatory and anti-bacterial effects, which are superior to those of gamma-aminobutyric acid agonists, such as benzodiazepines and propofol [7]. Furthermore, it also reduces neuronal apoptosis and promotes biomimetic sleep—all of which could improve clinical outcomes [10]. For potential risk factors, existing data suggested that a dexmedetomidine loading dose might cause heart arrythmias. However, despite extensive research, the potential benefits and risks of dexmedetomidine in sepsis patients remain controversial.
Recent four meta-analyses have shown controversial results, where two of these studies [11] [12] suggested a positive effect of dexmedetomidine on mortality in sepsis patients, while two other studies [13] [14] did not find a significant difference in mortality between dexmedetomidine and the other sedative agents. However, these conclusions are limited by the number of included studies, and the effects of dexmedetomidine on the incidence of delirium, adverse events, and the length of intensive care unit (ICU) stay remains controversial. Furthermore, trial sequential analysis (TSA) [15] and cumulative meta-analyses were not performed in the previous systematic reviews and meta-analyses.
Methods
Protocol and registration
The protocol for this study was pre-registered on PROSPERO (CRD42022303354), and the findings are reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (Additional file 1).
Systematic search
We conducted a comprehensive search on PubMed, EMBASE, Web of Science, Google Scholar, and unpublished sources including PROSPERO, Clinicaltrials.gov, and the Cochrane Library from inception until February 16, 2022 for randomized controlled trials (RCTs) investigating the role of dexmedetomidine compared with placebo or other sedative agents as therapy in adult sepsis patients. We did not apply language restrictions. We included the following three search terms: “dexmedetomidine,” “sepsis,” and “randomized controlled trials” (Additional file 1: Appendix for the search strategy, appendices S1–S5). We used the Medical Subject Headings database to identify synonyms and examined the reference list of full-text articles for additional relevant studies. We also considered conference proceedings, such as the American Association for the Surgery of Trauma, the Critical Care Medicine, and the European Society of Intensive Care and Emergency Medicine.
Study selection
Study inclusion criteria are described below. Population: adult patients with sepsis receiving intravenous (IV) sedation in an ICU unit, either with or without mechanical ventilation. Sepsis was defined as per authors’ definition. (Table 1). Intervention: IV dexmedetomidine at any dose. Comparison: received IV sedative drugs regardless of the dose. Outcome: included prespecified outcomes for efficacy on the basis of the meta-analysis group consensus. The primary outcome was all-cause mortality (including ICU, hospital, 7/28/30/90-day mortality). For outcomes reported at multiple timepoints, we chose the longest reported follow-up timepoints. Secondary outcomes included the duration of mechanical ventilation and ventilator-free days; length of ICU stay; biological results (serum interleukin [IL]-6, tumor necrosis factor [TNF]-α, alanine aminotransferase, and creatinine changes at 24 h); incidence of delirium; and incidence of the total adverse events, including tachycardia, bradycardia, and hypotension. Design: RCT.
The exclusion criteria were as follows: (1) conference abstracts, comments, editorials, case reports, and systematic reviews, and articles, where the full text was unavailable; and (2) if two or more studies were based on the same patient cohort, we selected the study with the highest number of patients or the most recently published of the studies.
Data collection process and data items
Two reviewers (Z and M) aggregated the data independently and in duplicate using a pre-specified standardized data abstraction form. A third reviewer (Liu) adjudicated disagreements. We collected data on trial characteristics, demographic data, acute physiology and chronic health evaluation II (APACHE II) [16], sequential organ failure assessment (SOFA) [3], intervention and control procedures, and outcomes of interest. APACHE II used a point score based on the initial values of 12 routine physiologic measurements, age, and the patient’s previous health status to provide a general measure of disease severity [17].
Risk of bias assessment in individual studies
We assessed the risk of bias (RoB) independently and in duplicate using the Cochrane Risk of Bias 2.0 tool for RCTs. We used the tool to assess the RoB in the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. We ranked each domain as “low,” “some concerns,” or “high”. We determined the overall RoB for each trial on the basis of the highest risk attributed to any one domain. We assessed the certainty of evidence for each outcome using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [18]. In accordance with the GRADE methods, we used terminology consistent with the overall certainty of evidence, which includes stronger language for high certainty of evidence and the less certain language (“probably” or “may”) for moderate or low certainty of evidence. We used the Guideline Development Tool (https://www.gradepro.org) to formulate the summary of findings table.
Summary measures and synthesis of results
Statistical analyses were performed using Review Manager Software 5 (Review Manager [RevMan] Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) and STATA software V.16.0 (STATA Corporation, College Station, TX, USA) [19]. We used DerSimonian and Laird random-effects models to conduct the meta-analysis [20]. We presented the results as the relative risk (RR) for dichotomous outcomes, and we presented the mean difference (MD) or standardized mean difference (SMD) with the 95% confidence interval (CI) to outline continuous outcomes. We also presented the absolute difference with the 95% CI, which we used for the GRADE ratings. The median and interquartile range and the mean and standard deviation were determined in accordance with the methods described by McGrath et al. [21].
We assessed the heterogeneity between the selected trials by visual inspection of the forest plots, the Chi-squared test for homogeneity (where p < 0.1 indicates important heterogeneity), and the I2 statistic (for which a value of 50% or greater was considered to reflect potentially important heterogeneity) [22]. Funnel plots were created to assess the publication bias using the Egger’s test. We performed a predefined subgroup analysis comparing studies with a high RoB to those with low RoB as well as comparing the APACHE II scores [17], sedation < 24 h and sedation > 24 h, and control drug (dexmedetomidine vs propofol/others), and another subgroup analysis requested by peer review on the basis of the sedation level [23] [24]. Finally, we conducted a sensitivity analysis to investigate the robustness of the result as requested by peer reviewers, analyzing the subgroup based on the mortality outcome and excluding studies that used benzodiazepines as a comparator.
We conducted a cumulative meta-analysis on the basis of the publication year by updating the pooled risk ratio when the result of a new trial were published for the primary outcome [25]. This statistical method was used to detect the dynamic trend of the association result, and it further supported the meta-analysis conclusion. We conducted a TSA [15] using a random effects model for mortality. For the TSA, we used the statistical significance level of 5%, a power of 80%, and a relative risk reduction of 15%. We used a model variance-based heterogeneity correction, and we performed this analysis using Trial Sequential Analysis v.0.9.5.10 beta software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, https://www.ctu.dk/tsa).
Results
Study selection
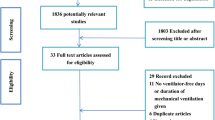
The searches yielded 263 citations (Fig. 1). After duplicates were removed and the titles and abstracts reviewed, 131 articles were excluded. Among the remaining 132 studies, full-text articles of 129 were available and 110 of them were excluded after reviewing the full-text manuscript. After several review stages, 19 eligible studies were included in the analysis [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. There were 1929 patients included in this study. Baseline characteristics of the included trials are summarized in Table 1.
Study description
The selected studies were published between 2009 and 2020. The number of included participants from each study ranged from 36 to 422. All patients were in the ICU and met the sepsis criteria. The mean participant age ranged from 43 to 75 years, with male participants accounting for 58.9% of the dexmedetomidine group and 56.8% of the control group. Sepsis was defined as sepsis-1 in three articles [26, 28, 36], sepsis-2 in eight articles [27, 29, 30, 33,34,35, 41, 44], and sepsis-3 in four articles [37, 38, 40, 43], and as septic shock in two articles [31, 32]. In two articles [39, 42], sepsis was defined in accordance with the 2014 Chinese Guideline of Sepsis and Septic Shock. The dexmedetomidine dose varied among the studies, whereby three [37, 42, 44] out of the six [27, 28, 30, 37, 42, 44] studies administered a loading dose of dexmedetomidine. Sixteen studies used propofol [26, 28,29,30,31,32,33,34,35,36,37,38,39, 41, 42, 44] and three studies used benzodiazepines as a comparator [27, 40, 43].
Five of the included trials had a high RoB [32, 35, 36, 38, 42]. Among them, two studies had a high RoB because of incomplete reporting regarding randomization, intervention descriptions, and reported result selection [32, 42], and three of them had a high RoB due to incomplete reporting of the randomization and concern about selection of the reported results [35, 36, 38]. The other trials had either a low RoB or particular concerns (Fig. 2 and Additional file 1: e-Fig. S1). After discussion among the meta-analysis group, we removed the five studies with a high RoB and then performed the meta-analysis. Table 2 and Additional file 1: e-Tables S2–S7 present the pooled outcomes with the associated GRADE certainty of evidence.
Primary outcomes
Eleven studies (n = 1222) showed results for mortality [26,27,28,29,30,31, 33, 34, 37, 39, 40], among which seven studies explored the 28-day or 30-day mortality, [27,28,29,30, 34, 37, 39] two studies focused on the 90-day mortality [26, 33], one study reported the 7-day mortality [40], and one study included ICU mortality of unknown duration [31] (Additional file 1: e-Table S1). A pooled analysis showed that the dexmedetomidine group had a lower occurrence of mortality (RR 0.83; 95% CI [0.69, 0.99]; high certainty) compared with the control group, with no significant heterogeneity (I2 = 1%) (Fig. 3). Table 2 shows the summary of findings for all outcomes including the certainty of evidence. Using a funnel plot and Egger’s test (Additional file 1: e-Fig. S2), we did not find any publication bias. The TSA results demonstrated that the information size needed to detect an intervention effect was 2781 patients. The cumulative Z curve did not cross either the conventional boundary for benefit or the trial sequential monitoring boundary for benefit (Fig. 4). A cumulative meta-analysis was conducted to assess changes over time (Fig. 5). A statistically significant decrease in mortality was first observed in studies that were performed from 2009 to 2016 (RR 0.51 95% CI [0.26, 0.98]). As the number of studies increased, the RR value approached 1.
A subgroup analysis was conducted on the basis of APACHE II scores ≤ 20 and > 20, control drug (dexmedetomidine vs other sedatives), and sedation level (deep or light). We found that the patients’ APACHE II scores in each study (≤ 20 or > 20) had no significant effect on mortality (Additional file 1: e-Fig. S3a). In addition, the sedation level (deep or light) did not demonstrate any credible subgroup effects (Additional file 1: e-Fig. S3b). Dexmedetomidine significantly reduced sepsis patients mortality compared with benzodiazepines but not with propofol (RR 0.36, 95% CI [0.18, 0.70]) except for propofol (RR 0.89, 95% CI [0.74, 1.07]; Additional file 1: e-Fig. S3c).
The sensitivity analyses excluding the study reporting 7-day mortality[40] showed that there was no significant difference between the dexmedetomidine and the other sedatives on mortality (RR 0.86; 95% CI [0.72, 1.03]; Additional file 1: e-Fig. S4a). The sensitivity analyses excluding the study reporting 90-day mortality[33] showed that the use of dexmedetomidine was associated with lower mortality compared to other sedatives (RR 0.71; 95% CI [0.55, 0.92], Additional file 1: e-Fig. S4b). After excluding the two studies reporting 7-day mortality[40] and 90-day mortality[33], the use of dexmedetomidine was also associated with lower mortality (RR 0.75; 95% CI [0.58, 0.98], Additional file 1: e-Fig. S4c).
Secondary outcomes
Length of ICU stay.
Nine studies (n = 659) [26,27,28,29,30,31, 34, 37, 45] included the length of ICU stay in their evaluation index. Our results indicated that dexmedetomidine did not reduce the length of the ICU stay compared with the other sedatives (SMD − 0.22; 95% CI [− 0.85, 0.41], high certainty) (Additional file 1: e-Fig.SS5a). We performed sensitivity analyses excluding Pandharipande’s study [27] that compared dexmedetomidine to benzodiazepines, and we found no substantially altered pooled estimates or conclusions (SMD − 0.23; 95% CI [− 0.87, 0.40], Additional file 1: e-Fig. S5b).
Duration of mechanical ventilation.
Six studies (n = 460) [26, 28, 30, 34, 36, 37] explored the impact of dexmedetomidine on the duration of mechanical ventilation. The meta-analysis did not show a reduction in mechanical ventilation time with dexmedetomidine use compared with that with the use of other sedatives (SMD 0.12; 95% CI [− 1.10, 1.35], high certainty) (Additional file 1: e-Fig. S6).
Duration of ventilator-free days.
Three studies (n = 686) [27, 28, 33] included ventilator-free days as indicator, and the meta-analysis results indicated that dexmedetomidine did not increase ventilator-free days compared with the other sedatives (MD 1.68; 95% CI [− 1.50, 4.85], very low certainty) (Additional file 1: e-Fig. S7a). After excluding Pandharipande’s study [27], a sensitivity analysis was conducted, and the results did not change significantly (SMD 0.29; 95% CI [− 1.81, 2.39]; Additional file 1: e-Fig. S7b).
IL-6, TNF-α, alanine aminotransferase, and creatinine level changes at 24 h.
Four studies (n = 352) reported the 24-h changes in IL-6 and TNF-α levels [26, 41, 43, 44]. Three studies (n = 219) reported the 24-h changes in alanine aminotransferase, and creatinine levels [31, 37, 39]. Random-effect models were used in the four outcomes, and the results showed significantly lower IL-6 and TNF-α levels at 24 h in the dexmedetomidine group compared with those in the other sedatives group (SMD − 2.15; 95% CI [− 3.25, − 1.05], low certainty; SMD − 1.07; 95% CI [− 1.92, − 0.22], moderate certainty; Additional file 1: e-Figs. S8a and S9a). However, random model analysis indicated that dexmedetomidine did not lead to a significant change in alanine aminotransferase and creatinine levels at 24 h (p = 0.17 and 0.30, respectively; low certainty; e-Fig. S10). The sensitivity analysis excluded Wu’s study [43] used benzodiazepines as a comparator and the results did not change (IL-6: SMD − 2.50; 95% CI [− 4.11, − 0.90]; TNF-α: SMD − 0.58; 95% CI [− 0.83, − 0.32], e-Figs. S8b and S9b).
Incidence of delirium
Two studies (n = 264) [28, 37] explored the incidence of delirium related to dexmedetomidine. Overall, 45/131 (34.35%) patients in the dexmedetomidine group reported that they experienced delirium compared with 46/133 (34.59%) patients in the control group. The meta-analysis showed that dexmedetomidine was not significantly associated with a lower risk of delirium compared with the other sedation types (risk ratio 0.98; 95% CI [0.72, 1.33], low certainty; Additional file 1: e-Fig. S11).
Overall incidence of adverse events
Six studies (n = 581) included the incidence of adverse events [27, 28, 30, 37, 39, 44]. There was no difference in the incidence of adverse events between the dexmedetomidine and propofol groups (RR 1.27, 95% CI [0.69, 2.36], moderate certainty; Additional file 1: e-Fig. S12a). We performed sensitivity analyses excluding Pandharipande’s study [27] and found no substantial changed in the pooled estimates (RR 1.43, 95% CI [0.59, 3.51], Additional file 1: e-Fig. S12b). For arrhythmia and hypotension, the pooled RRs were 2.69 (95%CI [1.19, 6.08], high certainty; e-Fig. S12c) and 1.04 (95% CI [0.46, 2.36], low certainty; Additional file 1: e-Fig. S12d). The research findings showed that dexmedetomidine was significantly associated with a higher risk of arrhythmia but not with a higher risk of hypotension compared with other sedatives.
Discussion
This systematic review and meta-analysis showed that dexmedetomidine sedation in sepsis patients could significantly decrease mortality and IL-6 and TNF-α levels at 24 h compared with other sedatives. Dexmedetomidine might lead to an increased incidence of arrythmias, but it was not associated with an increased incidence of total adverse events. There were no significant differences in the length of ICU stay, duration of mechanical ventilation, incidence of delirium, and the alanine aminotransferase or creatinine at 24 h. Considering the differences in pharmacological profiles, dexmedetomidine has known strengths including its anesthesia-inducing effect without inhibiting respiration, its anti-inflammation effects, and its low allergenic potential compared with propofol [46]. Dexmedetomidine already has a wide indication field in clinical practice, while propofol was not as widely used in septic shock patients [47, 48]. This study demonstrated that dexmedetomidine has advantages in treating sepsis patients by improving their overall survival.
Several systematic reviews and meta-analyses on this research topic have been previously conducted [11] [12] [13] [49] [14]. Among previous meta-analyses, Huang et al. was the most comprehensive study [13], and it included 15 RCTs with 1,871 patients in the analysis. Huang et al. showed that dexmedetomidine use did not significantly reduce mortality (RR 0.97, 95%CI [0.83, 1.13]) [13]. In Huang et al.’s study, nearly half of the studies were assessed as having a high RoB using the Cochrane Risk of Bias 2.0 tool, and we suspect that this non-significant result may be influenced by these high-RoB studies. A strength of our meta-analysis is that we systematically reviewed the current literature on the basis of previous meta-analyses and excluded studies with a high RoB. Our cumulative meta-analysis for the primary outcomes showed that from a dynamic perspective, although the RR value changed over time, the conclusion was relatively stable over time, and an advantage of dexmedetomidine use in treating sepsis patients was observed.
Comparing the safety profile of dexmedetomidine with that of the other sedation types, there were no significant differences in the incidence of the total adverse events in sepsis patients, although the incidence of arrhythmia was significantly increased. This finding was not reported in previous studies. Theoretically, dexmedetomidine is an alpha2-adrenoceptor agonist that causes vasodilation and decreases the sympathetic response [50] and, therefore, potentially induces hemodynamic side effects. A possible explanation for our research findings is that only three [37, 42, 44] out of the six [27, 28, 30, 37, 42, 44] studies administered a loading dose of dexmedetomidine, which is associated with higher risk of arrhythmia due to a decrease in cardiac output that occurred following the loading dose secondary to a transient afterload increase caused by alpha2-adrenoceptor-mediated vasoconstriction [51]. The incidence of arrhythmia may be reduced by eliminating a dexmedetomidine loading dose, and close hemodynamic monitoring is still recommended.
A large amount of evidence has demonstrated the stimulating effect of dexmedetomidine on the central and peripheral receptors, causing a reduction in sympathetic nerve activity and plasma catecholamine concentration [52]. Its ability to reduce sympathetic tone and indirectly increase the parasympathetic activity is important in inhibiting inflammatory factor release and reducing cell apoptosis, thereby reducing the occurrence of inflammation and sepsis [53]. Results of our meta-analysis also suggest that 24 h after receiving dexmedetomidine, patients’ TNF-α and IL-6 levels were significantly lower compared with those of the control group. However, our meta-analysis results were not consistent with those of previous reports [54, 55], which showed that dexmedetomidine prevents liver and kidney damage resulting from sepsis. Further research is needed to confirm these results. In addition, the sample size included in this study was small.
This systematic review and meta-analysis have several strengths including a protocol that was written a priori, a comprehensive literature search including unpublished sources, independent screening, and data abstractions, and use of the GRADE assessment of the certainty of evidence.
However, there are also some limitations to this study. First, there was a lack of individual patient data, and we were unable to conduct the pre-planned subgroup analyses using the patient baseline characteristics, such as the underlying etiology of sepsis. Because there was a partial lack study data, we had to change the protocol regarding ventilator free-days as a co-primary outcome, and we could not conduct the pre-planned subgroup analyses on the basis of sedation < 24 h and sedation > 24 h. In addition, only a small number of studies reported data on pain management (8) and ventilation settings (5), and we were unable to complete the subgroup analysis on these items. Second, the variations in sepsis definition, dexmedetomidine regimens, sedation levels, sedation substances used as a comparator, adjunctive therapies (e.g., pain management and ventilation settings), and mortality timeline among the included studies might have caused the clinical heterogeneity, although the levels of statistical heterogeneity were low across all studies. Furthermore, the required sample size was not attained (1222 patients were in the analysis but 2781 patients were needed), although recent studies had a major impact on the CI ranges.
In summary, the findings of this study indicated an association between dexmedetomidine and decreased mortality in sepsis patients. Considering the limitations, more high-quality trials are needed to improve the methodology and corroborate the study findings. Further studies are required to determine the population that would benefit the most from this drug and its optimal dosing regimen and infusion duration.
Conclusions
Optimizing treatment for sepsis patients and improving their outcomes is a worldwide research goal. The findings of this study are valuable for clinical work on sepsis patients. The meta-analysis showed that dexmedetomidine sedation in sepsis patients could decrease mortality compared with benzodiazepines but not with propofol. In addition, dexmedetomidine can significantly decrease inflammatory cytokine levels in sepsis patients compared with other sedatives. Dexmedetomidine might lead to an increased incidence in arrythmias, but its safety profile did not show an increased incidence of total adverse events. Future clinical RCTs are needed to verify the efficacy of dexmedetomidine on the length of the hospital stay and mechanical ventilation time and to determine the sepsis patient population that would benefit the most from this treatment and its optimal dosing regimen.
Availability of data and materials
All data associated with this manuscript are included in the main text and supplementary materials.
Abbreviations
- CI:
-
Confidence interval
- TSA:
-
Trial Sequential Analysis
- RCT:
-
Randomized-controlled trial
- ALT:
-
Alanine transaminase
- Cr:
-
Creatine
- RoB:
-
Risk of bias
- RR:
-
Relative risk
- OR:
-
Odds ratio
- MD:
-
Mean difference
- SMD:
-
Standardized mean difference
- IQR:
-
Interquartile ranges
- SD:
-
Standard deviation
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–72.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247.
The First ESA European Sepsis Report — European Sepsis Alliance. https://www.europeansepsisalliance.org/news/2021/9/9/the-first-esa-european-sepsis-report.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11.
Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6.
Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–54.
Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185(5):486–97.
Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–66.
Mei B, Li J, Zuo Z. Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor. Brain Behav Immun. 2021;91:296–314.
Zhang WQ, Xu P, Zhan XH, Zheng P, Yang W. Efficacy of dexmedetomidine for treatment of patients with sepsis: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(18): e15469.
Chen P, Jiang J, Zhang Y, Li G, Qiu Z, Levy MM, Hu B. Effect of Dexmedetomidine on duration of mechanical ventilation in septic patients: a systematic review and meta-analysis. BMC Pulm Med. 2020;20(1):42.
Huang P, Zheng X, Liu Z, Fang X. Dexmedetomidine versus propofol for patients with sepsis requiring mechanical ventilation: a systematic review and meta-analysis. Front Pharmacol. 2021;12: 717023.
Wang C, Chen Q, Wang P, Jin W, Zhong C, Ge Z, Xu K. The effect of dexmedetomidine as a sedative agent for mechanically ventilated patients with sepsis: a systematic review and meta-analysis. Front Med. 2021. https://doi.org/10.3389/fmed.2021.776882.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39.
Tang W, Zha ML, Zhang WQ, Hu SQ, Chen HL. APACHE scoring system and pressure injury risk for intensive care patients: a systematic review and meta-analysis. Wound Repair Regen. 2022;30(4):498–508.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache, II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14:120.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020. https://doi.org/10.1177/0962280219889080.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44.
Al-Qamari A, Ault ML. chapter 37 - Pain control in the critically ill patient. In: Benzon HT, Raja SN, Liu SS, Fishman SM, Cohen SP, Hurley RW, Narouze S, Malik KM, Candido KD, editors. Essentials of Pain Medicine (Third Edition). Saint Louis: W.B. Saunders; 2011. p. 253–60.
Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327(4):248–54.
Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400.
Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, Herr DL, Maze M, Ely EW, Investigators M. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38.
Kawazoe Y, Miyamoto K, Morimoto T, Yamamoto T, Fuke A, Hashimoto A, Koami H, Beppu S, Katayama Y, Itoh M, et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA. 2017;317(13):1321–8.
Zhou F. Study on organ protection value of dexmedetomidine in patients with septic myocardial injury. Knowledge Prevention Treatment Cardiovascular Diseases. 2017;6:73–4.
Cioccari L, Luethi N, Bailey M, Shehabi Y, Howe B, Messmer AS, Proimos HK, Peck L, Young H, Eastwood GM, et al. The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: a subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit Care. 2020;24(1):441.
Memis D, Kargi M, Sut N. Effects of propofol and dexmedetomidine on indocyanine green elimination assessed with LIMON to patients with early septic shock: a pilot study. J Crit Care. 2009;24(4):603–8.
Liu J, Shi K, Hong J, Gong F, Mo S, Chen M, Zheng Y, Jiang L, Xu L, Tu Y, et al. Dexmedetomidine protects against acute kidney injury in patients with septic shock. Ann Palliat Med. 2020;9(2):224–30.
Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, Jackson JC, Hoskins AS, Pun BT, Orun OM, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. 2021;384(15):1424–36.
Sigler MB, Nugent KM. Comparison of dexmedetomidine and propofol in mechanically ventilated patients with sepsis: a pilot study. The Southwest Respiratory and Critical Care Chronicles. 2018;6:10.
Yu-xin LXyL. Study of organ protective effects in sepsis patients with myocardial injury with dexmedetomidine and propofol in ICU. J Community Med. 2016;14(01):11–4.
Meng J, Zhang G, Shang F, Sun H, Li B. Comparative study of propofol and dexmedetomidine on inflammatory responses in patients with sepsis. Chin J Critical Care Med. 2014;7(5):19–23.
Jian C, Chang QX, Li LI, Ying YE. Therapeutic effect of dexmedetomidine in sepsis patients with mechanical ventilation. China J Emergency Resuscitation and Disaster Med. 2019;14(5):442–5.
Yifei XW, Guofa L, Yue L, Yang Z, Huanggang M, Weidong Z, Yunhua Z. Effect of Dexmedetomidine -based early goal-oriented sedation strategy on gastrointestinal function in patients with sepsis. Chin J Critical Care Intensive Care Medicine. 2019;5(4):317–24.
Wei Guowen LB. Effects of dexmedetomidine on perioperative hemodynamics, lactate clearance rate, and hepatic and renal function in patients with septic shock. Guangxi Med J. 2020;42(10):1219–23.
Chun Z. Effects of Dexmedetomidine on the Plasma hs-CRP PCT IL-6 TFC levels and Oxygenation of Patients with ARDS Induced by Sepsis. Hebei Medicine. 2019;25(1):94–8.
Xianfeng HJC, Chi Z, Yiping P, Diansheng T, Fafa K, Zhanhong T. Effect and mechanism of dexmedetomidine on lungs in patients of sepsis complicated with acute respiratory distress syndrome. Chin Critical Care Med. 2018;30(2):151–5.
Zhang Lin GX. Effects of dexmedetomidine on inflammatory response and S100 beta protein in patients with sepsis associated encephalopathy. Chin J Multiple Organ Diseases. 2020;19(2):115–8.
Xiandan WWP, Jinbo Z, et al. Effects of dexmedetomidine sedation on inflammatory factors in patients with mechanical ventilation sepsis. J General Practice. 2018;16(4):675–7.
Wang Y, Handong Z, Chengliang Z. Effects of dexmedetomidine and propofol on agitation and inflammatory response in patients with sepsis associated encephalopathy Modern. J Integrated Traditional Chin and Western Med. 2016;5:544–5.
Zhang C, Wu X, Jiang Z, Yu G. Effects of dexmedetomidine sedation on immunomodulation and cytokine release in mechanically ventilated septic shock patients. J Hebei Medical University. 2013;34(1):33–6.
Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, Davies A, Delaney A, Ghosh A, van Haren F, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460–8.
Yu T, Peng X, Liu L, Li Q, Huang Y, Guo F, Yang Y, Qiu H. Propofol increases preload dependency in septic shock patients. J Surg Res. 2015;193(2):849–55.
Abdelmalik PA, Rakocevic G. Propofol as a risk factor for ICU-Acquired weakness in septic patients with acute respiratory failure. Can J Neurol Sci. 2017;44(3):295–303.
Liu Z, Zeng Y, Yang B, Liao P. Efficacy and safety of dexmedetomidine in sepsis patients requiring mechanical ventilation: a systematic review and meta-analysis. J Clin Pharm Ther. 2021;47:298.
Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43(12):2064–74.
Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29(2):201–7.
Barends R, Absalom A, Minnen B, Vissink A, Visser A. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. PLoS ONE. 2017;12(1):0169525.
Flanders CA, Rocke AS, Edwardson SA, Baillie JK, Walsh TS. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: a systematic review of animal and human studies. Crit Care. 2019;23(1):402.
Miyamoto K, Nakashima T, Shima N, Kato S, Ueda K, Kawazoe Y, Ohta Y, Morimoto T, Yamamura H. Effect of dexmedetomidine on lactate clearance in patients with septic shock: a subanalysis of a multicenter randomized controlled trial. Shock (Augusta, Ga). 2018;50(2):162–6.
Kang K, Gao Y, Wang SC, Liu HT, Kong WL, Zhang X, Huang R, Qi ZD, Zheng JB, Qu JD, et al. Dexmedetomidine protects against lipopolysaccharide-induced sepsis-associated acute kidney injury via an α7 nAChR-dependent pathway. Biomed Pharmacother. 2018;106:210–6.
Acknowledgements
Not applicable.
Funding
Supported by the Non-Profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant Number: 2019XK320035).
Author information
Authors and Affiliations
Contributions
YCL, HDZ and TZ conceived of the study idea. TZ and YCL coordinated the systematic review. QMM and SBD designed the search strategy. QMM and SBD screened abstracts and full texts. TZ and QMM acquired the data and judged risk of bias in the studies. TZ verified the data and performed the analyses. TZ and SBD created the GRADE evidence profiles. All authors interpreted the data analyses. All authors cowrote and revised the manuscript for intellectual content. All authors provided their final approval for manuscript submission. All authors agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read the manuscript and consented for this manuscript to be published by Critical Care.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. PRISMA checklist. Sample Search Strategy: Appendix S1 to Appendix S5.e-Table S1: mortality timeline. Appendix S6. Summary of Findings Table: e-Table S2 to e-Table S8. Appendix S6. Summary of Findings Table: e-Table 2 to e-Table 8. e-Table S9: Levels of IL-6 and TNF-α changes at 24 h. e-Table S10: Levels of Alanine Transaminase and Creatinine changes at 24 he-Figure 1: Summarizes the RoB for each individual trial. e-Figure 2: e-Figure_2 Publish bias assessments. e-Figure 3: Effect of dexmedetomidine on mortality. e-Figure 4: Sensitivity analysis based on mortality . e-Figure 5: Effect of dexmedetomidine on ICU stays. e-Figure 6: Forest plot of duration of mechanical ventilation. e-Figure 7: Forest plot of ventilator-free days. e-Figure S8: Effect of dexmedetomidine on levels of IL-6. e-Figure 9: Effect of dexmedetomidine on levels of TNF-α. e-Figure 10: Effect of dexmedetomidine on levels of ALT and Cr. e-Figure 11: Forest plot of Incidence of delirium. e-Figure 12: Forest plot of Incidence of adverse events.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Mei, Q., Dai, S. et al. Use of dexmedetomidine in patients with sepsis: a systematic review and meta-analysis of randomized-controlled trials. Ann. Intensive Care 12, 81 (2022). https://doi.org/10.1186/s13613-022-01052-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01052-2