Abstract
Objective
The purpose of this article is to systematically review the association between dry eye and sleep quality.
Methods
PubMed, EMBASE, Cochrane, Web of Science, and grey literature databases were searched for observational studies published before April 2023. Meta-analysis was performed using STAT15 software.
Results
A total of 21 studies with 419,218 participants were included. The results showed that the dry eye subjects had a worse sleep quality than the healthy population, with poorer subjective sleep quality, longer sleep latency, and a higher risk of unhealthy sleep duration such as insufficient sleep or excessive sleep. The Pittsburgh Sleep Quality Index (PSQI) scores of the dry eye subjects were significantly higher than those of the control subjects (WMD = 1.78, 95%CI: 1.06, 2.50, P < 0.001). The dry eye subjects scored higher than the control subjects in sleep quality, sleep latency, and sleep disturbance in PSQI; there was no difference between the dry eye individuals and control subjects in sleep duration, sleep efficiency, daytime dysfunction, and sleep medication scores. The risk of sleep disorders in the dry eye subjects was significantly higher than that in the non-dry eye subjects (RR = 2.20, 95%CI: 1.78, 2.72, P < 0.001); the risk of insufficient sleep in the dry eye subjects was higher than that in the control subjects (RR = 3.76, 95%CI: 3.15, 4.48, P < 0.001), and the prevalence of excessive sleepiness in dry eye subjects was higher than that in the control subjects (RR = 5.53, 95%CI: 3.83, 7.18, P < 0.001). The ESS scores of the dry eye subjects were significantly higher than those of the control subjects (WMD = 3.02, 95%CI: 2.43, 3.60, P < 0.01).
Conclusion
Our meta-analysis suggests that individuals with dry eye have a worse sleep quality than the healthy population, with poorer subjective sleep quality, longer sleep latency, and higher risk of unhealthy sleep duration such as insufficient sleep or excessive sleepiness.
Similar content being viewed by others
Introduction
Sleep is an essential physiological process for life, accounting for approximately one-third of human daily activities. In recent years, bad sleep quality, including sleep disorder, insomnia, circadian rhythm disorders, and excessive sleep, have become a persistent global social issue [1]. Previous studies have shown that sleep disorder was associated with higher risks of hypertension [2], diabetes [3], cancer [4], and other adverse outcomes [5]. Although pharmacological therapies have applied to the management of sleep disorder, chance to receive sleep therapy is limited owing to the lack of sleep therapists and sleep clinics [6]. Due to lifestyle changes and the dramatic increase in the population with sleep disorders, identifying potentially modifiable risk factors is clinically important for the primary prevention of sleep disorders.
Dry eye is a multifactorial ocular surface disease characterized by an imbalance in tear film homeostasis, accompanied by varying degrees of tear film instability, increased osmotic pressure, inflammation, and nerve damage [7]. Poor sleep quality in dry eye patients may be related to incomplete eyelid closure, eye discomfort, and mental stress [8]. The dry mouth symptoms of Sjogren’s syndrome patients, which require them to frequently get up at night to drink water or consume more fluids before sleep, may also be one of the culprits causing poor sleep quality. Accordingly, there are emerging studies that have indicated that dry eye was associated with the risk of sleep disorder [9, 10]. A cross-sectional study by Kawashima et al. [9] including 672 participants aged 26–64 years from Osaka reported that sleep quality measured by Pittsburgh Sleep Quality Index(PSQI) score in dry eye patients was significantly poorer than that in non-dry eye individuals. However, only one meta-analysis suggested that participants with dry eye had poorer sleep quality, more daytime sleep, shorter total sleep duration, and higher prevalence, incidence, and severity of sleep disorders compared to participants without dry eye [10].However, present studies were limited by the small sample size included, years only up to 2018, and the inaccurate selection criteria for the dry eye subjects.
Therefore, the purpose of the current study was to systematically review the association between dry eye and sleep disorder by performing a meta-analysis of the evidence across existing observational studies to scientific evidence for the prevention of sleep disorders.
Method
Literature search strategy
A comprehensive search was conducted in PubMed, EMBASE, Cochrane, Web of Science, and gray literature databases, and appropriate search strategies were developed using “dry eye” and “sleep disorder” as search targets to retrieve articles as comprehensively as possible (see Annex). The search was conducted up to April 2023. To ensure the comprehensiveness of the retrieved literature, no restrictions were placed on the publication date, location, or ethnicity. See Supplementary material 1 for specific search strategies.
Inclusion and exclusion criteria
The inclusion criteria for this study were: (1) subjects aged ≥ 18 years; (2) case subjects diagnosed with dry eye or Sjogren’s syndrome, with diagnostic criteria provided, and the control subjects consisting of healthy individuals; (3) provision of the number of bad sleep quality or sleep scores for both case and control subjectss; (4) study type is observational research.
Exclusion criteria: (1) inappropriate article types, such as reviews, meta-analyses, conference abstracts, case reports, etc.; (2) animal experiments; (3) unobtainable data or inappropriate data types; (4) duplicate publications or articles without full-text availability; (5) control subjects with other eye diseases.
Data extraction
Two researchers independently screened the articles based on titles and abstracts, and then further screened the full texts according to the inclusion and exclusion criteria. In case of disagreement, a third researcher was consulted for resolution. Information extracted included author(s), country, study type, data collection period, publication year, type of dry eye, number of participants in the dry eye and control subjectss, age, female proportion, and sleep-related judgment criteria.
Quality assessment
Quality assessments were conducted for the finally included articles based on their types. For cohort and case-control studies, the Newcastle-Ottawa Scale (NOS) was used to [11, 12] assess the selection of cases and controls/cohorts, comparability, exposure and outcome. The assessment included appropriateness and representativeness of case or cohort selection, determination and reliability of exposure, comparability of cases or cohorts, response rate or follow-up rates and duration, consistency of investigation and outcome measurement methods, etc., totaling 9 points, with 2 points for “comparability”. The final scores were categorized as: 0–3 points = low quality; 4–6 points = medium quality; 7–9 points = high quality [13]. For cross-sectional studies, the Agency for Healthcare Research and Quality (AHRQ) scale was used for evaluation, with a total of 11 items and 11 points, covering the definition of information sources, inclusion and exclusion criteria, time frame and continuity of patient identification, subjective factors of evaluators, quality assurance assessment, confounding and missing data, and patient response rate and completeness. For each question in the questionnaire, 1 point was awarded for a “yes” answer, and no points were awarded for a “no” or “unclear” answer. The final scores were related to the article quality as follows: low quality = 0–3; medium quality = 4–7; high quality = 8–11 [13].
Statistical method
Data were analyzed using the STATA15 software. Continuous variables were expressed as weighted mean difference (WMD), and non-continuous variables were reported as relative risk (RR). Both were provided with 95% confidence intervals (CI), and forest plots were drawn. Heterogeneity between studies was assessed using the Q test and I2 test: if the test result was P ≥ 0.10 and I2 < 50%, homogeneity among studies was assumed, and a fixed-effects model was chosen; if P < 0.10 and I2 ≥ 50%, heterogeneity was considered among the included studies, and a random-effects model was chosen. For outcome measures reported in more than 10 included articles, Egger’s test was used to assess potential publication bias. Sensitivity analysis was performed by sequentially excluding individual articles. If the results obtained after excluding individual articles did not show significant deviation, the results were considered relatively accurate. A P-value of < 0.05 was considered statistically significant.
Result
Literature screening process and results
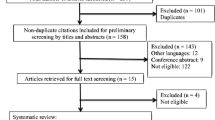
A total of 2236 related articles were retrieved, and after deduplication, 1511 articles remained. By reading the titles and abstracts, 64 articles remained after excluding those that did not meet the inclusion and exclusion criteria. After reading the full articles, 43 articles were excluded, including 9 articles with unavailable full texts, 9 articles with unsuitable data types, 14 articles with unsuitable study subjects, and 11 articles with unclear disease definitions or unsuitable article types. A total of 21 articles were finally included in this study [8, 9, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The literature screening process and results are shown in Fig. 1.
Study characteristics
This study included a total of 419,218 patients, with 152,567 patients in the dry eye subjects and 266,651 patients in the healthy control subjects. The study characteristics are shown in Table 1, with all articles being of medium to high quality. Among them, there were 12 cross-sectional studies [8, 9, 14,15,16,17,18, 21, 22, 25,26,27], 6 cohort studies [19, 23, 29, 31, 32], and 3 case-control studies [24, 28, 30]. The studies involved cases from the United States (1) [17], Australia (1) [28], China (3) [30,31,32], India (1) [24], Italy (1) [26], Japan (3) [9, 14, 27], South Korea (5) [8, 16, 21, 25, 31], the Netherlands (2) [23, 29], Singapore (1) [22], Sweden (1) [18], Turkey (1) [15], and the United Kingdom (1) [19]. There were 14 studies with a dry eye-only experimental subjects [8, 9, 14, 17, 21,22,23,24,25, 27, 30,31,32] and 7 studies with a Sjögren’s syndrome subjects [15, 16, 18, 19, 26, 28, 29]. The judgment of sleep disorder was based on the PSQI questionnaire (including the CPSQI questionnaire) in 8 studies [9, 14,15,16, 23, 26, 30, 31], the ESS questionnaire in 5 studies [15, 16, 19, 22, 28], the ISI in 2 studies [17, 22], the IRLS in 1 study [15], the ICD-10 disease code in 1 study [32], other self-assessment questionnaires in 4 studies [8, 21, 24, 27], the 15-item Dutch questionnaire on sleep quality in 1 study [29], the Uppsala Sleep Inventory in 1 study [18], and polysomnography in 1 study [28].
Quality assessment of the included literature showed that the cross-sectional study quality scores ranged from 6 to 10, indicating that the included studies were of medium to high quality. Cohort studies scored 8–9 points, representing high-quality articles. Case-control studies scored 6–8 points, representing high-quality articles.
Total PSQI score
The PSQI (Pittsburgh Sleep Quality Index) is a widely used and recognized questionnaire for assessing sleep quality [1], which is subjectively completed by the patients. The higher the score, the worse the sleep quality. In the included articles, 7 articles [9, 14,15,16, 26, 30, 31] mentioned the PSQI total score and used it to evaluate the sleep quality of the patients. There was significant heterogeneity between the studies (I2 = 89.1%, P < 0.001), so a random-effects model was used (see Fig. 2A). It was found that the PSQI score of the dry eye subjects was significantly higher than that of the control subjects (WMD = 1.78, 95%CI: 1.06, 2.50, P < 0.001), indicating that the sleep quality of dry eye patients was worse than that of healthy people, and this difference was statistically significant. A sensitivity analysis was conducted on the included articles for this indicator (Fig. 2B), and it was found that the exclusion of individual articles did not significantly affect the main results. At the same time, since the number of included articles was less than 10, Egger’s test was not performed.
PSQI Subitem scores
The PSQI questionnaire scores are divided into subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, daytime dysfunction, and use of sleep medication. Each domain has a score of 0–3 points. The higher the score, the worse the patient’s performance in that item. A random-effects model was used for the analysis (Fig. 3).
Four articles mentioned the subjective sleep quality scores [14, 27, 30, 31]. The combined results showed that the subjective sleep quality scores of dry eye subjects patients were significantly higher than those of the control subjects (WMD = 0.24, 95%CI: 0.10, 0.38, P = 0.001). Two articles mentioned sleep latency scores [30, 31], and the dry eye subjects had significantly higher scores than the control subjects (WMD = 0.19, 95%CI: 0.12, 0.26, P < 0.001). Two articles mentioned sleep duration scores [30, 31], and there was no statistically significant difference in the sleep duration scores between the dry eye subjects and the control subjects (WMD = 0.36, 95%CI: -0.13, 0.86, P = 0.151). Three articles mentioned sleep efficiency scores [14, 30, 31], and there was no statistically significant difference in the sleep efficiency scores between the dry eye subjects and the control subjects (WMD = 0.16, 95%CI: -0.03, 0.36, P = 0.093). Three articles mentioned sleep disturbance scores [14, 30, 31], and the dry eye subjects had significantly higher scores than the control subjects (WMD = 0.21, 95%CI: 0.12, 0.29, P < 0.001). Three articles mentioned daytime dysfunction scores [14, 30, 31], and there was no statistically significant difference in the sleep duration scores between the dry eye subjects and the control subjects (WMD = 0.27, 95%CI: -0.02, 0.55, P = 0.064). Three articles mentioned used sleep medication scores [14, 30, 31], and there was no statistically significant difference in the sleep medication scores between the dry eye subjects and the control subjects (WMD = 0.01, 95%CI: -0.05, 0.07, P = 0.719).
Sleep disorder
A total of 8 articles [9, 17, 22, 23, 30,31,32] reported the number of individuals with sleep disorder (Fig. 4A). After testing for heterogeneity between the subjectss (I2 = 99.5%, P < 0.001), a random-effects model was used. The results showed that the risk of sleep disorders in the dry eye subjects was significantly higher than in the non-dry eye subjects (RR = 2.20, 95%CI: 1.78, 2.72, P < 0.001), with statistically significant differences.
Insufficient sleep
In this study, sleep duration of less than 5 h was considered insufficient sleep (Fig. 4B). Four articles [8, 21, 22, 25] reported the number of individuals with insufficient sleep (I2 = 89.7%, P < 0.001), and a random-effects model was used. The results showed that the incidence of insufficient sleep in dry eye patients (RR = 3.76, 95%CI: 3.15, 4.48, P < 0.001) was higher than that in the control subjects, with statistically significant differences.
Excessive sleep
In this study, sleep duration of 9 h or more was considered excessive sleep (Fig. 4C). Three articles [8, 21, 25] reported the number of individuals with excessive sleep based on sleep duration (I2 = 93.5%, P < 0.001). According to the results, the incidence of excessive sleep in dry eye patients was higher than that in the control subjects (RR = 5.53, 95%CI: 3.83, 7.18, P < 0.001), with statistically significant differences.
Some articles [15, 16, 28] also used the ESS (Epworth Sleepiness Scale) questionnaire to investigate the likelihood of daytime sleepiness (Fig. 4D). The ESS questionnaire consists of 8 questions, mainly about the patient’s typical daytime sleepiness situation [16]. Cho et al. [16] concluded that dry eye patients had higher ESS scores and were more likely to experience daytime sleepiness. According to the analysis, dry eye patients are more likely to experience excessive sleepiness (WMD = 3.02, 95%CI: 2.43, 3.60, P < 0.01), with statistically significant differences.
Other Sleep-Related scales
Gudbjörnsson et al. [18]used the Uppsala Sleep Inventory to assess sleep conditions, which is an 87-item survey questionnaire completed by the patients themselves. Patients’ performances in the questionnaire were divided into five levels (five-point scale). Gudbjörnsson et al. [18] chose the top two levels of severity for analysis, namely “’great”/“very great” and " often “/“ very often”. The results showed that patients with pSS (primary Sjogren’s syndrome) had more tense muscles when falling asleep, and experienced anxiety and racing thoughts compared to healthy individuals. At the same time, they were more likely to wake up at night and feel more tired during the day.
The ISI (Insomnia Severity Index) questionnaire was also included in the literature of this study to assess patients’ insomnia [17]. Galor et al. [17] found that the ISI scores of dry eye patients (13.3 ± 8.3) were significantly higher than those of non-dry eye patients (7.3 ± 7.3), suggesting that dry eye patients are more likely to suffer from insomnia. Additionally, the severity of eye pain symptoms was related to the severity of insomnia. Lim et al. [22] observed that there was a significant association between dry eye and insomnia (OR = 1.08, 95%CI: 1.05, 1.11, P < 0.001).
Discussion
Compared to previously published systematic reviews, our analysis only included healthy controls and analyzed 22 studies involving 419,218 patients to draw conclusions. Current research results show that the sleep quality of dry eye patients is significantly worse than that of the healthy population, and the risk of sleep disorders is significantly higher than that of the normal population. Based on PSQI questionnaire scores, dry eye patients have poorer subjective sleep quality, longer sleep latency, and have sleep disorders; however, there is no difference in sleep duration, sleep efficiency, daytime dysfunction, or use of sleeping medications. Dry eye patients are more likely than healthy individuals to have the risk of unhealthy sleep duration, such as insufficient sleep or excessive sleep.
Our literature review confirms the previous conclusion by Au et al. [10]that dry eye patients are more likely to have sleep disorder. However, there is a slight discrepancy regarding whether dry eye patients are more prone to daytime issues. Au et al. [10] believed that dry eye patients are more likely to have daytime sleepiness, while we found no difference between dry eye patients and the healthy population in terms of daytime dysfunction. This is related to our different definitions of daytime behavior. Besides, Au et al.‘s [10]systematic review included control subjectss with other eye diseases, while our control subjects consisted only of healthy individuals. There are some contradictions in the conclusions of this study. Based on the PSQI questionnaire, there is no difference in total sleep duration between dry eye patients and healthy people, but dry eye patients are more likely to have extreme sleep duration according to statistics. This may be related to an increased sample size or the subjectivity of the questionnaire.
Poor sleep qualities in dry eye patients may be due to light exposure and discomfort caused by incomplete eyelid closure or pain caused by inflammation [8]. Dry mouth discomfort experienced by dry eye and pSS patients is also a contributing factor. At the same time, dry eye patients are more likely to suffer from anxiety and depression [33], with a prevalence of about 29%, making it one of the eye diseases most likely to cause depression [34]. Emotional disorders, such as stress, can also cause sleep disturbance. In a study by Ayaki et al. [35], dry eye patients were provided with treatments including eye drops, eye care, nutritional supplements, and daily advice. The patients’ PSQI scores decreased, and their sleep quality improved, further supporting the findings of this study.
Additionally, there is a bidirectional association between dry eye and sleep disorders; poor sleep or sleep deprivation may also lead to the onset or exacerbation of dry eye symptoms. An intervention study by Lee et al. [36] showed that sleep deprivation (SD) induces increased tear osmolarity, shortened tear film break-up time, and reduced tear secretion, thereby further triggering the onset and development of dry eye. Physiologically, sleep disorders often lead to autonomic nervous system dysfunction, affecting the parasympathetic function in the lacrimal gland and reducing tear secretion [31, 37].
Although this study tried to include high-quality studies from different countries, there are still some limitations. Firstly, most of the included articles used questionnaires to determine whether patients had dry eye or sleep disorders, and questionnaires have a certain degree of subjectivity, with different respondents having different standards. Secondly, most of the included articles were cross-sectional studies, with little discussion on the mechanisms and causal relationships underlying the increased incidence of sleep disorders in dry eye patients. Finally, dry eye is more common in women and elderly patients, and the original studies included in this study adjusted for the gender factor, leading to the inclusion of more elderly women in the study population compared to other subjects.
Conclusion
Our meta-analysis indicates that dry eye patients have a lower sleep quality than the healthy population, with poorer subjective sleep quality, longer sleep latency, and a higher risk of unhealthy sleep duration such as insufficient sleep or excessive sleep.
However, so far, there is not enough evidence to establish a causal relationship and related mechanisms between dry eye and sleep disorder. In the future, more large-scale prospective studies are needed to provide more assistance in patient management and treatment.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Latreille MKP. Sleep disorders. Am J Med. 2019;132(3):292–9.
Han B, Chen WZ, Li YC, Chen J, Zeng ZQ. Sleep and hypertension. Sleep Breath = Schlaf Atmung. 2020;24(1):351–6.
Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab Clin Exp. 2018;84:56–66.
Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. 2021;56:101409.
Cappuccio FP, Miller MA. Sleep and Cardio-Metabolic Disease. Curr Cardiol Rep. 2017;19(11):110.
Peersmann SHM, Grootenhuis MA, van Straten A, Kerkhof GA, Tissing WJE, Abbink F, de Vries ACH, Loonen J, Kremer LCM, Kaspers GJL et al. Prevalence of Sleep disorders, risk factors and Sleep Treatment needs of adolescents and young adult Childhood Cancer patients in Follow-Up after treatment. Cancers 2022, 14(4).
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
An Y, Kim H. Sleep disorders, mental health, and dry eye disease in South Korea. Sci Rep. 2022;12(1):11046.
Kawashima M, Uchino M, Yokoi N, Uchino Y, Dogru M, Komuro A, Sonomura Y, Kato H, Kinoshita S, Tsubota K. The association of sleep quality with dry eye disease: the Osaka study. Clin Ophthalmol (Auckland NZ). 2016;10:1015–21.
Au NH, Mather R, To A, Malvankar-Mehta MS. Sleep outcomes associated with dry eye disease: a systematic review and meta-analysis. Can J Ophthalmol J Canadien D’ophtalmologie. 2019;54(2):180–9.
Mao DH, Miao JK, Zou X, Chen N, Yu LC, Lai X, Qiao MY, Chen QX. Risk factors in Predicting prognosis of neonatal bacterial Meningitis-A systematic review. Front Neurol. 2018;9:929.
Baldacci S, Santoro M, Coi A, Mezzasalma L, Bianchi F, Pierini A. Lifestyle and sociodemographic risk factors for gastroschisis: a systematic review and meta-analysis. Arch Dis Child. 2020;105(8):756–64.
Hu Q, Li SJ, Chen QL, Chen H, Li Q, Wang M. Risk factors for acute kidney Injury in critically ill neonates: a systematic review and Meta-analysis. Front Pead. 2021;9:666507.
Ayaki M, Kawashima M, Negishi K, Kishimoto T, Mimura M, Tsubota K. Sleep and mood disorders in women with dry eye disease. Sci Rep. 2016;6:35276.
Balkarli A, Semiz M, Uslu AU, Yalcinkaya S, Can B, Sahin M, Kucuk A. An Assessment of sleep disturbances and quality of life in primary Sjögren’s syndrome and its relationship with urinary incontinence. 2016.
Cho KJ, Kim HK, Lim MH, Baek HS, Yang YA, Kang BH, Lee JY, Kim JY, Kim MS, Lee CMJJP. Depression, ADHD, job stress and sleep problems with dry eye disease in Korea. 2015, 18(6):1–5.
Galor A, Seiden BE, Park JJ, Feuer WJ, McClellan AL, Felix ER, Levitt RC, Sarantopoulos CD, Wallace DM. The Association of Dry Eye Symptom Severity and Comorbid Insomnia in US veterans. Eye Contact Lens. 2018;44(Suppl 1):S118–24.
Gudbjörnsson B, Broman JE, Hetta J, Hällgren R. Sleep disturbances in patients with primary Sjögren’s syndrome. Br J Rheumatol. 1993;32(12):1072–6.
Hackett KL, Newton JL, Frith J, Elliott C, Lendrem D, Foggo H, Edgar S, Mitchell S, Ng WF. Impaired functional status in primary Sjögren’s syndrome. Arthritis Care Res. 2012;64(11):1760–4.
Han KT, Nam JH, Park EC. Do Sleep disorders positively correlate with Dry Eye Syndrome? Results of national claim data. Int J Environ Res Public Health 2019, 16(5).
Lee W, Lim SS, Won JU, Roh J, Lee JH, Seok H, Yoon JH. The association between sleep duration and dry eye syndrome among Korean adults. Sleep Med. 2015;16(11):1327–31.
Lim EWL, Chee ML, Sabanayagam C, Majithia S, Tao Y, Wong TY, Cheng CY, Tong L. Relationship between sleep and symptoms of tear dysfunction in Singapore Malays and indians. Investig Ophthalmol Vis Sci. 2019;60(6):1889–97.
Magno MS, Utheim TP, Snieder H, Hammond CJ, Vehof J. The relationship between dry eye and sleep quality. Ocul Surf. 2021;20:13–9.
Nithish Raj K, Bhaskaran B, Panimalar V, Veeramani A, Divya N. Association between dry eye symptoms and insomnia. Int J Res Pharm Sci. 2020;11(Special Issue 4):2516–9.
Park H-W, Kim T-H. The Association between Sleep Duration and Dry eyes by general characteristics: the 7th Korea National Health and Nutrition Examination Survey. Korean J Vis Sci. 2021;23(4):555–65.
Priori R, Minniti A, Antonazzo B, Fusconi M, Valesini G, Curcio G. Sleep quality in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2016;34(3):373–9.
Takahashi A, Negishi K, Ayaki M, Uchino M, Tsubota K. Nocturnal lagophthalmos and Sleep Quality in patients with Dry Eye Disease. Life (Basel Switzerland) 2020, 10(7).
Usmani ZA, Hlavac M, Rischmueller M, Heraganahally SS, Hilditch CJ, Lester S, Catcheside PG, Antic NA, Chai-Coetzer CL, McEvoy RD. Sleep disordered breathing in patients with primary Sjogren’s syndrome: a group controlled study. Sleep Med. 2012;13(8):1066–70.
van Oers ML, Bossema ER, Thoolen BJ, Hartkamp A, Dekkers JC, Godaert GL, Kruize AA, Derksen RH, Bijlsma JW, Geenen R. Variability of fatigue during the day in patients with primary Sjögren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(5):715–21.
Wu M, Liu X, Han J, Shao T, Wang Y. Association between Sleep Quality, Mood Status, and ocular surface characteristics in patients with Dry Eye Disease. Cornea. 2019;38(3):311–7.
Yu X, Guo H, Liu X, Wang G, Min Y, Chen SS, Han SS, Chang RT, Zhao X, Hsing A et al. Dry eye and sleep quality: a large community-based study in Hangzhou. Sleep 2019, 42(11).
Zheng Q, Li S, Wen F, Lin Z, Feng K, Sun Y, Bao J, Weng H, Shen P, Lin H, et al. The Association between Sleep Disorders and incidence of Dry Eye Disease in Ningbo: Data from an Integrated Health Care Network. Front Med. 2022;9:832851.
Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. 2016;30(12):1558–67.
Zheng Y, Wu X, Lin X, Lin H. The prevalence of Depression and depressive symptoms among Eye Disease patients: a systematic review and Meta-analysis. Sci Rep. 2017;7:46453.
Ayaki M, Toda I, Tachi N, Negishi K, Tsubota K. Preliminary report of improved sleep quality in patients with dry eye disease after initiation of topical therapy. Neuropsychiatr Dis Treat. 2016;12:329–37.
Lee YB, Koh JW, Hyon JY, Wee WR, Kim JJ, Shin YJ. Sleep deprivation reduces tear secretion and impairs the tear film. Investig Ophthalmol Vis Sci. 2014;55(6):3525–31.
Chiaro G, Calandra-Buonaura G, Cecere A, Mignani F, Sambati L, Loddo G, Cortelli P, Provini F. REM sleep behavior disorder, autonomic dysfunction and synuclein-related neurodegeneration: where do we stand? Clin Auton Research: Official J Clin Auton Res Soc. 2018;28(6):519–33.
Acknowledgements
Not applicable.
Funding
This research was supported by “The Youth Beijing Scholars program”, Natural Science Foundation of China (821704052, 8217040515).
The fund was not involved in any study design, data collection, analysis and interpretation, report writing, and article submission for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: Yixuan Gu, Ao Li, Lei Tian. Data curation: Yixuan Gu, Ao Li, Lei Tian. Formal analysis: Yixuan Gu, Ao Li, Ying Jie. Funding acquisition: Yixuan Gu, Jingyi Wang, Ying Jie. Investigation: Jingyi Wang, Ying Jie. Methodology: Yixuan Gu, Jingyi Wang, Ying Jie. Project administration: Kai Cao, Yihan Guo, Ying Jie. Resources: Kai Cao, Yihan Guo, Yiran Hao. Software: Kai Cao, Yiran Hao. Supervision: Kai Cao, Lei Tian. Validation: Kai Cao, Lei Tian. Visualization: Ao Li, Lei Tian. Writing -original draft: Yixuan Gu, Kai Cao, Ao Li, Jingyi Wang, Yihan Guo, Yiran Hao, Lei Tian, Ying Jie. Writing-review & editing: Yixuan Gu, Kai Cao, Ao Li, Jingyi Wang, Yihan Guo, Yiran Hao, Lei Tian, Ying Jie.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gu, Y., Cao, K., Li, A. et al. Association between sleep quality and dry eye disease: a literature review and meta-analysis. BMC Ophthalmol 24, 152 (2024). https://doi.org/10.1186/s12886-024-03416-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03416-7