Abstract
Background
The current standard first-line treatment for hormone receptor-positive/human epidermal growth factor receptor 2 negative (HR + /HER2 −) advanced breast cancer (ABC) is a combination of aromatase inhibitor (AI) plus CDK4/6 inhibitors (CDK4/6i). Direct comparison trials of different CDK4/6i are scarce. This real-world study compared the effectiveness of first-line AI plus ribociclib versus palbociclib.
Methods
This multicenter retrospective cohort study, conducted in six cancer centers in Thailand, enrolled patients with HR + /HER2 − ABC treated with first-line AI, and either ribociclib or palbociclib. Propensity score matching (PSM) was performed. The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), overall response rate (ORR), time to chemotherapy (TTC), and adverse events.
Results
Of the 250 patients enrolled, 134 patients with ribociclib and 49 patients with palbociclib were captured after PSM. Baseline characteristics were well-balanced between groups. Median PFS in patients receiving ribociclib and palbociclib were 27.9 and 31.8 months, respectively (hazard ratio: 0.87; 0.55–1.37). The median OS in the AI + ribociclib arm was 48.7 months compared to 59.1 months in the AI + palbociclib arm (hazard ratio: 0.55; 0.29–1.05). The median TTC in the AI + palbociclib group was 56 months, but not reached in the AI + ribociclib group (p = 0.42). The ORR of AI + ribociclib and AI + palbociclib were comparable (40.5% vs. 53.6%, p = 0.29). Patients receiving palbociclib demonstrated a higher proportion of neutropenia compared to those receiving ribociclib, despite a similar dose reduction rate (p = 0.28). Hepatitis rate was similar between the ribociclib (21%) and palbociclib groups (22%). Additionally, a low incidence of QT prolongation was observed in both the ribociclib (5%) and palbociclib groups (4%).
Conclusion
This preliminary analysis of a real-world study demonstrated the comparable effectiveness of ribociclib and palbociclib with AI as an initial therapy for HR + /HER2 − ABC. No statistically significant difference in PFS, OS, and TTC was found in patients treated with AI combined with palbociclib or ribociclib. Longer follow-up and further prospective randomized head-to-head studies are warranted.
Similar content being viewed by others
Background
Breast cancer is the most prevalent cancer among females globally, including Thailand [1]. Its diverse subtypes hinge on staining for hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2), with HR-positive and HER2-negative (HR + /HER2 −) breast cancer being the most prevalent [2]. Controlling disease, bolstering overall survival (OS), and improving the quality of life (QoL) are the primary aims of advanced breast cancer (ABC) treatment. Developing the treatment for locally advanced/metastatic breast cancer (LA/MBC) relies on factors, including tumor subtype, disease burden, Eastern Cooperative Oncology Group performance status(ECOG-PS), comorbidities, and economic considerations [3].
The initial treatment typically is upfront hormonal therapy for patients with HR + /HER2 − LA/MBC experiencing non-visceral crises. Selective estrogen receptor modulators, non-steroidal aromatase inhibitors (NSAIs), steroidal aromatase inhibitors (SAIs), and selective estrogen receptor downregulators are among the variety of options available [2]. Recent studies indicate that initiating treatment with upfront hormonal therapy improves progression-free survival (PFS) and enhances patients’ QoL. First-line tamoxifen provided a PFS of approximately 8 months [4], whereas PFS from NSAIs and fulvestrant were approximately 12 months [4, 5] and 14 months, respectively [4].
Cyclin-dependent kinase (CDK) is a crucial molecule for cancer cell division. The interaction of cyclin D1 with CDK4 and CDK6 in the cell cycle causes hyperphosphorylation of the retinoblastoma gene (Rb), thereby activating the cell to pass from the G-phase checkpoint to the S phase (replication phase) of the cell cycle. Cyclin D-CDK4/6-Rb pathway alterations, such as cyclin D amplification, Rb gene loss or mutation, or P16 loss, cause uncontrolled cell cycle progression. Consequently, cancer cells divide rapidly and metastasize [6].
Currently, drugs that inhibit the CDK4/6-Rb pathway (CDK4/6 inhibitors [CDK4/6i]), such as palbociclib, ribociclib, and abemaciclib, have been approved as an effective therapy for ER + /HER2 − ABC. Studies have revealed the benefit of CDK4/6i when combined with NSAI as first-line treatment which prolongs PFS and increases overall response rates (ORR) compared to AI monotherapy. Data from current studies indicate the median PFS for palbociclib, ribociclib, and abemaciclib of 28, 25, and 28 months, respectively [7,8,9]. The ORR from the combination CDK4/6i and NSAI stands at 53%–59% compared to AI which typically yields an ORR of approximately 30%–40%. Regarding OS, palbociclib, ribociclib, and abemaciclib have reported median OS of 53.9, 63.9, and 66.8 months [7, 8, 10,11,12,13], respectively. Notably, ribociclib is the only CDK4/6i that exhibited a significant OS improvement when compared to AI monotherapy in the first-line setting. Additionally, these agents in combination with fulvestrant provide gains in PFS and ORR in the second-line setting [14,15,16].
The side effects of CDK4/6i vary according to the specific drug. The main side effects of palbociclib include neutropenia, whereas ribociclib includes neutropenia, QT prolongation, and hepatotoxicity. Abemaciclib is more associated with diarrhea with less frequent myelosuppression [7,8,9].
Presently, prospective studies reported no evidence, and real-world evidence (RWE) that directly compares the efficacy and toxicities between CDK4/6i types when combined with AI for treating patients with HR + /HER2 − LA/MBC remains limited.
Palbociclib, ribociclib, and abemaciclib obtained Thai FDA approval in 2017, 2018, and 2020, respectively. However, reported outcomes concerning their efficacy in the first-line setting, as well as comparative efficacy among the different CDK4/6i, remain lacking. Furthermore, we aim to investigate the toxicity profile of these CDK4/6i in Thai patients which may diverge from that observed in reports from Western countries.
This multicenter study aimed to investigate the efficacy differences between ribociclib and palbociclib when combined with NSAI for first-line therapy of HR + /HER2 − LA/MBC in real-life clinical practice.
Patients and methods
Patients
Inclusion criteria were age of ≥ 18 years and histologically or cytologically confirmed HR + /HER2 − ABC diagnosis, defined as tumors expressing estrogen and/or progesterone receptors of > 1% and HER2 negativity determined by immunohistochemistry scores of 0, 1 + , or 2 + with negative results by in situ hybridization. Additionally, patients must have received first-line treatment with AI combined with ribociclib or palbociclib. This study included menopausal or premenopausal patients receiving ovarian function suppression. All patients included in the study were diagnosed with LA/MBC from January 1, 2017, to October 31, 2022, with the last follow-up cut-off date on September 30, 2023. Exclusion criteria were insufficient or missing data and previous chemotherapy for treatment in a metastatic setting.
Study design
This multicenter retrospective cohort study was conducted across six medical institutions/centers in Thailand. The study aimed to compare the efficacy of ribociclib and palbociclib when combined with AI as a first-line therapy for HR + /HER2 − ABC. The primary endpoint was OS by propensity score-match (PSM) analysis, whereas secondary endpoints were PFS, subgroup analysis of OS, time to chemotherapy (TTC), response rate, CDK4/6i dose modification rate, and toxicity. The Institutional Review Board of all participating institutions approved the study.
Statistical analysis
The chi-square test or Fisher’s test was used to compare qualitative variables, whereas the Student’s t-test was used to compare quantitative variables. The Mann–Whitney U test was utilized to compare medians. PSM was conducted to balance baseline characteristics. The Kaplan–Meier method was used to estimate PFS, OS, and TTC with group comparisons conducted using the log-rank test. A Cox proportional hazard model was used to estimate hazard ratios.
PSM analysis was conducted to minimize potential selection bias due to the lack of randomization. Propensity scores for AI with palbociclib vs. AI with ribociclib were estimated using a logistic regression model based on clinically selected covariates, including age, ECOG-PS, de novo metastasis, visceral metastasis, and level of estrogen receptor (ER) expression (< 50% or ≥ 50%). Propensity score-adjusted analyses were conducted in the sensitivity analysis. The results are presented as hazard ratios and 95% confidence intervals (CI). The p-value of < 0.05 was considered statistically significant. All analyses were conducted using STATA version 14.
Results
Patients’ clinical characteristics
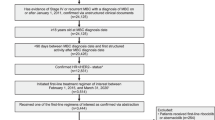
Figure 1 illustrates the patient consort diagram, indicating that an initial 539 patients with HR + /HER2 − ABC receiving first-line therapy were collected from six tertiary care centers. Of these patients, 250 received first-line treatment with a combination of AI and CDK4/6i. Out of these 250 patients, PSM analysis revealed 183 matched patients for analysis, including 134 patients receiving AI combined with ribociclib and 49 patients treated with AI combined with palbociclib.
Patient consort diagram. Abbreviations: CRA: Chulabhorn Royal Academy, CU: Chulalongkorn Memorial Hospital, PSU: Prince of Songkhla University, RA: Ramathibodi Hospital, SPR: Sawanpracharak Hospital, SI: Siriraj Hospital, AI: aromatase inhibitor, HR: hormone receptor, HER-2: Human Epidermal Growth Factor Receptor 2, MBC: metastatic breast cancer, CDK4/6i: cyclin-dependent kinases 4 and 6 inhibitor
Baseline characteristics were well-balanced between the two groups (Table 1). The median age in the ribociclib and palbociclib groups was 58 and 56 years, respectively. The majority of patients demonstrated an ECOG-PS score of 0–1 (84% in the ribociclib group vs. 87% in the palbociclib group). The proportion of patients with high ER expression (≥ 50%) was comparable in both groups (93% vs. 86%). Visceral metastases were reported in 55% and 53% of patients receiving ribociclib and palbociclib, respectively. Additionally, the majority of patients exhibited fewer than three metastatic sites (78% vs. 75%) (Table 1).
Comparative effectiveness analysis of palbociclib and ribociclib
Overall survival
The data cut-off date for OS analysis was September 30, 2023. The median follow-up time was 29 months (95% CI: 26.15–31.85). Death events occurred in 45 (33%) of 134 and 15 (30%) of 49 patients in the ribociclib and palbociclib groups, respectively.
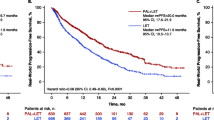
The unadjusted analysis of the entire cohort revealed the comparable median survival between the ribociclib and palbociclib groups at 51.2 months and 57.6 months, respectively (hazard ratio: 0.72, 95% CI: 0.44–1.17, p = 0.18) (Fig. 2A). Following PSM analysis, the adjusted median OS demonstrated a trend toward shorter OS with ribociclib + AI (48.7 months among patients receiving ribociclib and 59.1 months in the palbociclib group (hazard ratio of death: 0.55, 95% CI: 0.29–1.05, p-value: 0.07) (Fig. 2B).
A subgroup analysis of OS revealed no preferential benefit of either palbociclib or ribociclib (Fig. 3) across baseline characteristics of the patients. OS benefit was observed with ribociclib among patients with ≥ 3 metastasis sites and patients with coronary disease. However, the 95% CI was very wide and should be interpreted with caution.
Exploratory analysis of overall survival in subgroup. Abbreviations: AI: aromatase inhibitor, yrs: years, ECOG PS: Eastern Cooperative Oncology Group performance status, CAD: coronary artery disease, ER: estrogen receptor, PR: progesterone receptor, DFI: disease-free interval, ET endocrine therapy, HR: hazard ratio, CI: confidence interval
Progression free survival
Of 250 patients with HR + /HER2 − ABC receiving first-line AI + CDK4/6i, the median PFS was 26.9 (95% CI: 23.5–32.6) and 29.6 (95%CI 18.2–50.8) months (hazard ratio: 0.97, 95% CI: 0.7–1.4) in the ribociclib and palbociclib groups, respectively. A consistent indifference in the median PFS was observed from 180 patients available for analysis after PSM, including 27.9 months (95% CI: 21.8–38.3) and 31.8 months (95% CI: 19.7–57.4) in the ribociclib and palbociclib groups, respectively (hazard ratio: 0.87, 95%CI 0.55–1.37) (Fig. 4).
Univariate and multivariate analysis of overall survival
A univariate analysis identified age at diagnosis, ECOG-PS, menopausal status, comorbidities, de novo metastasis, and the number of metastatic sites to be associated with OS. Only four factors remained independently associated with OS after multivariable analysis (Table 2). The favorable prognostic factors include having one metastatic site and postmenopausal status, whereas adverse prognostic factors include worsening of ECOG-PS and the presence of coronary artery disease. Notably, different CDK4/6i types (ribociclib vs. palbociclib) were not associated with OS outcome in both univariate and multivariate analyses.
Time to chemotherapy and total lines of treatment
Time to first chemotherapy was comparable between both groups (Fig. 5). The median TTC was 56 months (95% CI: 39.37–72.63) in the palbociclib group while in the ribociclib group it was not reached (p = 0.42). Patients who received palbociclib had a trend to receive more subsequent therapy with a median of three lines (95% CI: 2.05–4.08) compared to two (95% CI: 1.8–2.8) lines in the ribociclib group (p = 0.3).
Response rate
The ORRs in the AI + ribociclib and AI + palbociclib groups were 40.5% and 53.6%, respectively (p = 0.29). Similarly, the disease control rate was excellent in both groups (89.6% in the ribociclib group and 92.7% in the palbociclib group, p = 0.29). The median time to response was 15.5 (95% CI: 13.57–17.71) and 11.7 (95% CI: 11–37.14) weeks among patients receiving AI + ribociclib and AI + palbociclib (p = 0.12), respectively.
Toxicity
Neutropenia and anemia are common toxicities observed in both groups. Grades 3–4 neutropenia was significantly less frequent among patients with ribociclib therapy (48% vs. 69%, p = 0.02). Additionally, grades 3–4 thrombocytopenia occurred in only 2% of patients in the ribociclib group compared to 8% in the palbociclib group (p = 0.001). Grades 3–4 anemia was numerically more frequently observed in patients receiving palbociclib (6% vs. 3%, p = 0.44). Abnormal aspartate transaminase/alanine aminotransferase elevation, mostly in grades 1–2, was seen in 19% and 22% of patients in the ribociclib and palbociclib groups, respectively (p = 0.59). QTc prolongation was reported in 5.1% and 4% of patients in the ribociclib and palbociclib groups, respectively (p = 0.91) (Table 3).
Real-world practice of CDK4/6i dosing and dose modification
Nearly all patients (98.6%) in the palbociclib group received the full starting dose of 125 mg daily whereas 86.6% in the ribociclib group received the starting dose of 600 mg daily (p = 0.004). A higher proportion of patients receiving palbociclib experienced myelosuppression, but the rate of CDK4/6i dose reduction was similar (65% and 60% among ribociclib and palbociclib, respectively). All dose reductions occurred in patients who were started on full doses of both agents except for one patient receiving ribociclib at a starting dose of 400 mg who still required a dose adjustment. A similar proportion of patients in both groups required a one-dose level reduction (49% vs. 48%). Additionally, 16% and 11% of patients in the ribociclib and palbociclib groups, respectively, underwent a two-dose level reduction. The median time to dose reduction was 42 days (95% CI: 27.35–56.65) and 49 days (95% CI: 14.31–83.69) in the ribociclib and palbociclib groups, respectively (p = 0.01).
Discussion
CDK4/6i in combination with AI has revolutionized the treatment of HR + /HER2 − ABC and become a standard of care globally. An unsurpassed PFS gain over that of ET alone has been consistently reported in pivotal randomized controlled trials (RCTs) of palbociclib [7], ribociclib [8, 17], and abemaciclib [9] whereas the OS improvement was statistically and/or clinically significant with ribociclib [8, 10] and abemaciclib [13], respectively. However, no RCT and only a few RWEs directly compared the OS of ribociclib and palbociclib [18, 19].
Our study investigated the outcome of first-line treatment with palbociclib or ribociclib in combination with AI for HR + /HER2 − ABC. We collected real-world practice data from six centers across Thailand. A PSM was used to balance the patients’ clinicopathological characteristics and minimize the bias of the retrospective nature of real-world data. We also included ER expression levels using the cut-off level of 50% in both the baseline characteristics and efficacy analysis. The 50% cutoff was chosen as a surrogate for endocrine responsiveness which has been demonstrated in various settings [20,21,22,23]. We revealed no statistically significant differences in both OS and PFS between palbociclib and ribociclib as first-line treatment in our cohort. A multivariate Cox regression analysis, showing a hazard ratio for the survival of 0.51 (p = 0.06, 95% CI: 0.8–1.04) confirmed this result.
The decision to select one agent over the other remained upon physicians’ judgment/experiences together with the side effect profiles and the patients themselves due to the lack of direct RCT comparing the effectiveness of the currently available CDK4/6i. Thus, a comparative analysis of the RWE was used to decipher the dilemma and contribute complementary information to that of RCT. As abemaciclib was the last agent in this class to receive approval in Thailand, we only compared the efficacy of palbociclib and ribociclib in a real-world situation with the main interest in OS. Both the adjusted OS and PFS of our palbociclib cohort were not statistically different from that of ribociclib. These results were congruent with other RWE reports that compared ribociclib and palbociclib outcomes [18, 19]. Numerically, the OS and PFS of both groups were consistent with those of large pivotal trials (PALOMA-2, MONALEESA-2, and MONALEESA-7), except for a somewhat lower OS in our ribociclib cohort (48.7 months vs. 63.9 and 58.7 months in MONALEESA-2 and MONALEESA-7, respectively) [8, 10]. Notably, compared to other RWE of individual CDK4/6i, our study demonstrated longer PFS in both treatment arms [7, 17, 24,25,26,27,28]. Several factors may have contributed to these differences. Patients in our cohort exhibited a lower tumor burden, with > 80% having fewer than three metastatic sites and > 90% having high ER expression (ER ≥ 50%) compared to other trials, thereby representing an endocrine-sensitive population. These results demonstrate that palbociclib and ribociclib are highly effective in endocrine-sensitive patients with low tumor burden and high ER expression. Several factors may have contributed to the numerically shorter OS in ribociclib arm in our study. We revealed a higher number of subsequent therapies in the palbociclib group which could affect OS. In addition, as palbociclib was the first CDK4/6i approved in Thailand, which resulted in longer follow-up periods and potentially more accurate OS assessment in this group.
The results of our study were in contrast to an analysis by Jhaveri et al. [29], which uses a matching adjusted indirect comparison to individual data from MONALEESA-2 and PALOMA-2, demonstrating a greater OS in favor of ribociclib as a first-line regimen. However, its generalizability to a broader patient population in practice is limited, considering the strict and narrow inclusion/exclusion in RCT.
The ORR of the treatment was comparable between the two groups (48% with ribociclib vs. 53.6% with palbociclib). Our real-world finding aligned with MONALEESA and PALOMA-2 trials [7, 12, 17] and previous RWE trials [30,31,32,33,34].
Our study emphasized neutropenia as the primary adverse event as well as a higher myelosuppression incidence with palbociclib compared to ribociclib in terms of tolerability. This indicated a greater bone marrow toxicity in palbociclib compared to ribociclib, possibly due to pharmacokinetic variances [35, 36]. Nearly all patients in the palbociclib group received a full starting dose compared to only 86.6% in the ribociclib group. A similar rate of dose reduction was demonstrated, mostly due to myelosuppression, and this rate was congruent with other reports from Asian [25, 37] and Western populations alike [28, 38].
This study represents the first and largest multicenter cohort data in the real-world practice of first-line ribociclib + AI versus palbociclib + AI in metastatic HR + , HER- breast cancer in Southeast Asia, thereby providing valuable insights into the context of a non-Western population. Importantly, the absence of randomized phase 3 trials comparing the efficacy between palbociclib + AI and ribociclib + AI emphasizes the significance of this study in addressing this literature gap. Despite its strengths, this study contained limitations. Firstly, being retrospective in nature, inherent bias may exist in patient selection at the outset. However, we mitigated this bias by using a PSM. Secondly, our study lacked data on patient-reported outcomes, QoL, and economic outcomes. Thirdly, information on subsequent treatment regimens was incomplete. In addition, this study involves a relatively small number of patients and also with imbalance of numbers of patients in palbociclib arm as only ribociclib was reimbursed in Thailand. And lastly, the median follow-up time of the cohort is relatively short compared to others as the drugs have just received approval in Thailand in 2017. Thus, longer follow-up and update of the current result would be vital to confirm the real effectiveness of both agents.
In summary, our RWE from Thai population indicated no differences in overall outcomes, TTC, and response rates between ribociclib and palbociclib as a first-line therapy despite the inconsistent OS gain of first-line ribociclib and palbociclib in RCT.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABC:
-
Advanced breast cancer
- CDK:
-
Cyclin-dependent kinase
- ECOG:
-
Eastern Cooperative Oncology Group
- ER:
-
Estrogen receptor
- ET:
-
Endocrine therapy
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- LA/MBC:
-
Locally advanced/metastatic breast cancer
- NSAIs:
-
Non-steroidal aromatase inhibitors
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PR:
-
Progesterone receptor
- PS:
-
Performance status
- PSM:
-
Propensity score matching
- QoL:
-
Quality of life
- Rb:
-
Retinoblastoma gene
- RCT:
-
Randomized controlled trial
- RWE:
-
Real-world evidence
- SAIs:
-
Steroidal aromatase inhibitors
- TTC:
-
Time to chemotherapy
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Burstein HJ, Harris JR, Morrow M. Malignant tumors of the breast. In: DeVita VT, Jr., Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology 9th ed. Philadelphia: Wolters Kluwer Health | Lippincott Williams & Wilkins; 2011. p. 1401–46.
NCCN. Clinical practice guidelines in oncology: breast cancer. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast_block.pdf.
Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group J Clin Oncol. 2000;18(22):3758–67.
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101–9.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–46.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–50.
Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851–9.
Slamon DJ, Diéras V, Rugo HS, Harbeck N, Im SA, Gelmon KA, et al. Overall survival with palbociclib plus letrozole in advanced breast cancer. J Clin Oncol. 2024;42(9):994–1000.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48.
Goetz MP, Toi M, Huober J, Sohn J, Trédan O, Park IH, et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: final overall survival results of MONARCH 3. Ann Oncol. 2024;35:718–27.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–84.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–24.
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–36.
Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15.
Cejuela M, Gil-Torralvo A, Castilla M, Domínguez-Cejudo M, Falcón A, Benavent M, et al. Abemaciclib, palbociclib, and ribociclib in real-world data: a direct comparison of first-line treatment for endocrine-receptor-positive metastatic breast cancer. Int J Mol Sci. 2023;24(10):8488.
Kahraman S, Erul E, Seyyar M, Gumusay O, Bayram E, Demirel BC, et al. Treatment efficacy of ribociclib or palbociclib plus letrozole in hormone receptor-positive/HER2-negative metastatic breast cancer. Future Oncol. 2023;19(10):727–36.
Lu YS, Mahidin EIBM, Azim H, Eralp Y, Yap YS, Im SA, et al. Final results of RIGHT choice: ribociclib plus endocrine therapy versus combination chemotherapy in premenopausal women with clinically aggressive hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. J Clin Oncol. 2024;42(23):2812–21.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Dieci MV, Piacentini F, Dominici M, Omarini C, Goubar A, Ficarra G, et al. Quantitative expression of estrogen receptor on relapse biopsy for ER-positive breast cancer: prognostic impact. Anticancer Res. 2014;34(7):3657–62.
Makhlouf S, Quinn C, Toss M, Alsaleem M, Atallah NM, Ibrahim A, et al. Quantitative expression of oestrogen receptor in breast cancer: clinical and molecular significance. Eur J Cancer. 2024;197: 113473.
Abbasvandi F, Bayat M, Akbari A, Shojaeian F, Zandi A, Rahmani J, et al. Tumor characteristics and survival rate of HER2-low breast cancer patients: a retrospective cohort study. Sci Rep. 2023;13(1):16719.
Masuda N, Nishimura R, Takahashi M, Inoue K, Ohno S, Iwata H, et al. Palbociclib in combination with letrozole as first-line treatment for advanced breast cancer: a Japanese phase II study. Cancer Sci. 2018;109(3):803–13.
Harbeck N, Bartlett M, Spurden D, Hooper B, Zhan L, Rosta E, et al. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol. 2021;17(16):2107–22.
Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C, et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer. 2022;8(1):114.
Fernández-Cuerva C, Chinchilla-Alarcón T, Alcaraz-Sánchez JJ. Real-world effectiveness of ribociclib in metastatic breast cancer patients: Does dose affect survival? J Oncol Pharm Pract. 2023;29(7):1619–27.
Jhaveri K, O’Shaughnessy J, Fasching PA, Tolaney SM, Yardley DA, Sharma VK, et al. Matching-adjusted indirect comparison of PFS and OS comparing ribociclib plus letrozole versus palbociclib plus letrozole as first-line treatment of HR+/HER2- advanced breast cancer. Ther Adv Med Oncol. 2023;15:17588359231216096.
Staropoli N, Geuna E, Rinaldi G, Bisagni G, Scotti V, Faggioni G, et al. Real-world clinical outcomes of ribociclib in combination with a non-steroidal aromatase inhibitor and a luteinizing hormone-releasing hormone agonist in premenopausal HR+/HER2- advanced breast cancer patients: an Italian managed access program. Curr Oncol. 2022;29(9):6635–41.
Petrelli F, Ghidini A, Pedersini R, Cabiddu M, Borgonovo K, Parati MC, et al. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174(3):597–604.
Brufsky A, Mitra D, Davis KL, Nagar SP, McRoy L, Cotter MJ, et al. Treatment patterns and outcomes associated with palbociclib plus letrozole for postmenopausal women with HR(+)/HER2(-) advanced breast cancer enrolled in an expanded access program. Clin Breast Cancer. 2019;19(5):317-25.e4.
Seki H, Sakurai T, Maeda Y, Oki N, Aoyama M, Yamaguchi R, et al. Efficacy and safety of palbociclib and fulvestrant in Japanese patients with ER+/HER2- advanced/metastatic breast cancer. In Vivo. 2019;33(6):2037–44.
Taylor-Stokes G, Mitra D, Waller J, Gibson K, Milligan G, Iyer S. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: Results from the IRIS study. Breast. 2019;43:22–7.
Groenland SL, Martínez-Chávez A, van Dongen MGJ, Beijnen JH, Schinkel AH, Huitema ADR, et al. Clinical pharmacokinetics and pharmacodynamics of the cyclin-dependent kinase 4 and 6 inhibitors palbociclib, ribociclib, and abemaciclib. Clin Pharmacokinet. 2020;59(12):1501–20.
Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in Breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs. 2021;81(3):317–31.
Low JL, Lim E, Bharwani L, Wong A, Wong K, Ow S, et al. Real-world outcomes from use of CDK4/6 inhibitors in the management of advanced/metastatic breast cancer in Asia. Ther Adv Med Oncol. 2022;14:17588359221139678.
Wong V, de Boer R, Baron-Hay S, Blum R, Boyle F, Chua S, et al. Real-world outcomes of ribociclib and aromatase inhibitor use in first line hormone receptor positive, HER2-negative metastatic breast cancer. Clin Breast Cancer. 2022;22(8):792–800
Acknowledgements
We gratefully acknowledge the TSCO Breast Oncology Group for their pivotal role in initiating the idea for this study and Miss Pawina Wamalun from the Department of Medicine Siriraj Hospital for her assistance in data gathering.
Funding
Open access funding provided by Mahidol University
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Thanate Dajsakdipon(T.D.), Thiti Susiriwatananont(T.S.) and Concord Wongkraisri(C.W.). Data collection was performed by Thanate Dajsakdipon(T.D), Thiti Susiriwatananont(T.S.), Concord Wongkraisri(C.W.), Suthinee Ithimakin(S.I.), Napa Parinyanitikul(N.P.), Archara Supavavej(A.S.), Arunee Dechaphunkul(A.D.), Patrapim Sunpaweravong(P.S.), Sunee Neesanun(S.N.), Charuwan Akewanlop(C.A.) and Thitiya Dejthevaporn(T.D.) Data analysis was performed by Thanate Dajsakdipon(T.D.) and Thitiya Dejthevaporn(T.D.). The first draft of the manuscript was written by Thanate Dajsakdipon(T.D.). All authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by
1. Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, Reference number: COA.MURA 2023/216
2. Institutional Review Board, Faculty of medicine, Chulalongkorn university, Bangkok, Thailand, Reference number: COA.No. 1193/2023
3. Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, Reference number: COA no. SI 142/2023
4. Human Research Ethics Committee, Chulabhorn Royal Academy, Bangkok, Thailand, Reference number: EC 061/2566(2023)
5. Institutional Review Board, Prince of Songkla University, Songkhla, Thailand, Reference number: REC 66–218-14–1
6. Institutional Review Board, Sawanpracharak Hospital, Nakhonsawan, Thailand, Reference number: COA. 30/2566(2023)
Statement of human and animal rights
All procedures in this study were conducted in accordance with international guidelines for human research protection, such as the Declaration of Helsinki, the Belmont Report, the CIOMS Guidelines, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Statement of informed consent
This is a retrospective study, and informed consent is not applicable. Consent to participate was waived by the following ethics committees and institutional review boards:
• Human Research Ethics Committee, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
• Institutional Review Board (IRB), Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
• IRB, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
• Human Research Ethics Committee, Chulabhorn Royal Academy, Bangkok, Thailand
• IRB, Prince of Songkla University, Songkhla, Thailand
• IRB, Sawanpracharak Hospital, Nakhonsawan, Thailand
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dajsakdipon, T., Susiriwatananont, T., Wongkraisri, C. et al. Comparative effectiveness analysis of survival with first-line palbociclib or ribociclib plus AI in HR + /HER2- advanced breast cancer (CEPRA study): preliminary analysis of real-world data from Thailand. BMC Cancer 24, 1018 (2024). https://doi.org/10.1186/s12885-024-12765-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12765-x