Abstract
Background
Several trials have demonstrated the benefit of anti-CDK4/6 inhibitors plus endocrine therapy in estrogen receptor-positive (ER+) advanced breast cancer (BC), in first or subsequent lines of therapy. However, due to the lack of direct/indirect comparisons, there are no data demonstrating the superiority of one drug over the other. We compared the effectiveness of palbociclib, ribociclib, and abemaciclib in advanced ER + BC via an indirect adjusted analysis.
Methods
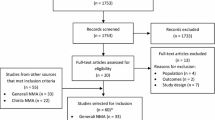
We performed electronic searches in the PubMed, EMBASE, and Cochrane databases for prospective phase 3 randomized trials evaluating anti-CDK4/6 inhibitors plus endocrine agents. We compared the results with an adjusted indirect analysis of randomized-controlled trials. Outcomes of interest were progression-free survival (PFS), overall response rate (ORR) and G3–4 toxicities occurring in ≥ 5% of patients.
Results
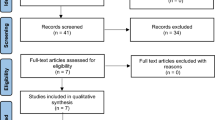
Six trials and six treatment arms including a total of 3743 participants, were included. For PFS and ORR analysis, the three agents were similar in both first- and second-line studies. All G3–4 toxicities were similar, with reduced risk of diarrhea for palbociclib versus abemaciclib (relative risk [RR] 0.13, 95% CI 0.02–0.92; P = 0.04) and of QTc prolongation for palbociclib versus ribociclib (RR 0.02, 95% CI 0–0.83; P = 0.03). Despite different inclusion criteria and length of follow-up, similar features were noticed among second-line studies with the exception of increased risk of anemia G3–4 and diarrhea G3–4 for abemaciclib.
Conclusions
Based on PFS and ORR results of this indirect meta-analysis, palbociclib, ribociclib, and abemaciclib are equally effective in either first- or second-line therapy for advanced ER + BC. They, however, ported different toxicity profiles.





Similar content being viewed by others
References
Slamon DJ, Neven P, Chia S et al (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36(24):2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
Sledge GW, Toi M, Neven P et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884. https://doi.org/10.1200/JCO.2017.73.7585
Cristofanilli M, Turner NC, Bondarenko I et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol 17(4):425–439. https://doi.org/10.1016/S1470-2045(15)00613-0
Goetz MP, Toi M, Campone M et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Finn RS, Martin M, Rugo HS et al (2016) Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/NEJMoa1607303
Hortobagyi GN, Stemmer SM, Burris HA et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748. https://doi.org/10.1056/NEJMoa1609709
Turner NC, Slamon DJ, Ro J et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med.NEJMoa1810527. https://doi.org/10.1056/NEJMoa1810527
Chen P, Lee NV, Hu W et al (2016) Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther 15(10):2273–2281. https://doi.org/10.1158/1535-7163.MCT-16-0300
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12. https://doi.org/10.1016/0197-2456(95)00134-4
Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50(6):683–691. https://doi.org/10.1016/S0895-4356(97)00049-8
Durairaj C, Ruiz-Garcia A, Gauthier ER et al (2018) Palbociclib has no clinically relevant effect on the QTc interval in patients with advanced breast cancer. Anticancer Drugs 29(3):271–280. https://doi.org/10.1097/CAD.0000000000000589
Rugo HS, Diéras V, Gelmon KA et al (2018) Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann Oncol 29(4):888–894. https://doi.org/10.1093/annonc/mdy012
Verma S, O’Shaughnessy J, Burris HA et al (2018) Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat 170(3):535–545. https://doi.org/10.1007/s10549-018-4769-z
Kiefer C, Sturtz S, Bender R (2015) Indirect comparisons and network meta-analyses: estimation of effects in the absence of head-to-head trials—part 22 of a series on evaluation of scientific publications. Dtsch Ärzteblatt Int 112(47):803–808. https://doi.org/10.3238/arztebl.2015.0803
Kish JK, Ward MA, Garofalo D et al (2018) Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res 20(1):1–8. https://doi.org/10.1186/s13058-018-0958-2
Stearns V, Brufsky AM, Verma S et al (2018) Expanded-access study of palbociclib in combination with letrozole for treatment of postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2018.07.007
Rugo HS, Turner NC, Finn RS et al (2018) Palbociclib plus endocrine therapy in older women with HR+/HER2: advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer 101:123–133. https://doi.org/10.1016/j.ejca.2018.05.017
Knudsen ES, Hutcheson J, Vail P, Witkiewicz AK (2017) Biological specificity of CDK4/6 inhibitors:dose response relationship, in vivo signaling, and composite response signature. Oncotarget 8(27):43678–43691. https://doi.org/10.18632/oncotarget.18435
Sonke GS, Hart LL, Campone M et al (2018) Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 167(3):659–669. https://doi.org/10.1007/s10549-017-4523-y
Zheng J, Amantea M, Wang D (2015) Effect of palbociclib concentration on heart rate-corrected QT interval in patients with cancer. Can Res 75:9 SUPPL. 1
Ribnikar D, Volovat SR, Cardoso F (2018) Targeting CDK4/6 pathways and beyond in breast cancer. Breast 8(43):8–17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable (no informed consent required).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrelli, F., Ghidini, A., Pedersini, R. et al. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat 174, 597–604 (2019). https://doi.org/10.1007/s10549-019-05133-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05133-y