Abstract
Objective
To investigate the impact of response to induction chemotherapy (IC) on survival outcomes in patients with locally advanced nasopharyngeal carcinoma (LANPC) and evaluate the efficacy of adding nimotuzumab to concurrent chemoradiotherapy (CCRT) based on different responses to IC.
Methods
We retrospectively included patients with stage III-IVA NPC who underwent IC with and without nimotuzumab during CCRT. Statistical analysis included the chi-square test, propensity score matching, Kaplan-Meier survival analysis, and Cox proportional hazards model.
Results
Among 383 identified patients, 216 (56.4%) received nimotuzumab during CCRT, while 167 (43.6%) did not. Following IC, 269 (70.2%) patients showed a complete response (CR) or partial response (PR), and 114 (29.8%) had stable disease (SD) or progressive disease (PD). The response to IC independently influenced disease-free survival (DFS) and overall survival (OS). Patients achieving CR/PR demonstrated significantly higher 3-year DFS (80.3% vs. 70.6%, P = 0.031) and OS (90.9% vs. 83.2%, P = 0.038) than those with SD/PD. The addition of nimotuzumab during CCRT significantly improved DFS (P = 0.006) and OS (P = 0.037) for CR/PR patients but not for those with SD/PD.
Conclusions
This study emphasizes the importance of IC response in LANPC and highlights the potential benefits of nimotuzumab during CCRT for improving survival outcomes in CR/PR patients. Tailored treatment approaches for SD/PD patients warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) originates from the epithelial cells lining the nasopharynx and is highly prevalence in specific regions, particularly Southeast Asia and Southern China [1, 2]. This malignancy is closely associated with Epstein-Barr virus (EBV) infection and often metastasizes to regional lymph nodes [3]. At the time of diagnosis, the majority of patients (approximately 70–80%) present with locally advanced NPC (LANPC) due to its subtle onset [3]. The standard treatment approach for LANPC involves induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CCRT) [4]. IC is utilized to reduce tumor burden, minimize distant metastasis risk, and enhance treatment outcomes [5]. However, despite treatment advancements, LANPC prognosis remains suboptimal, with a significant portion of patients experiencing disease recurrence [3]. Therefore, there is a pressing need for innovative therapeutic approaches to improve treatment efficacy.
Nimotuzumab (NTZ), a humanized monoclonal antibody targeting the epidermal growth factor receptor (EGFR), has shown promise in treating various cancers, including LANPC [6,7,8,9]. Previous studies have indicated that combining NTZ with standard chemotherapy or radiotherapy could enhance treatment efficacy and improve survival rates in LANPC patients [10,11,12,13]. Our recent study similarly revealed that adding NTZ to CCRT improved survival outcomes compared to CCRT alone in LANPC patients who underwent IC [14]. Additionally, several studies have highlighted a correlation between response to IC and patient survival. Patients achieving complete response (CR) or partial response (PR) to IC exhibited significantly better survival outcomes than those with stable disease (SD) or progressive disease (PD) [15, 16]. Nonetheless, whether the efficacy of NTZ varies among patients with different response to IC remains unclear. Thus, this study aimed to explore the influence of IC response on survival outcomes in LANPC patients and further evaluate the efficacy of NTZ in patients with different IC response categories.
Materials and methods
Patients
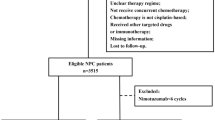
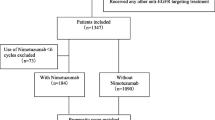
Patients diagnosed with NPC between December 2012 and October 2021 were retrospectively included in this study. Inclusion criteria were as follows: (1) pathologically confirmed NPC with aged ≥ 18 years; (2) stage III-IVA disease according to the 8th edition of the American Joint Committee on Cancer staging system; (3) Eastern Cooperative Oncology Group performance status of 0 or 1; (4) receipt of two or three cycles of IC followed by CCRT with or without concurrent NTZ; (5) EGFR-positive disease, (6) available imaging evaluation after IC; (7) data on smoking and alcohol history available; (8) adequate hematologic, liver, and renal function. Patients with a history of prior malignancy or concurrent malignant diseases were excluded. Additionally, patients who did not complete radiotherapy were also excluded. This study received approval from the Ethics Committee of the First Affiliated Hospital of Xiamen University (approval number: 3502Z20244ZD1001), and informed consent was obtained from all patients.
Measures
The following patient characteristics were included in the analysis: gender, age, smoking history, alcohol history, histology, tumor (T) stage, nodal (N) stage, clinical stage, and use of NTZ. Former and current smokers were defined as individuals who had smoked within the past year or had quit smoking more than one year ago.
Induction chemotherapy
IC regimens consisted of two or three cycles of TPF (docetaxel 75 mg/m2 or paclitaxel 135 mg/m2 or nab-paclitaxel 260 mg/m2 on day 1, cisplatin 25 mg/m2 on days 1–3, and 5-FU 600–750 mg/m2 per day as a continuous 120 h infusion or S1 capsules 40 mg/m2 bid on day 1–14), TP (docetaxel 75 mg/m2 or paclitaxel 135 mg/m2 or nab-paclitaxel 260 mg/m2 on day 1, cisplatin 25 mg/m2 on days 1–3), or GP regimens (gemcitabine 1000 mg/m2 on days 1 and 8, cisplatin 25 mg/m2 on days 1–3). The choice of IC regimens was primarily based on the preferences of the treating physicians.
Assessment of induction chemotherapy response
Response after IC was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. All patients underwent nasopharyngeal and neck magnetic resonance imaging (MRI) evaluation one week after completing the last cycle of IC. Changes in imaging of primary nasopharyngeal tumors and metastatic cervical lymph nodes before and after IC were evaluated for CR, PR, SD, and PD.
Concurrent chemoradiotherapy and nimotuzumab treatment
All patients underwent intensity-modulated radiotherapy (IMRT). Primary nasopharyngeal tumor, cervical metastatic lymph nodes, high-risk clinical target volume, and low-risk clinical target volume received doses of 70 Gy (Gy), 66–70 Gy, 62 Gy, and 56 Gy in 33 fractions, respectively. Each patient received two cycles of cisplatin-based (80 mg/m2 every 3 weeks) or lobaplatin-based (30 mg/m2 every 3 weeks) concurrent chemotherapy. NTZ (200 mg, weekly for 7 courses) was administered during CCRT. The decision-making of the concurrent chemotherapy regimens was mainly according to physician-specific preference. NTZ has been approved for use in patients with LANPC in China. In clinical practice, NTZ was routinely recommended during CCRT for these patients, although its administration depended largely on patient preference.
Follow-up
Follow-up visits occurred every three months during the first two years, every 6 months from the 3rd to 5th year post-treatment, and annually thereafter. Physical examination, nasopharynx and neck lymph node examinations, and auxiliary examinations including nasopharynx and neck MRI, endoscopy, chest CT, abdominal ultrasound, and bone emission computerized tomography were performed. PET-CT scans were conducted as necessary. Biopsies or needle biopsies were performed if disease recurrence was suspected. The primary endpoints of this study were disease-free survival (DFS) and overall survival (OS). DFS was calculated from the time of NPC diagnosis to disease recurrence or death from any cause. OS was defined as the time of NPC diagnosis until death from any cause.
Statistical analysis
The chi-square test or chi-square test for trend (Cochran-Armitage test) was used to analyze the correlation between patient characteristics and IC response. Propensity score matching (PSM) was employed to reduce bias. Kaplan-Meier survival analysis with a log-rank test was used to depict and compare survival curves. Variables with P values < 0.10 in the univariate Cox regression model were included in the multivariate Cox proportional analysis to identify prognostic factors related to survival outcomes. Sensitivity analyses were performed to evaluate the impact of NTZ on survival outcomes after stratifying by IC response. All statistical analyses were conducted using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA), with P values < 0.05 considered statistically significant.
Results
Patients characteristics
A total of 383 patients meeting the inclusion criteria were included (Table 1). Among them, 216 (56.4%) received NTZ, while 167 (43.6%) did not. The median age of patients was 48.0 years (range, 18–70 years). Of these, 278 (72.6%) were male, 338 (88.3%) had WHO III type classification, and 257 (67.1%) were at stage T3-4 disease. There were 83 (21.7%), 172 (44.9%), and 128 (33.4%) patients at N1, N2, and N3 stages, respectively.
Regarding primary nasopharyngeal tumors, the responses to IC were as follows: 79 (20.6%) for CR, 243 (63.4%) for PR, 58 (15.1%) for SD, and 3 (0.8%) for PD. For cervical lymph nodes, there were 52 (13.6%), 234 (61.1%), 93 (24.3%), and 4 (1.0%) patients with CR, PR, SD, and PD to IC, respectively. Overall, 269 (70.2%) patients achieved CR/PR, while 114 (29.8%) had SD/PD. There were no significant differences observed in terms of age (P = 0.478), gender (P = 0.188), smoking history (P = 0.430), alcohol consumption history (P = 0.678), histology (P = 0.891), T stage (P = 0.522), N stage (P = 0.059), and clinical stage (P = 0.207) between patients with CR/PR and SD/PD after IC. Notably, patients with SD/PD after IC were less likely to receive NTZ compared to those with CR/PR (46.5% vs. 60.6%, P = 0.011). In addition, patients who received gemcitabine-based IC showed a better response to IC compared to those treated with taxane-based IC (P < 0.001).
Survival analysis
The median follow-up time was 41.2 months (range, 5-127 months), with 3-year DFS and OS rates of 77.3% and 88.5%, respectively. The 3-year DFS for patients with CR/PR and SD/PR was 80.3% and 70.6%, respectively (P = 0.031) (Fig. 1A), while the 3-year OS rates were 90.9% and 83.2%, respectively (P = 0.038) (Fig. 1B). Additionally, the 3-year DFS for patients with and without NTZ were 81.5% and 71.5%, respectively (P = 0.016) (Fig. 2A), and the 3-year OS rates were 91.4% and 85.6%, respectively (P = 0.070) (Fig. 2B).
Prognostic analysis
Cox regression analysis was performed to investigate prognostic factors influencing DFS and OS (Table 2). Factors with P-values less than 0.10 in the univariate Cox regression analysis for DFS included age, T stage, N stage, response to IC, and receipt of NTZ. These factors were incorporated into the multivariate Cox regression model, revealing the T stage, N stage, and response to IC as independent prognostic factors affecting survival. Patients with SD/PD after IC had significantly worse DFS compared to those with CR/PR (hazard ratio [HR] 1.610, 95% confidence interval [CI] 1.041–2.490, P = 0.032).
For OS, factors with P-values less than 0.10 in the univariate Cox regression analysis included age, T stage, response to IC, and receipt of NTZ. These factors were included in the multivariate Cox regression model, indicating response to IC as an independent prognostic factor affecting OS. Patients with SD/PD after IC had significantly worse OS compared to those with CR/PR (HR 1.741, 95% CI 1.039–2.917, P = 0.035).
Sensitivity analyses
Sensitivity analyses were conducted to evaluate the impact of NTZ on survival outcomes after stratifying by treatment response to IC. Among patients achieving CR/PR, NTZ treatment was identified as an independent prognostic factor influencing both DFS and OS (Table 3). Patients receiving NTZ exhibited better DFS (HR 0.459, 95% CI 0.263–0.799, P = 0.006) and OS (HR 0.429, 95% CI 0.194–0.949, P = 0.037). Furthermore, 3-year DFS and OS rates were significantly higher for patients receiving NTZ compared to those not receiving it (DFS: 85.4% vs. 72.1%, P = 0.005; OS: 94.4% vs. 87.1%, P = 0.020) (Fig. 3A and B). T stage, N stage, and age were also identified as independent prognostic factors affecting survival. A 1:1 PSM was employed to reduce potential bias, including variables such as age, gender, smoking history, alcohol history, histology, T stage, N stage, and IC regimens. A total of 80 pairs of patients were fully matched (Table 4). Results further indicated that patients receiving NTZ treatment exhibited better DFS (P = 0.036) (Fig. 3C) and OS (P = 0.015) (Fig. 3D).
Comparison of disease-free survival (A, before propensity score matching; C, after propensity score matching) and overall survival (C, before propensity score matching; D, after propensity score matching) between those with and without nimotuzumab during concurrent chemoradiotherapy in the CR/PR groups before and after propensity score matching
For patients with SD/PD after IC, no significant correlation was found between those with and without NTZ regarding DFS (3-year DFS: 69.7% vs. 70.6%) (HR 1.015, 95% CI 0.520–1.983, P = 0.964) (Fig. 4A) and OS (3-year OS: 83.1% vs. 83.1%) (HR 1.098, 95% CI 0.491–2.457, P = 0.820) (Fig. 4B).
Discussion
In this study, we investigated the impact of response to IC on survival outcomes in patients with LANPC. Furthermore, we examined whether the addition of NTZ during CCRT could enhance survival benefits for patients exhibiting different responses to IC. Our findings indicated that patients achieving CR/PR after IC experienced significantly better DFS and OS compared to those with SD/PD after IC. Moreover, our findings showed that the addition of NTZ during CCRT conferred survival benefits exclusively to patients who achieved CR/PR, but not to those with SD/PD.
IC represents a standard treatment for LANPC [4]. Although NPC is typically responsive to chemotherapy, not all patients exhibit sensitivity to these treatments. In our study, 70.2% of patients achieved CR/PR after IC, while 29.8% showed SD/PD. Prospective study using the TPF regimen reported a CR/PR rate of 90.9%, with 9.1% exhibiting SD [15]. Another prospective study using the GP regimen found a CR/PR rate of 94.5% after IC, with SD and PD rates of 4.2% and 1.3%, respectively [16]. However, in real-world studies like Liu et al., observed a CR/PR rate of 76.9% after IC, with a SD/PD rate of 23.1% [24]. Additionally, Jiang et al. reported CR/PR and SD/PD rates of 68.3% and 31.7% following IC, respectively [19], which was consistent with our findings. Furthermore, our study indicated that IC regimens predominantly including gemcitabine resulted in significantly higher tumor response rates compared to those incorporating taxanes. Therefore, variations in patient demographics and treatment protocols may contribute to differing outcomes following IC.
The secondary analyses from the aforementioned two prospective studies consistently demonstrated a strong correlation between IC response and patient survival in LANPC. Patients achieving CR/PR after IC exhibited significantly better outcomes compared to those with SD/PD after IC [15, 16]. This correlation was consistently observed across retrospective studies conducted in both endemic and non-endemic areas [17,18,19,20]. Our study underscores the critical role of IC response in determining survival outcomes of LANPC patients. Specifically, patients achieving CR/PR showed significantly improved DFS and OS, aligning with previous studies that highlight the crucial role of favorable response to IC in improving LANPC prognosis. The primary objective of IC is to reduce tumor size, minimize distant metastasis risk, and potentially improve locoregional control efficacy. Patients demonstrating favorable IC responses generally experience better long-term outcomes. Therefore, IC response could serve as a potential surrogate endpoint for LANPC. Conversely, patients with SD/PD following IC require aggressive treatment strategies, highlighting the importance of closely monitoring treatment response during IC to facilitate timely adjustments in treatment plans.
The mechanism of action of NTZ involves EGFR pathway blockade, which is pivotal for cancer cell growth and survival [6, 21]. By inhibiting EGFR activation, NTZ enhances the cytotoxic effects of chemotherapy and radiotherapy [22, 23]. This dual-targeting approach likely contributes to the observed survival benefits in patients receiving NTZ. Notably, NTZ has shown promising efficacy across various cancers, including LANPC, head and neck cancer, cervical, and esophageal cancers, with a favorable safety profile [6,7,8,9]. Our study showed that adding NTZ to CCRT improves survival outcomes in chemotherapy-sensitive patients who respond favorably to IC, suggesting its potential as a valuable adjunctive therapy. This finding suggests that NTZ may enhance treatment efficacy by inhibiting EGFR. Importantly, tumor response to IC may inform survival outcomes and guide personalized treatment strategies, particularly for patients achieving CR/PR.
For patients showing suboptimal response to IC, several studies have explored various strategies, including increasing cisplatin dosage during CCRT, employing dual-drug combination chemotherapy during CCRT, and escalating cycles of IC. However, none have shown a clear survival advantage [24,25,26]. Therefore, further investigation into methods to enhance efficacy is warranted for these patients. In our study, patients with SD/PD after IC were less likely to receive NTZ compared to those with CR/PR (46.5% vs. 60.6%, P = 0.011). The underlying reasons for this phenomenon remain unclear. Factors such as physician preferences, patient preferences, or treatment accessibility may influence treatment decisions. Similarly, patients experiencing SD/PD after IC often exhibit chemotherapy insensitivity, representing a subgroup with the highest rate of disease progression. Identifying more suitable treatment options for these patients is imperative. Thus, incorporating an EGFR inhibitor during CCRT could present a viable therapeutic avenue. A study by Niu et al. found that adding NTZ during CCRT in IC-resistant LANPC patients resulted in 3-year DFS and OS of 79.3% and 94.0%, respectively, with predominantly mild hematologic acute toxicities and rare severe toxicities [27]. However, the absence of a control group in this study precludes definitive determination of the exact efficacy of NTZ in patients with suboptimal response to IC. Contrarily, our study found no survival improvement with NTZ addition during CCRT in patients assessed as SD/PD after IC. Notably, patients exhibiting unsatisfactory tumor responses after IC demonstrated poorer prognoses and inferior response to NTZ compared to those with satisfactory tumor responses. Hence, clinical attention should be focused on patients experiencing SD/PD after IC.
NPC patients often harbor several drug-resistant genes [28, 29]. While several preclinical studies have attempted to reverse chemoresistance [30, 31], no formal drugs currently enhance cisplatin sensitivity. Immune checkpoint inhibitors may offer additional survival benefits to IC-insensitive patients. A recent study found that incorporating sintilimab (a PD-1 inhibitor) during IC, CCRT, and adjuvant therapy significantly improved event-free survival compared to those treated with IC and CCRT alone in LANPC [32]. However, studies involving avelumab (a PD-L1 inhibitor) or pembrolizumab (a PD-1 inhibitor) alongside standard-of-care CCRT did not enhance survival outcomes relative to CCRT alone in head and neck cancer patients [33, 34]. Therefore, further exploration is warranted to optimize the integration of immune checkpoint inhibitors into the treatment of LANPC at the optimal time. Several studies have found that adding immune checkpoint inhibitors during the IC phase could increase the CR rate to approximately 40–50% [35,36,37,38], significantly higher than the rates achieved with TPF or GP regimens (typically around 10%) [15, 16]. However, identifying the optimal patients who may benefit from immune checkpoint inhibitors during IC necessitates further investigation. Radiomics has been employed in several studies to predict IC response [39,40,41], though additional research is required to validate these findings. Moreover, carbon-ion radiotherapy may represent a potential treatment avenue for IC-insensitive patients [42, 43].
It is crucial to acknowledge the limitations of our study. Firstly, its retrospective nature renders it susceptible to inherent biases. Confirming our findings and establishing the causal relationship between treatment response and survival outcomes necessitate randomized controlled trials. Secondly, the small sample size in our study may limit the generalizability of our findings. Future studies with larger cohorts are necessary to validate our findings. Thirdly, we did not include EBV-DNA levels for analysis in this study because EBV-DNA testing was not conducted at our institution before 2017. However, in our previous studies, we have found that EBV-DNA levels before treatmet, residual EBV-DNA levels after IC, and residual EBV-DNA levels after IMRT have a significant impact on the survival outcomes of NPC patient [44, 45].
Conclusions
In conclusion, our study highlights the significance of treatment response to IC in LANPC and underscores the potential benefits of NTZ during CCRT for improving survival outcomes in patients achieving CR/PR to IC. Tailoring individualized treatment strategies for patients with SD/PD to IC warrants further exploration. These findings emphasize the significance of closely monitoring treatment response and adjusting treatment strategies accordingly.
Data availability
The data supporting the findings of the article are available within the article.
References
Zhang Y, Rumgay H, Li M, Cao S, Chen W. Nasopharyngeal Cancer incidence and mortality in 185 countries in 2020 and the projected Burden in 2040: Population-based global epidemiological profiling. JMIR Public Health Surveill. 2023;9:e49968.
Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 201736(1):90.
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in Combination with Radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO Guideline. J Clin Oncol. 2021;39(7):840–59.
Ng WT, Corry J, Langendijk JA, Lee AWM, Mäkitie A, Mendenhall WM, et al. Current management of stage IV nasopharyngeal carcinoma without distant metastasis. Cancer Treat Rev. 2020;85:101995.
Liang R, Yang L, Zhu X. Nimotuzumab, an Anti-EGFR monoclonal antibody, in the Treatment of Nasopharyngeal Carcinoma. Cancer Control. 2021;28:1073274821989301.
Yuan Y, Chen J, Fang M, Guo Y, Sun X, Yu D, et al. Nimotuzumab combined with chemoradiotherapy for the treatment of cervical cancer: a meta-analysis of randomized controlled trials. Front Oncol. 2022;12:994726.
Patil VM, Noronha V, Joshi A, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. 2019;125(18):3184–97.
Qi S, Mao Y, Jiang M. A phase I study evaluating combined nimotuzumab and neoadjuvant chemoradiotherapy followed by surgery in locally advanced esophageal cancer. Cancer Chemother Pharmacol. 2019;84(5):1115–23.
Cai Z, Chen D, Qiu W, Liang C, Huang Y, Zhou J, et al. Concurrent chemoradiotherapy combined with nimotuzumab in stage III-IVa nasopharyngeal carcinoma: a retrospective analysis. J Cancer Res Clin Oncol. 2023;149(6):2327–44.
Lin M, You R, Liu YP, Zhang YN, Zhang HJ, Zou X, et al. Beneficial effects of anti-EGFR agents, Cetuximab or Nimotuzumab, in combination with concurrent chemoradiotherapy in advanced nasopharyngeal carcinoma. Oral Oncol. 2018;80:1–8.
You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent chemoradiotherapy with or without Anti-EGFR-Targeted Treatment for Stage II-IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow-up. Theranostics. 2017;7(8):2314–24.
You R, Sun R, Hua YJ, Li CF, Li JB, Zou X, et al. Cetuximab or Nimotuzumab plus intensity-modulated radiotherapy versus cisplatin plus intensity-modulated radiotherapy for stage II-IVb nasopharyngeal carcinoma. Int J Cancer. 2017;141(6):1265–76.
Wang RJ, Ke RQ, Yu YF, Lu GZ, Wu SG. Addition of nimotuzumab to concurrent chemoradiotherapy after induction chemotherapy improves outcomes of patients with locally advanced nasopharyngeal carcinoma. Front Pharmacol. 2024;15:1366853.
Peng H, Chen L, Li WF, Guo R, Mao YP, Zhang Y, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a randomized phase 3 clinical trial. Cancer. 2017;123(9):1643–52.
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Final overall survival analysis of Gemcitabine and Cisplatin Induction Chemotherapy in nasopharyngeal carcinoma: a Multicenter, Randomized Phase III Trial. J Clin Oncol. 2022;40(22):2420–5.
Peng H, Chen L, Zhang Y, Li WF, Mao YP, Liu X, et al. The Tumour response to Induction Chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated Radiation Therapy in Nasopharyngeal Carcinoma. Sci Rep. 2016;6:24835.
Xu Y, Wu Z, Ye W, Xiao Y, Zheng W, Chen Q, et al. Prognostic value of serum uric acid and tumor response to induction chemotherapy in locally advanced nasopharyngeal carcinoma. BMC Cancer. 2021;21(1):519.
Jiang YT, Chen KH, Liang ZG, Yang J, Wei SQ, Qu S, et al. A nomogram based on tumor response to induction chemotherapy may predict survival in locoregionally advanced nasopharyngeal carcinoma. Head Neck. 2022;44(6):1301–12.
Ou D, Blanchard P, El Khoury C, De Felice F, Even C, Levy A, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–21.
Chen X, Liang R, Zhu X. Anti-EGFR therapies in nasopharyngeal carcinoma. Biomed Pharmacother. 2020;131:110649.
Huang J, Yuan X, Pang Q, Zhang H, Yu J, Yang B, et al. Radiosensitivity enhancement by combined treatment of nimotuzumab and celecoxib on nasopharyngeal carcinoma cells. Drug Des Devel Ther. 2018;12:2223–31.
Qu L, Wang JH, Du JX, Kang P, Niu XQ, Yin LZ. Use of Nimotuzumab combined with cisplatin in treatment of nasopharyngeal carcinoma and its effect on expressions of VEGF and MMP-2. Clin Transl Oncol. 2021;23(7):1342–9.
Liu SL, Sun XS, Yan JJ, Chen QY, Lin HX, Wen YF, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother Oncol. 2019;137:83–94.
Zhang YN, Chen YP, Li JB, Lu TX, Han F, Chen CY. Concurrent chemotherapy using taxane plus cisplatin versus cisplatin alone in high-risk nasopharyngeal carcinoma patients with suboptimal response to induction chemotherapy. Ther Adv Med Oncol. 2023;15:17588359231177016.
Jiang YT, Chen KH, Liang ZG, Yang J, Qu S, Li L, et al. Individualized number of induction chemotherapy cycles for locoregionally advanced nasopharyngeal carcinoma patients based on early tumor response. Cancer Med. 2023;12(4):4010–22.
Niu X, Liu P, Zhou X, Ou D, Wang X, Hu C. Anti-epidermal growth factor receptor (EGFR) monoclonal antibody combined with chemoradiotherapy for induction chemotherapy resistant locally advanced nasopharyngeal carcinoma: a prospective phase II study. Transl Oncol. 2024;39:101797.
Hsu CH, Chen CL, Hong RL, Chen KL, Lin JF, Cheng AL. Prognostic value of multidrug resistance 1, glutathione-S-transferase-pi and p53 in advanced nasopharyngeal carcinoma treated with systemic chemotherapy. Oncology. 2002;62(4):305–12.
Larbcharoensub N, Leopairat J, Sirachainan E, Narkwong L, Bhongmakapat T, Rasmeepaisarn K, et al. Association between multidrug resistance-associated protein 1 and poor prognosis in patients with nasopharyngeal carcinoma treated with radiotherapy and concurrent chemotherapy. Hum Pathol. 2008;39(6):837–45.
Sun Y, Chen X, Zhou Y, Qiu S, Wu Y, Xie M, et al. Metformin reverses the drug resistance of cisplatin in irradiated CNE-1 human nasopharyngeal carcinoma cells through PECAM-1 mediated MRPs down-regulation. Int J Med Sci. 2020;17(16):2416–26.
Zhou D, Ye C, Pan Z, Deng Y. SATB1 Knockdown inhibits Proliferation and Invasion and decreases Chemoradiation Resistance in Nasopharyngeal Carcinoma cells by reversing EMT and suppressing MMP-9. Int J Med Sci. 2021;18(1):42–52.
Liu X, Zhang Y, Yang KY, Zhang N, Jin F, Zou GR, et al. Induction-concurrent chemoradiotherapy with or without sintilimab in patients with locoregionally advanced nasopharyngeal carcinoma in China (CONTINUUM): a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2024;403(10445):2720–31.
Machiels JP, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2024;25(5):572–87.
Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–62.
Yu YF, Lu GZ, Wang RJ, Song YK, Wu SG. Additional PD-1 inhibitor improves complete response to induction chemotherapy in locally advanced nasopharyngeal carcinoma. Front Immunol. 2024;15:1415246.
Jin YN, Qiang MY, Wang Y, Lin YJ, Jiang RW, Cao WW, et al. The efficacy and safety of adding PD-1 blockade to induction chemotherapy and concurrent chemoradiotherapy (IC-CCRT) for locoregionally advanced nasopharyngeal carcinoma: an observational, propensity score-matched analysis. Cancer Immunol Immunother. 2024;73(7):125.
Chen QY, Mai HQ, Tang LQ, Luo MJ, Zhao C, Mo HY, et al. Neoadjuvant chemotherapy plus tislelizumab followed by concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a single-arm, phase II trial. J Clin Oncol. 2022;40(16suppl):6068–6068.
Zhang Q, Lai M, Li F, Chen J, Chen G. 153P neoadjuvant therapy with tislelizumab plus chemotherapy followed by concurrent chemoradiotherapy in patients with stage IVa nasopharyngeal carcinoma: a single-arm, phase II trial. I mmuno-Oncol Technol. 2022;16:100265.
Liao H, Chen X, Lu S, Jin G, Pei W, Li Y, et al. MRI-Based back propagation neural network model as a powerful Tool for Predicting the response to induction chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma. J Magn Reson Imaging. 2022;56(2):547–59.
Yang Y, Wang M, Qiu K, Wang Y, Ma X. Computed tomography-based deep-learning prediction of induction chemotherapy treatment response in locally advanced nasopharyngeal carcinoma. Strahlenther Onkol. 2022;198(2):183–93.
Dong D, Zhang F, Zhong LZ, Fang MJ, Huang CL, Yao JJ, et al. Development and validation of a novel MR imaging predictor of response to induction chemotherapy in locoregionally advanced nasopharyngeal cancer: a randomized controlled trial substudy (NCT01245959). BMC Med. 2019;17(1):190.
Hu J, Huang Q, Gao J, Guan X, Hu W, Yang J, et al. Clinical outcomes of carbon-ion radiotherapy for patients with locoregionally recurrent nasopharyngeal carcinoma. Cancer. 2020;126(23):5173–83.
Li Y, Guan X, Xing X, Hu C. Survival outcomes and toxicity profiles among patients with nonmetastatic nasopharyngeal carcinoma treated with intensity-modulated radiotherapy (IMRT) versus IMRT + carbon-ion radiotherapy: a propensity score-matched analysis. Head Neck. 2024;46(7):1766–76.
Zhou P, Zhou J, Lian CL, Yu YF, Zhou R, Lin Q, et al. Residual plasma Epstein-Barr virus DNA after intensity-modulated radiation therapy is associated with poor outcomes in nasopharyngeal carcinoma. Future Oncol. 2023;19(33):2227–35.
Zheng H, Zhou P, Wang J, Yu YF, Zhou R, Lin Q, et al. Prognostic effect of residual plasma Epstein-Barr viral DNA after induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Cancer Med. 2023;12(14):14979–87.
Acknowledgements
Declared none.
Funding
This study was partly supported by the Xiamen Medical and Health Guidance Project (No. 3502Z20244ZD1001).
Author information
Authors and Affiliations
Contributions
Lin-Feng Guo, Ming-Yue Rao, and San-Gang Wu collected the data. Lin-Feng Guo, Yi-Feng Yu, Ming-Yue Rao, and San-Gang Wu analyzed and organized the data and wrote the manuscript. Qin Lin and San-Gang Wu assisted in revising the manuscript, approved to publish of the manuscript, and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University (approval number: 3502Z20244ZD1001), and we obtained informed consent from the patients.
Human and animal rights
This research was conducted on humans in accordance with the Helsinki Declaration of 1975, as revised in 2013 [http://ethics.iit.edu/ecodes/node/3931].
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, LF., Rao, MY., Yu, YF. et al. The addition of nimotuzumab during concurrent chemoradiotherapy improved survival outcomes in locally advanced nasopharyngeal carcinoma patients with optimal response to induction chemotherapy. BMC Cancer 24, 950 (2024). https://doi.org/10.1186/s12885-024-12731-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12731-7