Abstract
Purpose
We aimed to investigate the efficacy and side effects of concurrent chemoradiotherapy, with or without nimotuzumab, for the treatment of locally advanced nasopharyngeal carcinoma after neoadjuvant chemotherapy.
Methods
This study retrospectively enrolled 109 patients with NPC from our hospital from July 2019 to May 2021.All patients were treated with docetaxel, cisplatin, and fluorouracil(TPF) neoadjuvant chemotherapy for 2 cycles, and concurrent chemoradiotherapy was performed 2 weeks after chemotherapy. According to whether nimotuzumab was added in concurrent chemoradiotherapy, they were divided into the nimotuzumab group and the control group, with 52 cases in the nimotuzumab group and 57 cases in the control group.The efficacy and adverse reactions of the two groups were retrospectively analyzed.
Results
The objective remission and complete remission rates in the nimotuzumab and control groups were 100% vs 98.2% (p = 1.000), and 92.3% vs 78.9% (p = 0.049), respectively. The 3-year distant metastasis-free survival of the nimotuzumab and control groups was 91.6% and 77.3% (p = 0.047), respectively.The 3-year progression-free survival, locoregional relapse-free survival, and overall survival of the nimotuzumab and control groups were 87.6% vs 75.5% (p = 0.110), 90.5% vs 86.9% (p = 0.566), and 94.5% vs 87.1% (p = 0.295), respectively. In the nimotuzumab group, subgroup analysis showed that patients aged < 60 years (hazard ratio [HR] = 0.350, 95% confidence interval [CI]: 0.131–0.934, p = 0.036) and those with a neutrophil-to-lymphocyte ratio (neutrophil/lymphocyte ratio) ≤ 4 (HR = 0.365, 95% CI: 0.144–0.923, p = 0.033) achieved a better result. Additionally, multivariate analysis demonstrated that neutrophil/lymphocyte ratio was an independent risk factor for disease progression (HR = 7.485, p = 0.012) and distant metastasis (HR = 17.540, p = 0.009).No grade 4 adverse reactions were observed in either group. Grade 3 oral mucosal reactions, as well as pharyngeal and esophageal reactions were slightly higher in the nimotuzumab group than in the control group, but the difference was not statistically significant. No significant differences were observed in the incidence of adverse reactions such as leukopenia, HB reduction, thrombocytopenia between the two groups (P > 0.05).
Conclusion
The concurrent chemoradiotherapy plus nimotuzumab after neoadjuvant chemotherapy for locally advanced nasopharyngeal carcinoma achieved a higher complete remission rate and significantly improved distant metastasis-free survival compared with concurrent chemoradiotherapy alone. Additionally, an increasing trend was observed in progression-free survival, and the incidence of side effects was similar in both groups.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor in the head and neck region, particularly in China. Approximately 70–85% of patients with NPC are in the locally advanced stage at the time of initial diagnosis [1]. For patients with locally advanced NPC, concurrent chemoradiotherapy is the main treatment modality [2], and has been shown to improve the treatment efficacy sequentially after neoadjuvant chemotherapy in several studies [3,4,5]. Docetaxel, cisplatin, and fluorouracil (TPF) regimen is the first-line neoadjuvant chemotherapy for locally advanced NPC [6]. Neoadjuvant chemotherapy combined with concurrent chemoradiotherapy has become a type I recommended treatment for locally advanced high-risk NPC (NCCN recommendation) [7]. Although neoadjuvant chemotherapy improves overall efficacy, distant metastasis remains the main cause of treatment failure in locally advanced NPC [8].
High expression of the epidermal growth factor receptor (EGFR) is a poor prognostic factor for tumors [9], and has been observed in 80%-90% of patients with NPC [10]. Nimotuzumab is a non-intrinsically stimulating anti-EGFR monoclonal antibody that blocks the binding of EGFR to its ligand and exhibits anti-angiogenic, anti-tumor cell proliferative, and pro-apoptotic effects in EGFR-overexpressing tumors [11]. Wang et al. found that adding nimotuzumab to concurrent chemoradiotherapy for locally advanced NPC could improve the 5-year distant metastasis-free survival (DMFS) and overall survival (OS) [12]. Under the current neoadjuvant chemotherapy sequential concurrent chemoradiotherapy mode, there is a lack of relevant research on whether concurrent chemoradiotherapy combined with nimotuzumab provides further benefits.The purpose of this study was to investigate the efficacy and adverse reactions of concurrent chemoradiotherapy with or without nimotuzumab in locally advanced nasopharyngeal carcinoma after neoadjuvant chemotherapy, and to provide evidence-based medical evidence for the selection of treatment strategies for locally advanced nasopharyngeal carcinoma.
Data and methods
General information
This study included 115 patients with NPC admitted to our hospital from July 2019 to April 2021.The inclusion criteria were follows: (1) age 18–70 years; (2) pathological diagnosis of NPC (including non-keratinizing carcinoma [differentiated and undifferentiated], keratinizing squamous cell carcinoma, basal cell squamous cell carcinoma, and adenocarcinoma and excluding neuroendocrine carcinoma) and immunohistochemically suggested EGFR ( +); (3) NPC diagnosed as T1-2N2M0, T3N1-2M0, T1-3N3M0, and T4N0-3M0 based on imaging examination (the eighth version).The exclusion criteria were as follows: (1) patients with other tumors, such as double primary cancer; (2) The survival time of patients with other diseases ( such as coronary heart disease, cerebral infarction and other serious cardiovascular and cerebrovascular diseases) is shortened..
Treatment methods
All patients received paclitaxel liposome, cisplatin combined with fluorouracil ( TPF) regimen neoadjuvant chemotherapy and radical concurrent chemoradiotherapy. Immunohistochemical positive patients with locally advanced nasopharyngeal carcinoma after doctors and patients and their families to communicate the condition and treatment, according to the wishes of patients and their families whether to use nimotuzumab in concurrent chemoradiotherapy.According to whether nimotuzumab was added in concurrent chemoradiotherapy, they were divided into the nimotuzumab group and the control group, with 52 cases in the nimotuzumab group and 57 cases in the control group.This study was approved by the ethics committee of our hospital.Patients and their families agreed to this study.
Neoadjuvant chemotherapy: Patients received two cycles of TPF neoadjuvant chemotherapy before chemoradiotherapy with the following regimen: (paclitaxel liposome 135 mg/m2 d1, cisplatin 25 mg/m2 d1-3, fluorouracil 600 mg/m2 continuous intravenous infusion on d1-5, repeated every three weeks).
Concurrent chemoradiotherapy: Two weeks after neoadjuvant chemotherapy, patients in both groups were treated with the same intensity modulated radiation therapy (IMRT) using the same technique. The prescribed dose were as follows: 95% PGTVnx(planning gross target volume nasopharynx) 69.96–73.92 Gy/33f, 95% PGTVnd(planning gross target volume lymph node) 69.96 Gy/33f, 95% PTV1 (planning target volume 1)60.06 Gy/33f, and 95% PTV2(planning target volume 2) 50.4 Gy/28f.
Synchronous treatment: During radiotherapy, patients in the nimotuzumab group received concurrent chemotherapy using single-agent cisplatin (80 mg/m2 for three days, administered every 21 days [Q21D], for two 2 cycles) and concurrent targeted therapy using nimotuzumab (200 mg/m2 once a week for six times), while patients in the control group received concurrent chemotherapy using single-agent cisplatin (80 mg/ m2, for three days, Q21D for two cycles).
Efficacy and observation indicators
Short-term efficacy:The maximum diameter of the tumor was measured through imaging examination and evaluated according to the International Response Evaluation Criteria in Solid Tumor (RECIST) 1.1 standard, in which tumor diameters at baseline and after treatment were compared. The efficacy evaluation included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was defined as CR + PR.
Recent toxicity and side effects experienced by patients during concurrent chemoradiotherapy were recorded and evaluated based on the International Common Adverse Reaction Standard (3rd edition) for toxicity and side effects.
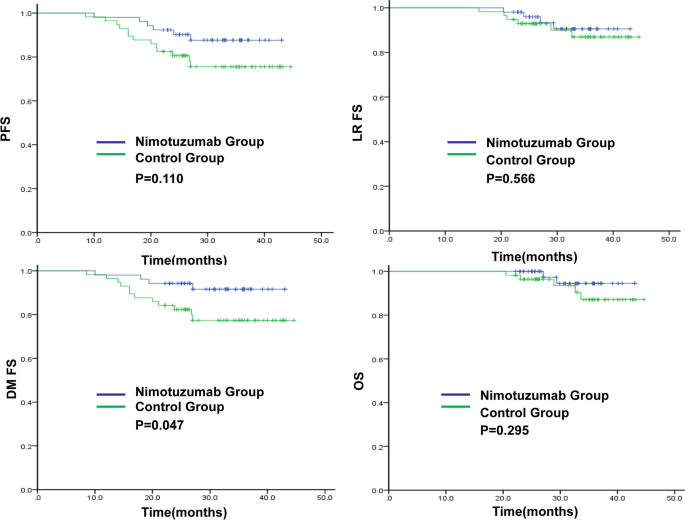
Long-term efficacy, including local recurrence, distant metastasis, and patient survival status, were determined at follow-up. The main endpoints was DMFS.The secondary endpoints were progression-free survival (PFS),OS and locoregional relapse-free survival (LRFS).
Follow-up observation
The patients were re-examined at specific intervals after the completion of treatment as follows:reviewed every three months within 2 years, at 6-month intervals for the next two to five years, and once every year after five years. The follow-up ended on February 28, 2023, with periods ranging from 20 to 45 months and a median of 32.1 months.
Statistical analysis
All data were analyzed using Statistical Package for the Social Sciences version 20.0. Enumeration data were expressed as % using χ2 test, continuity correction χ2 test, or Fisher exact probability method. PFS, LRFS, DMFS, and OS were evaluated with log-rank of univariate analysis and multivariate analysis Cox proportional risk model. The incidence of adverse reactions was analyzed using the chi-square test. P-values of < 0.05 was considered significant.
Results
Comparison of baseline data between the two groups
Differences in age, sex, pathological type, clinical stage, T stage, N stage, Epstein-Barr virus (EBV) DNA level, neutrophil-to-lymphocyte ratio (neutrophil/lymphocyte ratio), lactate dehydrogenase (LDH), HB, and EGFR expression were not statistically significant (all P > 0.05) (Table 1).
Comparison of short-term efficacy between the two groups
In the nimotuzumab group (52 cases), there were 48 and four cases of CR and PR, respectively, but no cases of SD and PD, with an objective remission rate of 100%. In the control group (57 patients), there were 45, 11, and one case(s) of CR, PR, and SD, respectively, but no cases of PD, with an objective remission rate of 98.2%. Compared with the complete remission rate of the two groups, the nimotuzumab group had a higher complete remission rate, the difference was statistically significant (χ2=3.876, P=0.049). One patient with SD in the control group had tumor-reduced (Table 2).
Comparison of long-term efficacy between the two groups
The 3-year DMFS rate in the nimotuzumab and control groups was 91.6% and 77.3%(P = 0.047), respectively.The 3-year PFS,LRFS and OS rates in the nimotuzumab and control groups were 87.6% vs 75.5% (P = 0.110), 90.5% vs 86.9% (P = 0.566) and 94.5% vs 87.1% (P = 0.295),respectively (Fig. 1). In the nimotuzumab group, there were six cases of disease progression, two of local recurrence, four of distant metastasis, and two of death. In the control group, there were 13 cases of disease progression, two of local recurrence, 12 of distant metastasis, and five cases of death.
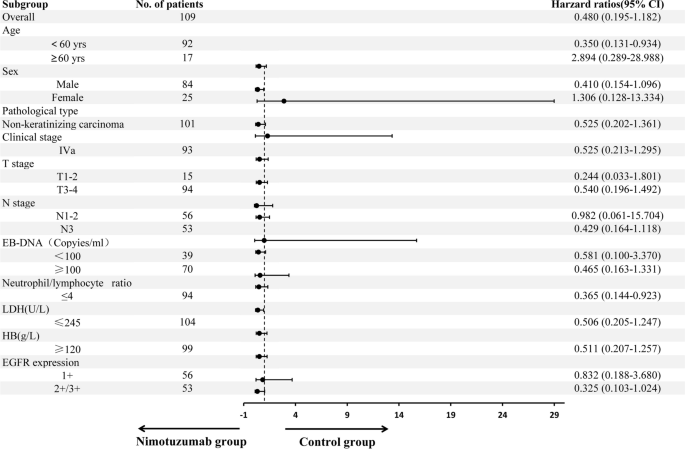
Subgroup analysis showed in patients with locally advanced NPC after neoadjuvant chemotherapy, concurrent chemoradiotherapy plus nimotuzumab targeted therapy had significant benefits to PFS for individuals aged < 60 years (hazard ratio [HR] = 0.350, 95% confidence interval [CI]: 0.131–0.934, P=0.036) and those with neutrophil/lymphocyte ratio ≤ 4 (HR = 0.365, 95% CI: 0.144–0.923, P = 0.033). There was a trend towards PFS benefit for patients with male (HR = 0.410, 95% CI: 0.154–1.096, P = 0.076),N3 (HR = 0.429, 95% CI: 0.164–1.118, P = 0.083), and those with EGFR (+ + / + + + + , HR = 0.325, 95% CI: 0.103–1.024, P = 0.055) (Fig. 2).
After radical chemoradiotherapy, EB DNA remained positive in four patients in the control group, while it was negative in the nimotuzumab group. The EB DNA levels after treatment,recent therapeutic effect and baseline factors were considered. Multivariate regression analysis showed that the neutrophil/lymphocyte ratio was an independent risk factor for disease progression (HR = 7.485, P = 0.012) and distant metastasis (HR = 17.540, P = 0.009) of locally advanced NPC, with a higher neutrophil/lymphocyte ratio being associated with an increased risk of disease progression and distant metastasis after treatment (Table 3).
Comparison of adverse reactions between the two groups
No grade 4 adverse reactions were observed in either group. Grade 3 oral mucosal reactions, as well as pharyngeal and esophageal reactions were slightly higher in the nimotuzumab group than in the control group, but the difference was not statistically significant. No significant differences were observed in the incidence of adverse reactions such as leukopenia, HB reduction, thrombocytopenia, elevated alanine aminotransferase (ALT), elevated aspartate aminotransferase (AST), elevated creatinine, nausea, vomiting, radiation dermatitis, weight loss, hyponatremia, hypokalemia, skin rash and infusion reaction between the two groups (P > 0.05) (Table 4).
Discussion
The results of this study show that the nimotuzumab group had significantly higher 3-year DMFS compared to the control group. Additionally, the 3-year PFS showed an increasing trend, suggesting that concurrent chemoradiotherapy combined with nimotuzumab after neoadjuvant chemotherapy can effectively reduce distant metastasis in locally advanced NPC, and may be beneficial to PFS and OS over time.
Concurrent chemoradiotherapy following neoadjuvant chemotherapy has emerged as the standard treatment for locally advanced NPC, with the TPF regimen recommended as a grade I neoadjuvant chemotherapy [13]. Although induction chemotherapy combined with concurrent chemoradiotherapy has achieved good results in the treatment of locally advanced NPC, distant metastasis remains a major cause of treatment failure [8]. Hence, further reduction of distant metastasis and improvement of the OS rate are important and difficult issues in the treatment of locally advanced NPC.
EGFR is highly expressed in 80%-90% of patients with NPC [10]. Activation of EGFR can promote proliferation, invasion, and metastasis of tumor cells, as well as inhibit apoptosis of tumor cells, thus inducing tolerance to radiotherapy and chemotherapy [14, 15]. At present, EGFR has become a therapeutic target for NPC. Huang et al. confirmed that the addition of radiotherapy to nimotuzumab improved the overall efficacy of NPC treatment compared to radiotherapy alone [16]. Wu et al. also confirmed that radiotherapy combined with nimotuzumab can improve the efficacy of treatment for locally advanced NPC compared with radiotherapy alone [17]. In a retrospective study conducted by Lu et al., concurrent chemoradiotherapy combined with nimotuzumab was demonstrated to improve DMFS and OS compared to concurrent chemoradiotherapy in patients with NPC and cervical lymph node metastasis [18]. Sun et al. conducted a prospective, randomized, controlled, double-blind, multicenter phase III clinical trial, which demonstrated that compared with concurrent chemoradiotherapy, nimotuzumab combined with concurrent chemoradiotherapy improved the efficacy of treatment for locally advanced NPC, and the 5-year OS rate increased from 64.3% to 76.9% (P = 0.042) [19]. These studies collectively support the efficacy of both radiotherapy alone and concurrent chemoradiotherapy plus nimotuzumab in the treatment of locally advanced NPC. Jiang et al. reported that concurrent chemoradiotherapy combined with nimotuzumab after induction chemotherapy improved the objective remission rate and 5-year PFS compared with concurrent chemoradiotherapy alone [20]. Similarly, a retrospective study by Wang et al. confirmed that concurrent chemoradiotherapy combined with nimotuzumab after induction chemotherapy for locally advanced NPC can prolong DMFS for up to five years. For patients with N2-3, the DMFS, and OS of the nimotuzumab group were significantly prolonged [21], which was similar to the results obtained in this study. Our study further confirmed that nimotuzumab combined with concurrent chemoradiotherapy after neoadjuvant chemotherapy improved the efficacy of treatment for locally advanced NPC.Adverse reactions observed in both the nimotuzumab and control groups were comparable and oral mucosal reactions, radiation dermatitis, as well as other reactions did not increase, similar to the results of a previous study [22].
The cumulative dose of cisplatin in concurrent radiochemotherapy is also debated. Lv et al. revealed no significant association between a 200 mg/m2 cumulative dose of cisplatin and improved survival, while a 160 mg/m2 cumulative dose of cisplatin may be appropriate [23]. Liu et al. revealed a better curative effect with a > 200 mg/m2 than ≤ 100 mg/m2 cumulative dose of cisplatin, but was comparable with 100–200 mg/m2 cumulative dose in concurrent radiochemotherapy [24]. These findings suggested that the higher cumulative dose of cisplatin did not always indicate a better curative effect. The present study adopted a 160 mg/m2 cumulative dose of cisplatin. On the contrary, some studies have revealed a better survival benefit with a higher cumulative dose of cisplatin. Jiang et al. revealed higher 3-year PFS and DMFS in > 200 mg/m2 cumulative dose of cisplatin than that in ≤ 200 mg/m2 cumulative dose in concurrent radiochemotherapy [25].
The subgroup analysis of this study revealed that patients with locally advanced NPC who were < 60 years old and had a neutrophil/lymphocyte ratio ≤ 4 gained a significant survival benefit when treated with neoadjuvant chemotherapy followed by concurrent chemoradiotherapy combined with nimotuzumab. Patients with male,N3 disease and EGFR expression (+ + / + + +) also showed a favorable trend in terms of survival benefits. Multivariate regression analysis identified the neutrophil/lymphocyte ratio as a risk factor for disease progression and distant metastasis in locally advanced NPC. The neutrophil/lymphocyte ratio is the ratio of absolute counts of neutrophils and lymphocytes in the peripheral blood, which may represent the balance between the pro-tumor inflammatory state and the anti-tumor immune response. Several studies have found that the neutrophil/lymphocyte ratio is an independent factor for tumor prognosis [26,27,28], confirming the results of this study. Other prognostic factors for NPC [29,30,31,32,33], such as EGFR expression, EB DNA level, LDH, T stage, and N stage, were not detected in this study, which may be related to the retrospective study,relatively small sample size and short follow-up time.
This study provides clinical evidence to support the benefit of concurrent chemoradiotherapy with nimotuzumab after neoadjuvant chemotherapy for locally advanced NPC. However, this study is limited in that it is a retrospective study with a relatively short follow-up duration. Long-term follow-up to observe the 5-year survival data is required. In addition, multicenter, randomized, double-blind, large-sample prospective studies are needed to confirm these findings.
Conclusion
Based on concurrent chemoradiotherapy after neoadjuvant chemotherapy for locally advanced NPC, nimotuzumab demonstrated a better complete remission rate, as well as significantly improved DMFS and PFS, with a notable increasing trend. The incidence of adverse reactions was comparable. Therefore, concurrent chemoradiotherapy with nimotuzumab after neoadjuvant chemotherapy may be a preferred treatment strategy for locally advanced NPC.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CTV1:
-
Clinical target volume 1
- CTV2:
-
Clinical target volume 2
- CR:
-
Complete response
- DMFS:
-
Distant metastasis-free survival
- EGFR:
-
Epithelial growth factor receptor
- EB-DNA:
-
Epstein barr virus deoxyribonucleicacid
- GTV:
-
Gross tumor volume
- HR:
-
Hazard ratio
- LRFS:
-
Locoregional relapse-free survival
- LDH:
-
Lactate dehydrogenase
- NPC:
-
Nasopharyngeal carcinoma
- NLR:
-
Neutrophil-to-lymphocyte ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PD:
-
Progressive disease
- SD:
-
Stable disease
- TPF:
-
Docetaxel,cisplatin,and 5-fluorouracil
References
Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophilto-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: a pooled analysis of two randomised controlled trials. Eur J Cancer. 2016;67:119–29.
Wang Rensheng, Pan Jianji, Ma Jun, et al. Chinese Guidelines for Radiotherapy of Nasopharyngeal Carcinoma (2020 Edition). Chinese J Cancer Prev Treat. 2021, 28 (3): 167–177.
Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20.
Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin inductionchemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–35.
Jin YN, Qiang MY, Liu MM, et al. Impact of cumulative cisplatin dose in childhood nasopharyngeal carcinoma based on neoadjuvant chemotherapy response in the intensity-modulated radiotherapy era: a real-world study. Cancer Cell Int. 2021;21:604–16.
Chen YP, Tang LL, Yang Q, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018;24(8):1824–33.
National Comprehensive Nancer Network. NCCN clinical practice guidelines in head and neck cancers, 2019. Available from:www.nccn.org.
Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patientswith nasopharyngeal carcinoma: an analysis of survival and treatmenttoxicities. Radiother Oncol. 2014;110(3):398–403.
Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy[J]. Cancer. 2002;94:1593–611.
Psyrri A, Seiwert TY, Jimeno A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am Soc Clin Oncol Educ Book. 2013;2013:246–55.
Talavera A, Friemann R, Gomez-Puerta S, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Can Res. 2009;69:5851–9.
Zhi-Qiang Wang, Qi Mei, Ji-Bin Li, et al. The long-term survival of patients with III-IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer. 2019;19(1):1122–34.
Tang LL, Chen YP, Chen CB, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021;41(11):1195–227.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Pang LY, Saunders L, Argyle DJ. Epidermal growth factor receptor activity is elevated in glioma cancer stem cells and is required to maintain chemotherapy and radiation resistance. Oncotarget. 2017;8:72494–512.
Huang Xiaodong, Yi Junlin, Gao Li, et al. Phase II clinical trial of anti-EGFR monoclonal antibody h-R3 combined with radiotherapy for advanced nasopharyngeal carcinoma. Chin J Radiat Oncol. 2007;29(3):197–201.
Wu Renrui, Wu Shaoxiong, Zhao Chong, et al. Phase II clinicaI triaI of h—R3 combined radiotherapy for locoregionally advanced nasopharyngeal carcinom. Chin J Cancer. 2007;26(8):874–9.
Jinlong Lu, Wei Jiazhang, Xiao Xin, et al. Efficacy of concurrent chemoradiotherapy combined with nimotuzumab in the treatment of nasopharyngeal carcinoma with cervical lymph node metastasis. Eur Arch Otorhinolaryngol. 2023;280(5):2479–88.
Chinese Society of Clinical Oncology. Nimotuzumab plus chemo-radiotherapy versus placebo plus chemo-radiotherapy in locally advanced nasopharyngeal cancinoma(NPC) patients [R]. Guangzhou: CSCO; 2023.
Jiang Danxian, Cao Jinxin, Guo Linying, et al. Induction chemotherapy with sequential nimotuzumab plus concurrent chemoradiotherapy in advanced nasopharyngeal carcinoma: a retrospective real-world study. Medicine. 2023;102:4 (e32732).
Wang Fangzheng, Sun Quanquan, et al. Additional induction chemotherapy to concurrent chemotherapy and intensity-modulated radiotherapy with or without nimotuzumab in first-line treatment for locoregionally advanced nasopharyngeal carcinoma: a propensity score matched analysis. J Cancer. 2018;9(3):594–603.
Cai Zhuochen, Chen Dongni, Qiu Wenze, et al. Concurrent chemoradiotherapy combined with nimotuzumab in stage III-IVa nasopharyngeal carcinoma: a retrospective analysis. J Cancer Res Clin Oncol. 2023;149(6):2327–44.
Lv J, Qi Z, Zhou G, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci. 2018;109(3):751–63.
Liu Sailan, Sun Xuesong, Yan Jinjie, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother Oncol. 2019;137:83–94.
Jiang Y, Chen K, Yang J, et al. Efficiency of high cumulative cisplatin dose in high- and low-risk patients with locoregionally advanced nasopharyngeal carcinoma[J]. Cancer Med. 2022;11(3):715–27.
Valero Cristina, Daniella K, Zanoni K, et al. Pretreatment peripheral blood leukocytes are independentpredictors of survival in oral cavity cancer. Cancer. 2020;126:994–1003.
Naszai M, Kurjan A, et al. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021;10(17):5983–97.
Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil-to-lymphocyte ratio andmutational burden as biomarkers of tumorresponse to immune checkpoint inhibitors. Nat Commun. 2021;12:729–38.
Hong Xiaohua, Wang Guangyao, Guanglan Xu, et al. Prognostic value of EGFR and p-EGFR in nasopharyngeal carcinoma. A systematic review and meta-analysis. Medicine (Baltimore). 2022;101(3):28507–28.
Su ZY, Siak PY, Leong CO, et al. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front Microbiol. 2023;14:1116143–60.
Chen Wenjie, Wenna Xu, Wang Haiyun, et al. Plasma Epstein-Barr virus DNA and risk ofnasopharyngeal carcinoma in a prospectiveseropositive population. BMC Cancer. 2021;21:651–8.
Zhang Mingwei, Wei Shushan, Li Su, et al. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population. A meta-analysis. Medicine (Baltimore). 2016;95(35):4494–502.
Chiang CL, Guo Q, Ng WT, et al. Prognostic Factors for Overall Survival in Nasopharyngeal Cancer and Implication for TNM Staging by UICC: A Systematic Review of the Literature. Front Oncol. 2021;11:703995.
Acknowledgements
Not applicable.
Funding
This work was supported by Hunan Natural Science Foundation (grant no.2020JJ8036).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZY, QZ, RL and HW. The first draft of the manuscript was written by ZY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the People 's Hospital of Xiangxi Tujia and Miao Autonomous Prefecture(ethic code: 2020–05). This study has obtained the informed consent of patients.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Z., Zuo, Q., Liu, R. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy with or without nimotuzumab in the treatment of locally advanced nasopharyngeal carcinoma: a retrospective study. BMC Cancer 23, 1140 (2023). https://doi.org/10.1186/s12885-023-11608-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11608-5