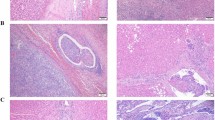
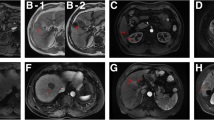
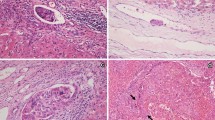
Abstract
Background
In this study, we aimed to establish nomograms to predict the microvascular invasion (MVI) and early recurrence in patients with small hepatocellular carcinoma (SHCC), thereby guiding individualized treatment strategies for prognosis improvement.
Methods
This study retrospectively analyzed 326 SHCC patients who underwent radical resection at Wuhan Union Hospital between April 2017 and January 2022. They were randomly divided into a training set and a validation set at a 7:3 ratio. The preoperative nomogram for MVI was constructed based on univariate and multivariate logistic regression analysis, and the prognostic nomogram for early recurrence was constructed based on univariate and multivariate Cox regression analysis. We used the receiver operating characteristic (ROC) curves, area under the curves (AUCs), and calibration curves to estimate the predictive accuracy and discriminability of nomograms. Decision curve analysis (DCA) and Kaplan-Meier survival curves were employed to further confirm the clinical effectiveness of nomograms.
Results
The AUCs of the preoperative nomogram for MVI on the training set and validation set were 0.749 (95%CI: 0.684–0.813) and 0.856 (95%CI: 0.805–0.906), respectively. For the prognostic nomogram, the AUCs of 1-year and 2-year RFS respectively reached 0.839 (95%CI: 0.775–0.903) and 0.856 (95%CI: 0.806–0.905) in the training set, and 0.808 (95%CI: 0.719–0.896) and 0.874 (95%CI: 0.804–0.943) in the validation set. Subsequent calibration curves, DCA analysis and Kaplan-Meier survival curves demonstrated the high accuracy and efficacy of the nomograms for clinical application.
Conclusions
The nomograms we constructed could effectively predict MVI and early recurrence in SHCC patients, providing a basis for clinical decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant tumor of the digestive system. The latest data from GLOBOCAN showed that its incidence and mortality rates ranked 6th and 3rd among all malignant tumors [1]. In the Barcelona Clinic Liver Cancer (BCLC) system, potential radical treatments recommended for patients with early-stage HCC include radical resection, radiofrequency ablation (RFA) and liver transplantation [2]. In the majority of cases, surgical resection remains the first choice for HCC [3], but the 5-year postoperative recurrence rate could be as high as 70%, with most patients experiencing early recurrence within 2 years after surgery, which is inevitable even in small HCC (SHCC) (tumor diameter ≤ 3 cm) [4,5,6].
Microvascular invasion (MVI) is a major risk factor for recurrence of HCC, and preoperative detection of MVI is of great significance in the choice of diagnostic, therapeutic options, and prognosis [7]. However, MVI could only be diagnosed by postoperative pathology with a certain lag [8]. Some studies have shown that AFP level, inflammatory indexes, and gadoxetic acid–enhanced magnetic resonance imaging (EOB-MRI) features (tumor diameter and tumor margin) have a close relationship with MVI [9,10,11]. Nevertheless, previous studies have mainly focused on HCC, and few have comprehensively evaluated the relevant characteristics of MVI in SHCC patients, and proposed a preoperative prediction model for MVI with a good predictive performance.
For now, a lot of staging systems for HCC have been developed, such as the BCLC system, TNM system, Hong Kong Liver Cancer (HKLC) system and Japan Integrated Staging (JIS) score [12,13,14,15], which plays an important role in preoperative evaluation and postoperative treatment. However, none of them focus on SHCC early recurrence accurately [16]. Given the high early recurrence rate of HCC, accurate assessment of early recurrence in SHCC is essential for individualized treatment strategies [17]. Moreover, numerous studies have shown that SHCC patients with a high risk of recurrence required postoperative adjuvant therapy and careful follow-up [18, 19]. Recurrence-free survival (RFS) is significantly prolonged in HCC patients after receiving appropriate postoperative adjuvant therapy, especially in SHCC [20,21,22]. Therefore, the development of an appropriate SHCC early recurrence risk system is urgent.
Therefore, we retrospectively analyzed the clinical data of 326 SHCC patients in our hospital, and established and validated two nomograms for MVI and early recurrence in SHCC. The innovation of this study is to non-invasively predict the preoperative probability of MVI and early postoperative recurrence in SHCC patients, providing more accurate guidance for the intervention and treatment of SHCC patients.
Methods
Study design and study population
This study retrospectively analyzed 326 SHCC patients who underwent radical resection at Wuhan Union Hospital between April 2017 and January 2022. This study was approved by Ethics Committee of Wuhan Union Hospital and did not require informed consent from participants (Ethics approval number: 2023 − 0586). According to the inclusion and exclusion criteria, 326 patients were enrolled in the study. Inclusion criteria included: (1) single tumor ≤ 3 cm in diameter or the sum of two tumors ≤ 3 cm in diameter; (2) patients underwent radical resection with definite pathological diagnosis; (3) received EOB-MRI preoperatively; (4) patients of clear mind and normal intelligence who could cooperate with the relevant examinations. Exclusion criteria included: (1) patients who underwent anti-tumor treatments such as surgical resection, transcatheter arterial chemoembolization (TACE), local ablation, targeted immunotherapy and liver transplantation before surgery; (2) patients with clinical and follow-up data missing; (3) pregnant and lactating female; (4) patients with surgical margin positive; (5) patients with other malignant tumors. For analysis, all 326 patients were randomly divided into a training set and a validation set at a 7:3 ratio (Fig. 1). The nomograms were established using the training set and its accuracy was validated using the validation set.
Collection of data and definition of variables
Baseline data collected included patient characteristics, laboratory index, inflammatory biomarkers, radiomics features, histopathologic characteristic, surgical information and follow-up data. Patient characteristics included age, sex, body mass index (BMI), etiology, cirrhosis, Child-Pugh grade, ALBI stage and BCLC grade. Laboratory index involved aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), albumin (ALB), platelet (PLT), prothrombin time (PT) and alpha-fetoprotein (AFP). Inflammatory biomarkers included platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), systemic inflammation response index (SIRI), systemic immune-inflammation index (SII), aspartate aminotransferase to neutrophil ratio index (ANRI), prognostic nutritional index (PNI). Radiomics features incorporated tumor diameter, tumor number, tumor location and tumor margin. Histopathologic characteristic involved MVI and Edmondson-Steiner grade. MVI was defined as a tumor cell nest that was only visible under the microscope in the tumor capsule blood vessels of the portal vein, hepatic vein, and endothelial lining. The “7-point” baseline sampling method was used for evaluation [23]. The three-tiered MVI grading system (MVI-TTG) classified specimens as M0 (no MVI detected), M1 (≤ 5 MVIs, all occurring in adjacent liver tissue ≤ 1 cm away from the main tumor), and M2 (> 5 MVIs or any MVI occurring in adjacent liver tissue ≤ 1 cm away from the main tumor) [24]. Surgical information included surgical methods and surgical margin. Among them, surgical methods included AR (anatomic resection) and NAR (non-anatomic resection). All laboratory index and radiomics features were obtained up to 1 week before surgery. The inflammatory biomarkers were calculated by the following formula: SIRI = (neutrophil × monocyte)/lymphocyte; SII = PLT × (neutrophil/lymphocyte); ANRI = AST/neutrophil; PNI = ALB + 5 × lymphocyte. The ALBI score was computed by the formula: ALBI = (log10(TBIL) × 0.66 + ALB× (− 0.085)). The cut-off value of the inflammatory biomarkers for predicting SHCC with MVI in our study were set by plotting the restricted cubic splines (PLR = 92.3; NLR = 1.8; SIRI = 0.6; SII = 241.2; PNI = 46.5; ANRI = 10.9), as shown in Fig. 2. Besides, AST, ALT, TBIL, ALB, PLT and PT cut-off value in our study were used as the upper limit of normal values for serologic tests in our institution.
The cut-off value of the inflammatory biomarkers for predicting SHCC with MVI. PLR, platelet-to-lymphocyte ratio (A); NLR, neutrophil-to-lymphocyte ratio (B); SIRI, systemic inflammation response index (C); SII, systemic immune-inflammation index (D); PNI, prognostic nutritional index (E); ANRI, aspartate aminotransferase to neutrophil ratio index (F)
Follow-up data
All patients underwent radical resection, defined as the complete resection of tumor tissue with negative surgical margin. After surgery, all patients were followed up monthly for the first three months, then every two months for the first year, and every three months thereafter. Laboratory index (including serum AFP level and blood tests) and imaging examinations (contrast-enhanced ultrasound, computed tomography or EOB-MRI) were conducted in follow-up examinations. Early recurrence was defined as the recurrence of HCC within 2 years after radical resection. HCC preoperative and recurrence diagnosis were both based on the criteria stipulated in the 2022 Standard for diagnosis and treatment of primary liver cancer in China [25]. The main end-point of our study was RFS, which was defined as the time from the date of radical resection to the date of tumor recurrence or the last follow-up without early recurrence within 2 years.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and compared by Student’s t test. Categorical variables were expressed as frequency and percentage, and the chi-square test or Fisher’s exact test was used for comparison. LASSO regression analysis was used for data dimensionality reduction and element selection. In the training set, independent risk factors for MVI were identified by univariable and multivariable logistic analysis, and independent prognostic factors of SHCC early recurrence were identified by univariable and multivariable Cox proportional hazard regression analysis. Subsequently, we established two nomograms to predict the risk of MVI and RFS in SHCC. ROC curves were plotted to assess nomogram’s differentiation and predictive efficacy in terms of area under the curve (AUC). Calibration curves were plotted to assess the agreement of nomograms, and decision curve analysis (DCA) was plotted to assess the clinical application value of nomograms by demonstrating net benefit for each risk threshold probability. We compared the ROC curves, the AUCs, the calibration curves, and the DCA results between the training and validation set to verify the stability of the nomogram. Finally, patients in the training set and validation set were assigned to either the high-risk group or the low-risk group based on the median risk score of the prognostic nomogram. RFS curves were calculated using the Kaplan–Meier method and compared with the Log-rank test. All statistical analyses were conducted using SPSS (version 26.0) and R software (version 4.3.1). Two-tailed P value < 0.05 was considered as a measure of statistical significance.
Results
Baseline clinical characteristics
A total of 326 patients with SHCC receiving radical resection were included in our study. Of these, 227 patients were assigned to the training set and 99 patients to the validation set. Patients baseline clinical characteristics were summarized in Table 1. There were no differences in clinical, radiologic, histopathologic characteristics or follow-up information between the training and validation sets (all P > 0.05). The median RFS was 19.2 months (95% CI: 12.3–26.1) for the training set and 18.3 months (95% CI:11.5–25.1) for the validation set (P = 0.249).
Independent risk factors for MVI
Patient characteristics, laboratory index, inflammatory biomarkers and radiomics features in Table 1 were included in Lasso regression analysis for element selection (Fig. 3). Table 2 illustrated that univariable logistic analysis demonstrated that age ≥ 60 years, TBIL ≥ 19µmol/L, AFP ≥ 200ng/mL, NLR ≥ 1.8, PNI ≥ 46.5, larger tumor diameter, and tumor margin non-smooth were significantly associated with MVI (all P < 0.05). The multivariable logistic analysis showed that AFP ≥ 200ng/mL, NLR ≥ 1.8, PNI ≥ 46.5, larger tumor diameter, and tumor margin non-smooth were independent risk factors for MVI (all P < 0.05).
Preoperative Nomogram for MVI Establishment and Validation
Based on above 5 independent risk factors, a nomogram for predicting the risk of MVI in patients with SHCC was constructed (Fig. 4). In the training set, the nomogram achieved an AUC of 0.749 (95%CI: 0.684–0.813) (Fig. 5A). In the validation set, the nomogram had an AUC of 0.856 (95%CI: 0.805–0.906) (Fig. 5D). There was no statistically significant difference in the AUC between the training and validation set (P > 0.05), indicating that the prediction nomogram had a high discriminative ability. The calibration curve showed good agreement between the predicted and actual probabilities in the training set (Fig. 5B). In the validation set, the calibration curve was slightly less consistent with the actual probabilities, but they were close to each other, demonstrating that the nomogram had a good level of reproducibility and reliability (Fig. 5E). DCA of the nomogram revealed the nomogram had a higher net benefit than categorizing all patients as MVI across almost all threshold probabilities (Fig. 5C and F).
The ROC curves for predicting the risk of SHCC with MVI in the training set (A) and validation set (D). The calibration curves for predicting the risk of SHCC with MVI in the training set (B) and validation set (E). The DCA for the training set (C) and the validation set (F). ROC receiver operating characteristic; DCA decision curve analysis
Independent prognostic factors for SHCC
Included patient characteristics, laboratory index, radiomics features, histopathologic characteristic and surgical information into the Lasso regression analysis (Fig. 6). The characteristics screened in the Lasso regression analysis were further revealed by univariable and multivariable Cox proportional hazard regression analysis. Our univariable Cox analysis revealed that ALB < 35 g/L, AFP ≥ 200 ng/mL, Child-Pugh B, BCLC A, larger tumor diameter, MVI, Edmondson-Steiner III-IV, NAR, and surgical margin < 1 cm were significantly associated with tumor recurrence (all P < 0.05). The multivariable Cox analysis showed that AFP ≥ 200 ng/mL, MVI as M2, Edmondson-Steiner III-IV, NAR, and surgical margin < 1 cm were independent prognostic factors of RFS in Table 3 (all P < 0.05).
Prognostic nomogram establishment and validation
According to the multivariable Cox analysis, AFP, MVI, Edmondson-Steiner, surgical methods and surgical margin were integrated to build the nomogram of RFS (Fig. 7). ROC analysis of the nomogram revealed that AUC of 1-year and 2-year RFS respectively reached 0.839 (95%CI: 0.775–0.903) and 0.856 (95%CI: 0.806–0.905) in the training set, and 0.808 (95%CI: 0.719–0.896) and 0.874 (95%CI: 0.804–0.943) in the validation set (Fig. 8). The calibration curves of nomogram revealed a strong consistency between actual observation and prediction (Fig. 9). In addition, the nomogram demonstrated a significant positive net benefit from the risk of early recurrence, indicating its great clinical practical value in predicting RFS of SHCC (Fig. 10). The Kaplan-Meier survival analysis of training set and validation set showed a distinct difference in survival rate (Fig. 11, P < 0.001).
Discussion
In this study, we developed and validated two nomograms based on readily available preoperative and postoperative clinical data, one for preoperative prediction of SHCC with MVI, and one for prediction of early recurrence risk of SHCC after radical resection. MVI is the main factor determining treatment strategies, so that preoperative prediction of SHCC with MVI can guide the selection of surgical methods, and prediction of postoperative early recurrence risk can also provide a basis for selection of postoperative adjuvant treatment plans, which is of great importance in prognosis improvement [26, 27]. By calculating AUC and plotting calibration curves, we have shown that both nomograms have good predictive performance and consistency, demonstrating a good predictive value, which was beneficial for preoperative non-invasive prediction of SHCC with MVI as well as the risk of early recurrence after radical resection, and provided a more accurate guidance for the intervention and treatment of SHCC patients. In addition, the DCA results indicated that the nomograms have good clinical application value and is beneficial for personalized treatment interventions.
MVI mainly refers to the nesting clusters of cancer cells seen microscopically in the endothelial cell-lined vascular lumen, which is the initial stage of portal vein cancerous embolism [28]. Postoperative pathology is still the gold standard for confirming the diagnosis of MVI. MVI mainly reflects the invasive nature of HCC, and it is an important predictor of postoperative recurrence of HCC. Shindoh et al [29] have demonstrated that even in SHCC, MVI is still an independent risk factor for poor prognosis, including increased risk of recurrence and decreased long-term survival. Therefore, preoperative prediction of MVI can not only guide the selection of surgical methods, but also provide a basis for the selection of new adjuvant plans before surgery, ultimately improving survival outcomes. As mentioned earlier, there are few studies on the occurrence of MVI in patients with SHCC. Zhang et al [30]found that fibrinogen, AFP, cirrhosis, tumor diameter and poor tumor border were independent risk factors of HCC with MVI, and similar to Zhang’s study, our study found that serum AFP level, tumor diameter and tumor margins were independent risk factors for SHCC patients with MVI.
Regarding tumor diameter, numerous studies have previously demonstrated that tumor size is an independent prognostic factor in HCC patients [31,32,33]. NLR, serving as an inflammatory indicator, has been reported to be associated with the poor prognosis of HCC [34, 35]. Interestingly, we found that tumor diameter and NLR were independent risk factors for MVI rather than independent prognostic factors for SHCC. The reason may be that the endpoint of our study is different from previous studies, cause our study only focused on predicting early recurrence. In addition, different study populations (our study only included SHCC) might be an another reason.
Edmondson-Steiner grade has been identified as an independent risk factor for HCC recurrence. Zhou et al [36] proved that the Edmondson-Steiner grade had important significance for the prognosis of HCC and might become a key prognostic indicator for HCC without MVI. Our study found that the early recurrence rate in Edmondson-Steiner III-IV stage patients was significantly higher than that of I-II, which confirmed this viewpoint. AFP is a specific tumor marker for HCC with a specificity of up to 93.3%for early diagnosis [37]. Relevant studies have proved that the higher the serum AFP level, the shorter the survival time of HCC patients, indicating the close relationship between AFP level and prognosis [38, 39]. The results of our study showed that serum AFP level was not only an independent risk factor for the occurrence of MVI, but also an independent risk factor for early recurrence of SHCC, which was consistent with previous research [40].
Surgical methods and surgical margin are another factor affecting HCC recurrence. Many studies have shown that AR has a better prognosis than NAR [41, 42], however, Eguchi et al [43] found that for SHCC, AR was not beneficial. Therefore, the therapeutic effect of AR remains controversial. Our results showed that AR improved patient prognosis and reduced early recurrence of HCC compared with NAR, the reason might be that AR could remove intrahepatic lesions and microvascular metastases. Famularo et al [44] found that the risk of early recurrence of HCC after AR was significantly reduced, especially in HCC with MVI. Therefore, if SHCC patients have sufficient liver function reserve and AR is technically feasible, AR should be considered first, and NAR should be considered as an alternative therapy for patients with limited liver function reserve [41]. In addition, Su et al [45] revealed that the RFS of wider surgical margin (≥ 1 cm) was higher than that of narrower surgical margin in HCC. Our results showed that wide resection margin (≥ 1 cm) can improve the prognosis of all patients, which is consistent with previous reported studies. Therefore, we suggested surgeons should use AR method as much as possible, and try to preserve the surgical resection margin width ≥ 1 cm for SHCC patients.
Our study has several limitations. First, this study was a single-center retrospective study with a limited sample size. Second, the cut-off values of some indicators in this study had a certain subjectivity, which might have a certain impact on the study results. Finally, most of the patients in this study suffered from hepatitis B virus-related hepatocellular carcinoma, which might have some selection bias. In the future, large-sample, multi-center prospective studies are planned to further improve and validate the results.
Conclusion
Our study developed and validated a preoperative nomogram for MVI prediction, and a prognostic nomogram for early recurrence in SHCC patients. These nomograms could better predict individual survival, guide follow-up management strategies and provide a basis for clinical decision making. Furthermore, based on the prognostic nomogram, we suggested that surgeons should choose AR while trying to maintain a surgical margin of ≥ 1 cm, which could reduce early recurrence and improve the prognosis of SHCC patients.
Data availability
The data used and evaluated in this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. The data are located in the controlled access data storage of Union Hospital of Tongji Medical College, Huazhong University of Science and Technology.
Abbreviations
- MVI:
-
Microvascular invasion
- SHCC:
-
Small hepatocellular carcinoma
- LH:
-
Laparoscopic hepatectomy
- RFA:
-
Radiofrequency ablation
- BCLC:
-
Barcelona Clinic Liver Cancer
- RFS:
-
Recurrence-free survival
- EOB-MRI:
-
Gadoxetic acid–enhanced magnetic resonance imaging
- AST:
-
Aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- ALB:
-
Albumin
- PLT:
-
Platelet
- PT:
-
Prothrombin time
- AFP:
-
Alpha-fetoprotein
- PLR:
-
Platelet-to-lymphocyte ratio
- NLR:
-
Neutrophil-to-lymphocyte ratio
- SIRI:
-
Systemic inflammation response index
- SII:
-
Systemic immune-inflammation index
- ANRI:
-
Aspartate aminotransferase to neutrophil ratio index
- PNI:
-
Prognostic nutritional index
- AR:
-
Anatomic resection
- NAR:
-
Non-anatomic resection
- RFS:
-
Recurrence-free survival
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Wei H, Jiang H, Qin Y, Wu Y, Lee JM, Yuan F, Zheng T, Duan T, Zhang Z, Qu Y, et al. Comparison of a preoperative MR-based recurrence risk score versus the postoperative score and four clinical staging systems in hepatocellular carcinoma: a retrospective cohort study. Eur Radiol. 2022;32(11):7578–89.
Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M, Festi D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70(3):440–8.
Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: Radiofrequency ablation vs. resection vs. Transplantation? Clin Mol Hepatol. 2019;25(4):354–9.
Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, Imbriani S, Claar E, Cozzolino D, Sasso FC, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. 2023;29(8):1243–60.
Sun X, Yang Z, Mei J, Lyu N, Lai J, Chen M, Zhao M. The guiding value of microvascular invasion for treating early recurrent small hepatocellular carcinoma. Int J Hyperthermia: Official J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2021;38(1):931–8.
Huang JY, Huang ZL, Yang Z, Zheng XP. Contrast-enhanced ultrasound predicts microvascular invasion in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis International: HBPD INT. 2022;21(6):609–13.
Lv K, Cao X, Du P, Fu JY, Geng DY, Zhang J. Radiomics for the detection of microvascular invasion in hepatocellular carcinoma. World J Gastroenterol. 2022;28(20):2176–83.
Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver cancer. 2019;8(5):373–86.
Gu Y, Zheng F, Zhang Y, Qiao S. Novel Nomogram based on inflammatory markers for the Preoperative Prediction of Microvascular Invasion in Solitary Primary Hepatocellular Carcinoma. Cancer Manage Res. 2022;14:895–907.
Xu X, Sun S, Liu Q, Liu X, Wu F, Shen C. Preoperative application of systemic inflammatory biomarkers combined with MR imaging features in predicting microvascular invasion of hepatocellular carcinoma. Abdom Radiol (New York). 2022;47(5):1806–16.
Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775–84.
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93.
Chuncharunee A, Siramolpiwat S. Validation of the Hong Kong Liver Cancer Staging System in patients with Hepatocellular Carcinoma after curative intent treatment. Asian Pac J cancer Prevention: APJCP. 2017;18(6):1697–701.
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated staging score (JIS score). J Gastroenterol. 2003;38(3):207–15.
Hu Z, Yuan Y, Hu Z, Liu Q, Fu Y, Hou J, Sun X, Li S, Duan W, Chen M. Development and Validation of Prognostic Nomograms for Hepatocellular Carcinoma after Hepatectomy based on inflammatory markers. J Hepatocellular Carcinoma. 2022;9:1403–13.
Mao S, Yu X, Shan Y, Fan R, Wu S, Lu C. Albumin-Bilirubin (ALBI) and Monocyte to lymphocyte ratio (MLR)-Based Nomogram Model to Predict Tumor recurrence of AFP-Negative Hepatocellular Carcinoma. J Hepatocellular Carcinoma. 2021;8:1355–65.
Hsiao JH, Tsai CC, Liang TJ, Chiang CL, Liang HL, Chen IS, Chen YC, Chang PM, Chou NH, Wang BW. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with hepatocellular carcinoma undergoing surgical treatment. Int J Surg (London England). 2017;45:35–41.
Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, Okabe H, Chikamoto A, Ishiko T, Baba H. Microvascular Invasion in small-sized Hepatocellular Carcinoma: significance for outcomes following Hepatectomy and Radiofrequency ablation. Anticancer Res. 2018;38(2):1053–60.
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Mo A, Lin B, Chen D. Efficacy of sequential TACE on primary hepatocellular carcinoma with microvascular invasion after radical resection: a systematic review and meta-analysis. World J Surg Oncol. 2023;21(1):277.
Li LQ, Su TS, Wu QY, Lin ZT, Liang SX. Therapeutic outcome of stereotactic body radiotherapy for Small Hepatocellular Carcinoma Lesions - a Systematic Review and network Meta-analysis. Clin Oncol (R Coll Radiol (G B)). 2023;35(10):652–64.
Wang F, Zhan G, Chen QQ, Xu HY, Cao D, Zhang YY, Li YH, Zhang CJ, Jin Y, Ji WB, et al. Multitask deep learning for prediction of microvascular invasion and recurrence-free survival in hepatocellular carcinoma based on MRI images. Liver International: Official J Int Association Study Liver. 2024;44(6):1351–62.
Sheng X, Ji Y, Ren GP, Lu CL, Yun JP, Chen LH, Meng B, Qu LJ, Duan GJ, Sun Q, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hep Intl. 2020;14(6):1034–47.
Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, et al. Guidelines for the diagnosis and treatment of primary Liver Cancer (2022 Edition). Liver cancer. 2023;12(5):405–44.
Nitta H, Allard MA, Sebagh M, Ciacio O, Pittau G, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, et al. Prognostic value and prediction of Extratumoral Microvascular Invasion for Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26(8):2568–76.
Yu Y, Wang XH, Hu WJ, Chen DH, Hu ZL, Li SQ, Patterns. Risk factors, and outcomes of recurrence after Hepatectomy for Hepatocellular Carcinoma with and without Microvascular Invasion. J Hepatocellular Carcinoma. 2024;11:801–12.
Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26(5):1474–93.
Shindoh J, Kobayashi Y, Kawamura Y, Akuta N, Kobayashi M, Suzuki Y, Ikeda K, Hashimoto M. Microvascular Invasion and a size cutoff value of 2 cm Predict Long-Term Oncological Outcome in multiple Hepatocellular Carcinoma: Reappraisal of the American Joint Committee on Cancer Staging System and Validation using the Surveillance, Epidemiology, and end-results database. Liver cancer. 2020;9(2):156–66.
Zhang J, Zeng F, Jiang S, Tang H, Zhang J. Preoperative prediction model of microvascular invasion in patients with hepatocellular carcinoma. HPB: Official J Int Hepato Pancreato Biliary Association. 2023;25(1):45–53.
Galdino-Vasconcelos MR, Feijó MS, Ferro HM, Gomes ACR, De Almeida Santos ME, Ferreira G, Jorge F, Trevizoli N, Diaz LG, De Campos PB et al. Preoperative Alpha-Fetoprotein and Radiological Total Tumor Diameter as Predictors of Hepatocellular Carcinoma Recurrence After Liver Transplantation. Transplantation proceedings. 2022; 54(5):1333–1340.
Xia F, Huang Z, Zhang Q, Ndhlovu E, Zhang M, Chen X, Zhang B, Zhu P. Early-stage ruptured Hepatocellular Carcinoma with different tumor diameters: small tumors have a better prognosis. Front Oncol. 2022;12:865696.
Usta S, Kayaalp C. Tumor Diameter for Hepatocellular Carcinoma: why should size matter? J Gastrointest cancer. 2020;51(4):1114–7.
Arvanitakis K, Mitroulis I, Germanidis G. Tumor-Associated neutrophils in Hepatocellular Carcinoma Pathogenesis, Prognosis, and Therapy. Cancers. 2021; 13(12).
Xu C, Wu F, Du L, Dong Y, Lin S. Significant association between high neutrophil-lymphocyte ratio and poor prognosis in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Front Immunol. 2023;14:1211399.
Zhou L, Rui JA, Zhou WX, Wang SB, Chen SG, Qu Q. Edmondson-Steiner grade: a crucial predictor of recurrence and survival in hepatocellular carcinoma without microvascular invasio. Pathol Res Pract. 2017;213(7):824–30.
Becker-Assmann J, Fard-Aghaie MH, Kantas A, Makridis G, Reese T, Wagner KC, Petersen J, Buggisch P, Stang A, von Hahn T, et al. Diagnostic and prognostic significance of α-fetoprotein in hepatocellular carcinoma. Der Chirurg; Z fur alle Gebiete Der Operativen Medizen. 2020;91(9):769–77.
Loosen SH, Kostev K, Demir M, Luedde M, Keitel V, Luedde T, Roderburg C. An elevated FIB-4 score is associated with an increased incidence of liver cancer: a longitudinal analysis among 248,224 outpatients in Germany. Eur J Cancer. 2022;168:41–50.
Tian S, Chen Y, Zhang Y, Xu X. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J Clin Lab Anal. 2023;37(1):e24823.
Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, et al. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol. 2019;29(11):5791–803.
Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg (London England). 2020;80:243–55.
Zeindler J, Hess GF, von Heesen M, Aegerter N, Reber C, Schmitt AM, Muenst S, Bolli M, Soysal SD, Kollmar O. Anatomic versus non-anatomic liver resection for hepatocellular carcinoma-A European multicentre cohort study in cirrhotic and non-cirrhotic patients. Cancer Med. 2024;13(5):e6981.
Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143(4):469–75.
Famularo S, Ceresoli M, Giani A, Ciulli C, Pinotti E, Romano F, Braga M, De Carlis L, Gianotti L. Is it just a Matter of Surgical extension to achieve the cure of Hepatocarcinoma? A Meta-analysis of propensity-matched and randomized studies for anatomic Versus Parenchyma-Sparing Liver Resection. J Gastrointest Surgery: Official J Soc Surg Aliment Tract. 2021;25(1):94–103.
Su CM, Chou CC, Yang TH, Lin YJ. Comparison of anatomic and non-anatomic resections for very early-stage hepatocellular carcinoma: the importance of surgical resection margin width in non-anatomic resection. Surg Oncol. 2021;36:15–22.
Acknowledgements
The authors would like to thank all the doctors, nurses and patients, who contributed to this study.
Funding
This study was supported by the National Natural Science Foundation of China (82203487).
Author information
Authors and Affiliations
Contributions
Xi Wang, Xinqun Chai, and Qinjunjie Chen conceived and designed the study. Xi Wang, Ruiya Tang and Ji Zhang collected clinical data and performed statistical analysis. Xi Wang and Qinjunjie Chen performed the research and drafted the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. All procedures were performed in accordance with the Declaration of Helsinki. Informed consent was waived by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China, because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Chai, X., Zhang, J. et al. Nomograms established for predicting microvascular invasion and early recurrence in patients with small hepatocellular carcinoma. BMC Cancer 24, 929 (2024). https://doi.org/10.1186/s12885-024-12655-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12655-2