Abstract
Background and aims
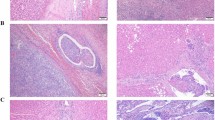
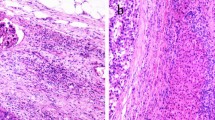
Microvascular invasion (MVI) is a key pathological factor that severely affects the postoperative prognosis of patients with hepatocellular carcinoma (HCC). However, no MVI classification schemes based on standardized gross sampling protocols of HCC are available at present.
Methods
119 HCC specimens were sampled at multiple sites (3-, 7-, and 13 points) for the optimum MVI detection rate. 16,144 resected HCCs were graded as M0, M1 or M2 by adopting three-tiered MVI grading (MVI-TTG) scheme based on the seven-point sampling protocol (SPSP). Survival analyses were performed on 2573 patients to explore the advantages of MVI-TTG.
Results
The MVI detection rate determined by SPSP was significantly higher than that determined by the 3-point sampling method (34.5% vs. 47.1%, p = 0.048), but was similar to that determined by the 13-point sampling method (47.1% vs. 51.3%, p = 0.517). Among 16,144 resected HCCs, the proportions of M0, M1 and M2 specimens according to SPSP were 53.4%, 26.2% and 20.4%, respectively. Postoperative survival analysis in 2573 HCC patients showed that the 3-year recurrence rates in M0, M1 and M2 MVI groups were 62.5%, 71.6% and 86.1%, respectively (p < 0.001), and the corresponding 3-year overall survival (OS) rates were 94.1%, 87.5% and 67.0%, respectively (p < 0.001). M1 grade was associated with early recurrence, while M2 grade was associated with both early and late recurrence. MVI-TTG had a larger area under the curve and net benefit rate than the two-tiered MVI grading scheme for predicting time to recurrence and OS.
Conclusions
SPSP is a practical method to balance the efficacy of sampling numbers and MVI detection rates. MVI-TTG based on SPSP is a better prognostic predictor than the two-tiered MVI scheme. The combined use of SPSP and MVI-TTG is recommended for the routine pathological diagnosis of HCC.





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2019;394:1145–1158.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579.
Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin. 2017;67:273–289.
Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–2047.
Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284–1293.
Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology (Baltimore, MD). 2015;61:191–199.
Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000–4013.
Wang H, Qian YW, Wu MC, Cong WM. Liver resection is justified in patients with BCLC intermediate stage hepatocellular carcinoma without microvascular invasion. J. Gastrointest. Surg. 2020;24(12):2737–47.
Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382.
Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, et al. Microvascular invasion in small-sized hepatocellular carcinoma: significance for outcomes following hepatectomy and radiofrequency ablation. Anticancer Res. 2018;38(2):1053–1060.
Xiao H, Chen ZB, Jin HL, Li B, Xu LX, Guo Y, et al. Treatment selection of recurrent hepatocellular carcinoma with microvascular invasion at the initial hepatectomy. Am J Transl Res. 2019;11:1864–1875.
Hu HT, Wang Z, Kuang M, Wang W. Need for normalization: the non-standard reference standard for microvascular invasion diagnosis in hepatocellular carcinoma. World J Surg Oncol. 2018;16:50.
Ratziu V, Bugianesi E, Dixon J, Fassio E, Ekstedt M, Charlotte F, et al. Histological progression of non-alcoholic fatty liver disease: a critical reassessment based on liver sampling variability. Aliment Pharmacol Ther. 2007;26:821–830.
Gill US, Pallett LJ, Kennedy PTF, Maini MK. Liver sampling: a vital window into HBV pathogenesis on the path to functional cure. Gut. 2018;67:767–775.
Pons E, Braun LM, Hunink MG, Kors JA. Natural language processing in radiology: a systematic review. Radiology. 2016;279:329–343.
Murff HJ, FitzHenry F, Matheny ME, Gentry N, Kotter KL, Crimin K, et al. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA. 2011;306:848–855.
Nadkarni PM, Ohnomachado L, Chapman WW. Natural language processing: an introduction. J Am Med Inform Assoc. 2011;18:544–551.
Yim W, Yetisgen M, Harris WP, Kwan SW. Natural language processing in oncology: a review. JAMA Oncol. 2016;2:797–804.
Torbenson MS. Liver. In. Westra WH, Phelps TH, Hruban RH, Isacson C. Surgical Pathology Dissection. An Illustrated Guide. 2nd Ed, Springer-Verlag New York Inc, 2003;76–81.
Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279–9287.
Yoo B, Fuchs BC, Medarova Z. New directions in the study and treatment of metastatic cancer. Front Oncol. 2018;8:258.
Zhou XD, Tang ZY, Ma ZC, Fan J, Wu ZQ, Qin LX, et al. Twenty-year survivors after resection for hepatocellular carcinoma-analysis of 53 cases. J Cancer Res Clin Oncol. 2009;135:1067–1072.
Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113.
Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38.
Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49:344–354.
Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074–2081.
Wei W, Jian PE, Li SH, Guo ZX, Zhang YF, Ling YH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (London). 2018;38(1):61.
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, MD). 2018;67:358–380.
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370.
Chen ZH, Zhang XP, Wang H, Chai ZT, Sun JX, Guo WX, et al. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB. 2019;21:935–944.
Huo TI, Liu PH, Hsu CY. Detecting microvascular invasion in HCC with contrast-enhanced MRI: is it a good idea? J Hepatol. 2018;68:862–263.
Cong WM, Dong H, Zhu YY, et al. Malignant tumors of the liver and intrahepatic bile ducts. In: Cong WM. Surgical pathology of hepatobiliary tumors. 1st ed. Springer Nature Singapore Pte Ltd. and People’s Medical Publishing House; 2017:145–282
Fujita N, Aishima S, Iguchi T, Mano Y, Taketomi A, Shirabe K, et al. Histologic classification of microscopic portal venous invasion to predict prognosis in hepatocellular carcinoma. Hum Pathol. 2011;42:1531–1538.
Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855.
Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–1009.
Hidaka M, Eguchi S, Okuda K, Beppu T, Shirabe K, Kondo K, et al. Impact of anatomical resection for hepatocellular carcinoma with microportal invasion (vp1): a multi-institutional study by the kyushu study group of liver surgery. Ann Surg. 2020;271(2):339–346.
Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. Hepato-Pancreato-Biliary Surg. 1996;10(2):97–103.
Park NH, Chung YH, Youn KH, Song BC, Yang SH, Kim JA, et al. Close correlation of p53 mutation to microvascular invasion in hepatocellular carcinoma. J Clin Gastroenterol. 2001;33:397–401.
Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26:1474–1493.
Feng LH, Dong H, Lau WY, Yu H, Zhu YY, Zhao Y, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143:293–303.
Li Z, Lei Z, Xia Y, Li J, Wang K, Zhang H, et al. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis B virus-related hepatocellular carcinoma. JAMA Surg. 2018;153(10):e182721.
Wang B, Xia CY, Lau WY, Lu XY, Dong H, Yu WL, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg. 2013;217:1054–1062.
Wang H, Feng LH, Qian YW, Cao ZY, Wu MC, Cong WM. Does microvascular invasion in Barcelona Clinic Liver Cancer stage A multinodular hepatocellular carcinoma indicate early-stage behavior? Ann Transl Med. 2019;7(18):428.
Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7:418–428.
Yang P, Si A, Yang J, Cheng Z, Wang K, Li J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165:721–730.
Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of surgical margin on the prognosis of early hepatocellular carcinoma (≤5 cm): a propensity score matching analysis. Front Med 2020;7(139).
Wang L, Wang W, Yao X, Rong W, Wu F, Chen B, et al. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget. 2017;8:79971–79981.
Acknowledgements
We would like to thank Prof. Han-Lin Wang (Department of Pathology and Laboratory Medicine, University of California at Los Angeles, USA) and Assoc Prof. Xu-Chen Zhang (Department of Pathology, Yale University School of Medicine, USA) for their constructive comments on this article.
Funding
This work is supported by National Natural Science Foundation of China (Grant No. 81472278), Funds for Creative Research Groups of National Natural Science Foundation of China (Grant No. 81521091), and National Key R&D Program of China (Grant No. 2016YFC0902400).
Author information
Authors and Affiliations
Consortia
Contributions
Concept and design: W-MC, XD, S-PL. Administrative support: W-MC, XD, S-PL. Data collection and acquisition: XS, YJ, G-PR, C-LL, J-PY, L-HC, BM, L-JQ, G-JD, QS, X-QY, S-SL, JY, BL, Z-BW, J-HZ, YS, X-SQ, LW, Z-SL, JC, C-YX, SH, C-YL, E-WX, J-SG, CP, DK, RQ, H-WG, Z-DW, QZ, HW. Data analysis: L-XL, XZ, HW, BW, W-JZ. Writing of article: XS, YJ, HW, W-MC. Final approval of manuscript: all authors
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest from financial, consulting, institutional or other relationships regarding this manuscript. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committees of all participating medical centers. Informed consent was obtained from all patients for their data to be used for research. The patients did not receive financial compensation
Ethical approval
TThis study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committees of all participating medical centers.
Informed consent
Informed consent was obtained from all patients for their data to be used for research. The patients did not receive financial compensation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12072_2020_10111_MOESM2_ESM.pdf
Supplementary Figure 2. Changes in the detection rate of MVI in HCC tissues before and after using SPSP in the LCPGC. (PDF 7 KB)
Rights and permissions
About this article
Cite this article
Sheng, X., Ji, Y., Ren, GP. et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int 14, 1034–1047 (2020). https://doi.org/10.1007/s12072-020-10111-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10111-4