Abstract
Objective
Single nucleotide polymorphisms (SNPs) are common in genes and can lead to dysregulation of gene expression in tissues, which can affect carcinogenesis. Many studies reporting the association between xeroderma pigmentosum group D (XPD) polymorphisms of rs13181 and rs1799793 with oral cancer risk, but with conflicting and inconclusive results.
Methods
We performed a comprehensive and systematic search through the PubMed, Elsevier, Web of science, and Embase databases, twelve studies were included in the meta-analysis to determine whether XPD rs13181 and rs1799793 polymorphism contributed to the risk of oral cancer.
Results
The pooled date indicated a significant association between the rs13181 polymorphism and oral cancer risk for the allele comparison model (odds ratio, OR = 1.60, 95% confidence intervals, CI = 1.09–2.35, P = 0.02), the dominant model (OR = 1.74, 95% CI = 1.08–2.82, P = 0.02), and the heterozygote model (OR = 1.59, 95% CI = 1.02–2.49, P = 0.04). For the XPD rs1799793 polymorphism, it is not associated with the incidence of oral cancer under any model. Subgroup analyses based on ethnicity indicated that the rs13181 polymorphism increased the risk of oral cancer among Asians according to the allele comparison model (OR = 1.97, 95% CI = 1.10–3.51, P = 0.02), the dominant model (OR = 2.35, 95% CI = 1.25–4.44, P = 0.008), the heterozygote model (OR = 2.05, 95% CI = 1.15–3.66, P = 0.01), and the homozygous model (OR = 2.47, 95% CI = 1.06–5.76, P = 0.04).
Conclusion
Our meta-analysis suggests a positive correlation between XPD rs13181polymorphism and the development of oral cancer among Asians, but a negative correlation among Caucasians populations.
Similar content being viewed by others
Introduction
In the last few decades, there has been an increasing incidence of oral cancer worldwide, with an estimated 377,713 new cases and 177,757 deaths recorded in 2020 [1]. The development of oral cancer is influenced by many factors such as diet and nutrition, familial and genetic predisposition, tobacco, alcohol, use of mouthwash, radiation, oral thrush, viruses, syphilis, immunosuppression, dental factors, occupational risks, and mate [2]. The genetic susceptibility factors of oral cancer are determined by family inheritance and ethnic characteristics, and many studies have shown that genetic polymorphism plays an important role in the occurrence and development of oral cancer [3,4,5,6]. Single nucleotide polymorphisms (SNPs) are common in genes and can lead to dysregulation of gene expression in tissues, which can affect carcinogenesis [5].
Xeroderma pigmentosum group D (XPD), also known as excision repair cross-complementation rodent repair deficiency group 2 (ERCC2), is located on chromosome 19q13.3, comprises of 23 exons and encodes 760 amino acids [7]. The XPD gene functions in the nucleotide excision repair (NER) pathway, alterations in this gene can cause defective DNA repair efficiency, ultimately leading to genomic instability and carcinogenesis [8]. Two SNPs in XPD have been shown to be involved in susceptibility to oral cancer: codon 312 (G > A substitution at position 23,951, exon 10, Asp > Asn, rs1799793) and codon 751 (A > C substitution at nucleotide position 35,931, exon 23, Lys > Gln, rs13181) [9]. The Lys751Gln variant affects an ATP-binding site of XPD and impairs its helicase activity, which is important for NER, but does not affect its transcriptional activity [7]. The function of Asp312Asn remains to be elucidated. Many studies have reported the association between polymorphisms of rs13181 and rs1799793 with oral cancer risk, but with conflicting and inconclusive results [7, 8, 10,11,12,13,14,15,16,17,18,19]. Thus, we performed this comprehensive meta-analysis to better illustrate the relationship between XPD polymorphism and oral cancer risk.
Materials and methods
Publication search strategy
A comprehensive and systematic search through the PubMed, Elsevier, Web of science, and Embase databases was performed using the following terms: “XPD” or “ERCC2”, “polymorphism” or “gene mutation” or “gene variation”, “oral cancer”, “oral neoplasms”, “mouth neoplasms” or “oral carcinoma”. The last search was updated on May 19th, 2024. All relevant publications were reviewed and the reference lists of articles were also searched for potentially relevant publications. There was no language or sample size limitations in the included studies. The study was registered in the International prospective register of systematic review (ID: 550,899).
Inclusion and exclusion criteria
Studies included should meet the following criteria: (1) cohort study or case–control study; (2) evaluation of XPD polymorphism and oral cancer risk; and (3) sufficient data to examine odds ratio (OR) with 95% confidence interval (95% CI). Major criteria for exclusion were as follows: (1) oral potentially malignant disorders not for oral cancer research; (2) studies not focused on XPD polymorphism; (3) studies with insufficient data for analysis or duplicated data; (4) reviews or meta-analyses; (5) studies where the distribution of genotypes among controls is not in Hardy–Weinberg equilibrium.
Data extraction
The eligible data in the studies were extracted by two investigators, and consensus was reached through discussion when divergences appeared. The following information was extracted: first author’s name, year of publication, ethnicity, numbers of cases and controls with the AA, AC, and CC genotypes for rs13181, the GG, GA, and AA genotypes for rs1799793, and the sample size of cases and controls. The ethnic populations were classified as either Asian or Caucasian.
Statistical analysis
We evaluated the risk using the allele comparison model (A vs. G for rs1799793; C vs. A for rs13181), the dominant model (GA + AA vs. GG for rs1799793; AC + CC vs. AA for rs13181), the recessive model (AA vs. GA + GG for rs1799793; CC vs. AC + AA for rsrs13181), the heterozygote model (GA vs. GG for rs1799793; AC vs. AA for rs13181), and the homozygote model (AA vs. GG for rs1799793; CC vs. AA for rs13181). Odds ratio (OR) with 95% confidence intervals (CI) were analyzed to measure the strength of association between the XPD rs13181 and rs1799793 polymorphism with oral cancer risk. The weighted mean difference with 95% CI was calculated. The statistical significance of the pooled OR was assessed using a Z test with a two-tailed P value of < 0.05 considered to be statistically significant. Stratified analysis by ethnicity was also carried out. Statistical heterogeneity, funnel plots, sensitivity analysis, and publication bias were analyzed as previously described [20]. All statistical tests for this meta-analysis were performed with Review Manager (RevMan) [Computer program]. Version5.4, The Cochrane Collaboration, 2020. and Stata Statistical Software 12 (StataCorp., T.X., USA).
Results
Characteristics of included studies
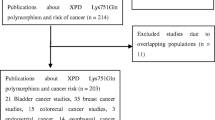
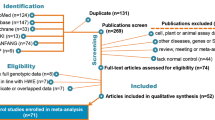
A total of one hundred and eighty-two records were identified based on the search strategy, and two additional records were added after screening reference lists. One hundred and thirteen records were screened, and twelve studies were included in this meta-analysis [7, 8, 10,11,12,13,14,15,16,17,18,19] (Fig. 1). A total of 1725 patients with oral cancer and 1833 controls were tested for the XPD rs13181 polymorphism, of which 476 patients with oral tumors and 491 controls were Caucasian (Table 1). Three studies including 659 oral cancer patients and 741 controls, focused on XPD rs1799793 polymorphism [7, 15, 19] (Table 2).
Quantitative synthesis
The pooled date indicated a significant association between the rs13181 polymorphism and oral cancer risk in the allele comparison model (OR = 1.60, 95% CI = 1.09–2.35, P = 0.02, Fig. 2), dominant model (OR = 1.74, 95% CI = 1.08–2.82, P = 0.02, Fig. 3), and heterozygote model (OR = 1.59, 95% CI = 1.02–2.49, P = 0.04, Fig. 4), but not in the recessive model (OR = 1.55, 95% CI = 0.92–2.59, P = 0.10, Fig. 5) and homozygous model (OR = 1.92, 95% CI = 1.00-3.68, P = 0.05, Fig. 6). Subgroup analyses were carried out according to the ethnicity, revealing that the rs13181 polymorphism increased the risk of oral cancer among Asians in the allele comparison model (OR = 1.97, 95% CI = 1.10–3.51, P = 0.02, Fig. 2), dominant model (OR = 2.35, 95% CI = 1.25–4.44, P = 0.008, Fig. 3), heterozygote model (OR = 2.05, 95% CI = 1.15–3.66, P = 0.01, Fig. 4), and homozygous model (OR = 2.47, 95% CI = 1.06–5.76, P = 0.04, Fig. 6). No association was found between the rs13181 polymorphism and oral cancer risk among the Caucasian population. For the XPD rs1799793 polymorphism under any model, there was no association with the incidence of oral cancer (Fig. 7).
Sensitivity analysis
In the sensitivity analysis, each individual study included in the meta-analysis was sequentially removed to observe its influence on the pooled ORs. We found that two studies significantly affected the pooled OR value [8, 10], suggesting relative instability in the results of this meta-analysis (Fig. 8 for allele model).
Publication bias
The symmetric shape of funnel plots suggested low publication bias (Fig. 9 for allele model). Begger’s funnel plot and Egger’s test were also performed. The statistical results showed no significant publication bias (P = 0.064 for allele model). Egger’s test was also conducted to assess the publication bias (P = 0.066 for allele model).
Discussion
Defects in DNA repair mechanisms can lead to genomic instability and increased cell proliferation, which are significant factors in caner development [21]. XPD is a crucial component of the mammalian transcription factor II H (TFIIH), involved in eukaryotic transcription initiation and DNA nucleotide excision repair processes. Research suggests that XPD polymorphisms directly impact TFIIH complex activity, leading to abnormal DNA repair and transcription defects [22]. Studies have linked XPD gene polymorphisms to various malignancies, including liver cancer, gastrointestinal tumors, and oral cancer [10, 23, 24].
To our knowledge, this meta-analysis is the first comprehensive examination of XPD rs13181 and rs1799793 polymorphisms in relation to oral cancer risk. Our findings indicate an association between XPD rs13181, but not rs1799793, polymorphism and oral cancer risk, particularly among Asian populations. The genetic polymorphism may increase the risk of oral cancer by 1.59 to1.74 times. Interestingly, our results were not consistent with those of previous meta-analyses. While pooled odds ratio from a study comprising 1093 cases and 2637 controls found no association between XPD Lys751Gln polymorphism and oral cancer risk across all genetic models [21], another meta-analysis including 1202 cases and 1145 controls indicated no significant associations between XPD rs1799793 and rs13181 polymorphisms and overall oral cancer risk. However, the rs13181 polymorphism might be associated with oral leukoplakia risk [9]. Our meta-analysis, incorporating 12 studies with a total of 1725 cases and 1833 controls, offers more extensive data than previous analyses. However, limitations persist. Limited data from Caucasian populations hindered subgroup analysis, as only one relevant study was found in a meta-analysis [9], and incomplete data were noted in another [21, 25].
Early detection and treatment of oral lesions can reduce the progression to oral cancer [12]. Our study suggests that screening for XPD rs13181 polymorphism in Asian populations may aid in early detection of oral cancer, facilitating timely intervention. Moreover, XPD rs13181 and rs1799793 polymorphisms may predict clinical outcomes in oral cancer patients undergoing postoperative radiotherapy [26]. Additionally, these polymorphisms could influence the clinical sensitivity of platinum-based chemotherapy [7], though limited data prevented meta-analysis.
While our meta-analysis provides valuable insights, it has limitations. Sensitivity analysis revealed relative instability, possibly due to two influential studies [8, 10], necessitating further more case-control studies. Factors such as gender, family history, environmental influences, and lifestyle, including tobacco and alcohol consumption, were not evaluated in our analysis, despite their known association with oral cancer [12]. Additionally, the sample size was relatively small, though larger than in previous meta-analyses [9, 21]. Although no publication bias was observed, positive results may be more likely to be published, potentially affecting the meta-analysis [20]. Despite these limitations, our study offers a comprehensive exploration of the relationship between XPD polymorphisms and oral cancer susceptibility.
Conclusion
In summary, our meta-analysis suggested that the XPD rs13181 polymorphism, but not rs1799793, is associated with oral cancer risk. Future studies with well-designed and larger population studies are needed to confirm these findings.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SNPs:
-
Single nucleotide polymorphisms
- XPD:
-
Xeroderma pigmentosum group D
- ERCC2:
-
Excision repair cross-complementation rodent repair deficiency group 2
- OR:
-
Odd ratio
- NER:
-
Nucleotide excision repair
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(2):458–63.
Hsieh MJ, Lo YS, Ho HY, Lin CC, Chuang YC, Chen MK. The Interaction between CLSPN Gene polymorphisms and Alcohol Consumption contributes to oral Cancer progression. Int J Mol Sci 2024, 25(2).
Yeh JC, Chen YT, Chou YE, Su SC, Chang LC, Chen YL, Lin CW, Yang SF. Interactive effects of CDKN2B-AS1 gene polymorphism and habitual risk factors on oral cancer. J Cell Mol Med. 2023;27(21):3395–403.
Tiwari S, Pandey R, Kumar V, Das S, Gupta V, Vishwakarma S, Nema R, Sindhuja T, Hashmi S, Kumar A. Association of single nucleotide polymorphism miRNA-146a (rs2910164) with increased predisposition to oral squamous cell carcinoma in central India population. Cancer Biomark A. 2023;38(2):203–14.
Mumlek I, Ozretić P, Sabol M, Leović M, Glavaš-Obrovac L, Leović D, Musani V. BIRC5 Gene Polymorphisms Are Associated with a higher stage of local and Regional Disease in oral and oropharyngeal squamous cell carcinomas. Int J Mol Sci 2023, 24(24).
Tejasvi MA, Maragathavalli G, Kumar PU, Ramakrishna M, Raghavan V, Ck AA. Impact of ERCC2 gene polymorphisms on OSCC susceptibility and clinical characteristics. Glob Med Genet. 2020;7(4):121–7.
Shrivastava P, Gosavi S, Ghatge D, Naik A, Marlapalle A, Krishna A. Comparative evaluation of XPD and XPG gene polymorphism in oral squamous cell carcinoma and tobacco chewers: an observational study. J oral Maxillofacial Pathology: JOMFP. 2022;26(4):518–23.
Zhang E, Cui Z, Xu Z, Duan W, Huang S, Tan X, Yin Z, Sun C, Lu L. Association between polymorphisms in ERCC2 gene and oral cancer risk: evidence from a meta-analysis. BMC Cancer. 2013;13:594.
Tata NH, Kshirsagar A, Nangare N. Characterization of genetic polymorphisms in oral cancer-related genes pertaining to oxidative stress, carcinogen detoxifying, and DNA repair: a case-control study. J Cancer Res Ther. 2022;18(4):1023–9.
Galíndez MF, Carrica A, Zarate AM, Secchi D, Don J, Barra JL, Brunotto M. DNA repair, NFKβ, and TP53 polymorphisms associated with potentially malignant disorders and oral squamous cell carcinoma in Argentine patients. Oral Surg oral Med oral Pathol oral Radiol. 2021;131(3):339–46.
Nigam K, Yadav SK, Samadi FM, Bhatt ML, Gupta S, Sanyal S. Risk modulation of oral pre Cancer and Cancer with polymorphisms in XPD and XPG genes in North Indian Population. Asian Pac J cancer Prevention: APJCP. 2019;20(8):2397–403.
Avci H, Iplik ES, Aydemir L, Acar S, Kiyak E, Unur M, Cakmakoglu B. Are XPD and XPG gene variants related to the mechanism of oral squamous cell carcinoma? Cell Mol Biol. 2018;64(15):94–9.
dos Santos Pereira J, Fontes FL, de Medeiros SR, de Almeida Freitas R, de Souza LB, da, Costa Miguel MC. Association of the XPD and XRCC3 gene polymorphisms with oral squamous cell carcinoma in a Northeastern Brazilian population: A pilot study. Arch Oral Biol 2016, 64:19–23.
Majumder M, Sikdar N, Ghosh S, Roy B. Polymorphisms at XPD and XRCC1 DNA repair loci and increased risk of oral leukoplakia and cancer among NAT2 slow acetylators. Int J Cancer. 2007;120(10):2148–56.
Bau D-T, Tsai M-H, Huang C-Y, Lee C-C, Tseng H-C, Lo Y-L, Tsai Y, Tsai F-J. Relationship between polymorphisms of nucleotide excision repair genes and oral cancer risk in Taiwan: evidence for modification of smoking habit. Chin J Physiol. 2007;50(6):294–300.
Ramachandran S, Ramadas K, Hariharan R, Rejnish Kumar R, Radhakrishna Pillai M. Single nucleotide polymorphisms of DNA repair genes XRCC1 and XPD and its molecular mapping in Indian oral cancer. Oral Oncol. 2006;42(4):350–62.
Kietthubthew S, Sriplung H, Au WW, Ishida T. Polymorphism in DNA repair genes and oral squamous cell carcinoma in Thailand. Int J Hyg Environ Health. 2006;209(1):21–9.
Buch S, Zhu B, Davis AG, Odom D, Siegfried JM, Grandis JR, Romkes M. Association of polymorphisms in the cyclin D1 and XPD genes and susceptibility to cancers of the upper aero-digestive tract. Mol Carcinog. 2005;42(4):222–8.
Zhong G, Luo X, Li J, Liao Y, Gui G, Sheng J. Update on the association of miR-149 rs2292832 C > T polymorphism with gastric cancer risk: a meta-analysis study of gastrointestinal cancers. Medicine. 2023;102(38):e35202.
Cui J, Li D, Shen L, Zhang W, Xu X. XPD Lys751Gln polymorphism is not associated with oral cancer risk: evidence from a meta-analysis. Tumour Biology: J Int Soc Oncodevelopmental Biology Med. 2014;35(7):6335–41.
Sun GF, Ding H. NOP2-mediated m5C methylation of XPD is associated with hepatocellular carcinoma progression. Neoplasma. 2023;70(3):340–9.
Zhou Q, Fu Y, Wen L, Deng Y, Chen J, Liu K. XPD polymorphisms and risk of Hepatocellular Carcinoma and gastric Cancer: a Meta-analysis. Technol Cancer Res Treat. 2021;20:1533033821990046.
Du H, Guo N, Shi B, Zhang Q, Chen Z, Lu K, Shu Y, Chen T, Zhu L. The effect of XPD polymorphisms on digestive tract cancers risk: a meta-analysis. PLoS ONE. 2014;9(5):e96301.
Matullo G, Dunning AM, Guarrera S, Baynes C, Polidoro S, Garte S, Autrup H, Malaveille C, Peluso M, Airoldi L, et al. DNA repair polymorphisms and cancer risk in non-smokers in a cohort study. Carcinogenesis. 2006;27(5):997–1007.
Mahimkar MB, Samant TA, Kannan S, Tulsulkar J, Pai PS, Anantharaman D. Polymorphisms in GSTM1 and XPD genes predict clinical outcome in advanced oral cancer patients treated with postoperative radiotherapy. Mol Carcinog. 2012;51:E94–103.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Project development: Wu Long; Data collection or management: Wu Long; Wenli Zeng; Wanting Xu; Data analysis and Interpretation: Wu Long; Wenli Zeng; Wanting Xu; Manuscript writing: Wenli Zeng; Manuscript editing: Wu Long; Study Supervision: Wu Long. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, W., Xu, W. & Long, W. The association between XPD rs13181 and rs1799793 polymorphism and oral cancer risk: evidence from a meta-analysis. BMC Cancer 24, 738 (2024). https://doi.org/10.1186/s12885-024-12503-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12503-3