Abstract
Background
The objective of the present study is to comprehensively evaluate the impact of the rs1800975 A/G polymorphism within the human xeroderma pigmentosum group A (XPA) gene on susceptibility to overall cancer by performing an integrative analysis of the current evidence.
Methods
We retrieved possible relevant publications from a total of six electronic databases (updated to April 2020) and selected eligible case–control studies for pooled assessment. P-values of association and odds ratio (OR) were calculated for the assessment of association effect. We also performed Begg’s test and Egger’s test, sensitivity analysis, false-positive report probability (FPRP) analysis, trial sequential analysis (TSA), and expression/splicing quantitative trait loci (eQTL/sQTL) analyses.
Results
In total, 71 case–control studies with 19,257 cases and 30,208 controls from 52 publications were included for pooling analysis. We observed an enhanced overall cancer susceptibility in cancer cases compared with negative controls in the Caucasian subgroup analysis for the genetic models of allelic G vs. A, carrier G vs. A, homozygotic GG vs AA, heterozygotic AG vs. AA, dominant AG + GG vs. AA and recessive GG vs. AA + AG (P < 0.05, OR > 1). A similar positive conclusion was also detected in the “skin cancer” or “skin basal cell carcinoma (BCC)” subgroup analysis of the Caucasian population. Our FPRP analysis and TSA results further confirmed the robustness of the conclusion. However, our eQTL/sQTL data did not support the strong links of rs1800975 with the gene expression or splicing changes of XPA in the skin tissue. In addition, even though we observed a decreased risk of lung cancer under the homozygotic, heterozygotic and dominant models (P < 0.05, OR < 1) and an enhanced risk of colorectal cancer under the allelic, homozygotic, heterozygotic, dominant (P < 0.05, OR > 1), our data from FPRP analysis and another pooling analysis with only the population-based controls in the Caucasian population did not support the strong links between the XPA rs1800975 A/G polymorphism and the risk of lung or colorectal cancer.
Conclusions
Our findings provide evidence of the close relationship between the XPA rs1800975 A/G polymorphism and susceptibility to skin cancer in the Caucasian population. The potential effect of XPA rs1800975 on the risk of developing lung or colorectal cancer still merits the enrollment of larger well-scaled studies.
Similar content being viewed by others
Background
The nucleotide excision repair (NER) system participates in the removal of the bulky adducts of DNA lesions from the genome under environmental stimuli, such as UV irradiation, tobacco, alkylating agents or pollutants, and xeroderma pigmentosum group A (XPA) acts as an essential NER member [1, 2]. XPA protein, as a zinc finger DNA binding protein and an important damage verifier, can bind the NER core repair factors to identify the damage site of the DNA substrate [2,3,4]. Abnormal DNA repair mechanisms or mutated NER proteins are involved in the process of mutagenesis and oncogenesis and are often linked to a group of clinical disorders [1, 2]. The human XPA rs1800975 T/C polymorphism is a common single nucleotide polymorphism (SNP) in the 5′-untranslated region of the XPA gene [5]. In the present study, we are interested in comprehensively exploring the possible effect of the XPA rs1800975 genetic variant on the susceptibility to different cancer diseases, such as skin cancer, lung cancer, breast cancer, esophageal cancer, gastric cancer, colorectal cancer or endometrial cancer.
There are different reports with distinct conclusions regarding the genetic relationship between the XPA rs1800975 polymorphism and cancer susceptibility in varied populations. For example, the XPA rs1800975 polymorphism was reported to be related to the risk of lung cancer in Norwegian [6], Germany [7, 8] or Korean populations [9] but not in patients from Belgium [10] or the USA [11]. These results merit a comprehensive evaluation by means of a meta-analysis.
To the best of our knowledge, to date, only two meta-analyses regarding the association between the XPA rs1800975 polymorphism and susceptibility to overall cancer diseases have been previously reported in 2012 [12, 13]. Nevertheless, no more than 36 case–control studies were enrolled for the prior meta-analysis. Therefore, we performed an updated comprehensive meta-analysis in 2020 based on the guidelines of preferred reporting items for systematic reviews and meta-analyses (PRISMA) [14]. In total, 71 case–control studies following the principle of Hardy–Weinberg equilibrium (HWE) were enrolled for pooling, and a series of stratified analyses, Begg’s test, Egger’s test, sensitivity analysis, FPRP analysis and TSA test, expression pattern, eQTL and sQTL analysis were conducted.
Methods
Database retrieval
Potentially relevant publications from six online databases, including PubMed, Excerpta Medica Database (EMBASE), Cochrane, China National Knowledge Infrastructure (CNKI), WANFANG and VIP, were retrieved until April 8, 2020. We did not set up any geographical or language restrictions for publications. Additional file 1: Table S1 shows our specific search terms during the database retrieval.
Screening criteria
The articles were then screened and evaluated for eligibility, according to our screening criteria. The inclusion criteria were as follows: genotypic frequency data for the XPA rs1800975 polymorphism in both cases and controls. The exclusion criteria included duplicate information; cell, plant or animal assay data; other diseases, genes or SNPs; review, meeting or meta-analysis; lack of normal control; lack of full genotypic data; and the genotypic distribution in controls was not in line with HWE.
Data extraction and quality evaluation
We utilized a table to independently extract the basic information, including first author, publication year, country, race, genotypic distribution, cancer type, control source, genotyping method, genotype frequency, and sample size. Possible disagreements were resolved by full discussion, and missing data were obtained by attempting to contact the corresponding author via e-mail. The P value of HWE in controls was obtained by the Chi square test. We evaluated the methodological quality of studies using the criteria of the Newcastle–Ottawa quality assessment scale (NOS) with a score ranging from one to nine. If the NOS score was less than five, the study was considered to be of poor quality.
Heterogeneity and association test
If the I2 value (variation in ORs attributable to heterogeneity) > 50% and the P-value of heterogeneity < 0.05, we adopted a random-effect model for the test of association. Otherwise, a fixed-effect model was used, owing to the absence of significant interstudy heterogeneity. P-values of association, OR and 95% CI (confidence interval) were calculated for the allelic (G vs. A), carrier (G vs. A), homozygotic (GG vs AA), heterozygotic (AG vs. AA), dominant (AG + GG vs. AA) and recessive (GG vs. AA + AG) models. In addition, subgroup analyses for race, control source and genotyping method were conducted. In the subgroup analysis, a minimum of three case–control studies should be included to obtain a relatively scientific and reliable conclusion.
Publication bias assessment
Begg’s test and Egger’s test were carried out for the quantitative evaluation of potential publication bias. We finally obtained the P-values for Begg’s test and Egger’s test, Begg’s funnel plot (pseudo 95% confidence limit) and Egger’s publication bias plot. If there is a basic symmetrical funnel plot and yielded P-values were larger than 0.05, the absence of significant publication bias was suggested.
Data sensitivity
We also conducted sensitivity analyses under the above six genetic models. After the sequential removal of each case–control study, the obvious change in the estimates showed the lack of statistical stability. STATA 12.0 software (StataCorp, College Station, USA) was used for the above statistical analysis.
False-positive report probability test
As the relevant information of former studies [15,16,17], a false-positive report probability (FPRP) test was carried out for the assessment of the true genetic relationship probability under the parameters of FPRP threshold value with 0.2, power OR with 1.5, and prior probability levels with “0.25, 0.1, 0.01, 0.001, 0.0001, 0.00001″. If the FPRP value < 0.2 under the prior probability level of 0.1, a worthy outcome between XPA rs1800975 and cancer risk was considered.
Trial sequential analysis
We applied a trial sequential analysis (TSA) approach to adjust random and systematic error risk and provided the optimal sample size for pooling by means of TSA viewer software (Copenhagen Trial Unit, Copenhagen), similar to several reported studies [17,18,19]. The TSA plot with a two-sided boundary type was obtained by the parameters of type I error probability with 5%, statistical test power with 80%, and relative risk reduction with 20%. For the genetic model of AG + GG vs. AA, if the cumulative Z-curve crossed the TSA monitoring boundary and touched the line of required information size, the power of the results with robustness was regarded.
Expression pattern analysis
Based on the dataset of GTEx (Genotype-Tissue Expression) analysis release V8 (dbGaP accession phs000424.v8.p2) [20], we analyzed the expression profile of XPA gene (ENSG0000136936.10) across multiple tissues, such as heart, brain, lung, stomach or colon. Log10 [TPM (Transcripts Per Million) +1] was utilized for scale. Besides, we applied the TIMER (Tumor Immune Estimation Resource) approach [21] to compare the expression difference of the XPA gene between tumor and adjacent normal tissues across all TCGA (The Cancer Genome Atlas) tumors. Wilcoxon test was used for the assessment of statistical significance. The results were visualized by the violin plot or box-plot.
The eQTL and sQTL analysis
Based on the dataset of GTEx [20], we also analyzed the “Significant Single-Tissue” eQTL (expression quantitative trait loci) and sQTL (splicing quantitative trait loci) in all tissues, for the XPA gene and the rs1800975 SNP. The values of sample number, NES (Normalized Effect Size), p-value, m-value were obtained. When m-value was larger than 0.9, an eQTL effect was considered [22]. The violin plots of eQTL and sQTL, and multi-tissue eQTL plots of the cross-tissue meta-analysis were provided, respectively. The normalized intron-excision ratio was used for the scale of sQTL.
Results
Enrolled case–control studies
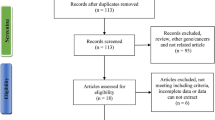
A schematic illustration of eligible case–control study selection is shown in Fig. 1. We initially obtained 400 publications from six databases. Then, duplicate publications were excluded, and the remaining 269 publications were screened. Of them, we further removed 195 publications using our screening criteria. A total of 22 full-text articles were also excluded due to “lack full genotypic data”, “not in line with HWE” or “duplicate or overlapped data”. We finally extracted a total of 71 case–control studies from 52 publications [6,7,8,9,10,11, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] for our integrated analysis. Table 1 lists the main characteristics of the enrolled case–control studies with good methodological quality (NOS score ≥ 5).
Overall meta-analysis results
As shown in Table 2, our overall meta-analysis enrolled a total of 71 case–control studies with 19,257 cases and 30,208 controls under the recessive model (GG vs. AA + AG) and 69 case–control studies with 19,039 cases and 29,707 controls under the other genetic models. The heterogeneity under the carrier G vs. A model (Table 2, I2 = 22.3%, P = 0.056) led to the utilization of a fixed-effects pooling model, and a random-effects pooling model was applied for others. For the pooling results shown in Table 2, a statistically significant difference in the susceptibility to cancer between cases and controls was detected under the allelic (P = 0.026, OR = 1.07), carrier (P = 0.009, OR = 1.04) and recessive (P = 0.001, OR = 1.12) genetic models. However, negative results were observed under other models (Table 2, P > 0.05). We failed to obtain evidence regarding the relationship between the XPA rs1800975 polymorphism and the overall risk of cancer in the overall population.
Subgroup analysis results
Next, we conducted a series of subgroup meta-analyses stratified by race, control source and genotyping method. As shown in Table 3, an increased cancer risk in cases was observed compared with negative controls in the Caucasian subgroup analysis under the models of allelic G vs. A (P < 0.001, OR = 1.12), carrier G vs. A (P = 0.001, OR = 1.08), homozygotic GG vs AA (P < 0.001, OR = 1.24), heterozygotic AG vs. AA (P = 0.046, OR = 1.10), dominant AG + GG vs. AA (P = 0.004, OR = 1.16) and recessive GG vs. AA + AG (P < 0.001, OR = 1.16). A similar positive conclusion was detected in the subgroup analysis of the “population-based control, PB” under the allelic, carrier, homozygotic and recessive models (Table 3, P < 0.05, OR > 1). For the PCR-RFLP subgroup analysis, we only observed an increased risk of cancer in the carrier (Table 3, P = 0.016, OR = 1.06) and recessive (P = 0.018, OR = 1.16) models.
As shown in Tables 4 and 5, compared with controls, a decreased lung cancer risk was detected in cases under the GG vs AA (P = 0.032, OR = 0.87), AG vs. AA (P = 0.014, OR = 0.86), AG + GG vs. AA (P = 0.021, OR = 0.87) models, but not allelic G vs. A (P = 0.155), carrier G vs. A (P = 0.345), and GG vs. AA + AG (P = 0.755) models. For the subgroup of digestive system cancer, a positive association was detected under the carrier (Table 4, P = 0.013, OR = 1.09) and recessive (Table 5, P = 0.025, OR = 1.26) models. Moreover, we observed an enhanced risk of colorectal cancer under allelic (Table 4, P = 0.021, OR = 1.20), homozygotic (P = 0.007, OR = 1.68), heterozygotic (Table 5, P = 0.041, OR = 1.46), and dominant (P = 0.016, OR = 1.54) conditions, implying the potential effect of the AG genotype of XPA rs1800975 on the risk of colorectal cancer.
Interestingly, as shown in Tables 4 and 5, we detected a significant difference between skin cancer cases and controls under the allelic (P < 0.001, OR = 1.17), carrier (P = 0.005, OR = 1.12), homozygotic (P < 0.001, OR = 1.36), heterozygotic (P = 0.029, OR = 1.18), dominant (P = 0.001, OR = 1.27), and recessive (P < 0.001, OR = 1.20) models. There was a similar positive association in the “skin BCC” subgroup under the allelic, carrier, homozygotic, dominant, and recessive models (all P < 0.05, OR > 1). These data suggested that XPA rs1800975 may be associated with a high susceptibility to skin cancer, especially skin BCC.
There were no significant differences between cases and controls in the majority of comparisons (Tables 2, 3, 4, P > 0.05), indicating that XPA rs1800975 does not seem to contribute to the risk of specific cancer types, such as breast cancer, esophageal cancer, gastric cancer, reproductive system cancer, endometrial cancer, or head and neck cancer. Forest plots of subgroup analyses by race (Fig. 2 of allelic model; Additional file 2: Fig. S1 of carrier model; Additional file 3: Fig. S2 of dominant model), control source (Additional file 4: Fig. S3 of allelic model; Additional file 5: Fig. S4 of carrier model; Additional file 6: Fig. S5 of dominant model), and cancer type (Fig. 3 of allelic model; Additional file 7: Fig. S6 of homozygotic model; Additional file 8: Fig. S7 of heterozygotic model; Additional file 9: Fig. S8 of dominant model) are presented as examples.
FRAP and TSA results
To strengthen our results in the subgroup analysis of “lung cancer”, “colorectal cancer”, and “skin cancer”, we performed the FPRP test. As shown in Table 6, under the 0.1 prior probability level, the FPRP value for lung cancer was less than 0.20 under the heterozygotic and dominant models but not the homozygotic model, suggesting the lack of notable associations. We found that the subjects in different populations or the mixed source-based controls were included for the pooling analysis of lung cancer. Considering the above positive results in the subgroup of “Caucasian” and “PB”, we also performed another pooling analysis limited to the Caucasian population. As shown in Additional file 1: Table S2, when we only included the Caucasian subjects for the pooling analysis, we did not observe positive conclusions (all P > 0.05). A similar negative conclusion was further detected in the meta-analysis using PB-based controls in the Caucasian population (Additional file 1: Table S3, P > 0.05). Collectively, this evidence did not support the strong association between lung cancer risk and XPA rs1800975.
With regard to colorectal cancer, we only observed that the FPRP value was less than 0.20 in the allelic and homozygotic models, under the prior probability level of 0.1 (Table 6). There are only three case–control studies [36, 40, 43] in the Caucasian population in the pooling analysis. After removing one study with the HB-based control [36], only two studies with 460 cases and 921 controls were enrolled for the pooling analysis (Additional file 1: Table S3). Although we observed an increased risk of colorectal cancer under the homozygotic, heterozygotic and dominant models (Additional file 1: Table S3, P < 0.05, OR > 1), this does not exceed our minimum requirement for pooling analysis, which requires at least three case–control studies. We cannot obtain a relatively scientific conclusion regarding the potential links of XPA rs1800975 and colorectal cancer risk.
As shown in Table 6, under the 0.1 prior probability level, the FPRP values for skin cancer were all less than 0.20, confirming notable associations. Caucasian subjects and PB-based controls were enrolled in all case–control studies. We further performed the TSA test, and the TSA plot in Fig. 4 shows that the cumulative Z-curve of the dominant model can cross both the lines of the TSA monitoring boundary and the required information size, suggesting a credible conclusion regarding the association between XPA rs1800975 and skin susceptibility.
Publication bias and sensitivity analysis results
For the evaluation of publication bias, the two-sided P-value of Begg’s and Egger’s test > 0.05 (Table 2) and the absence of obvious asymmetry of funnel plots under each genetic model (Fig. 5a, b show the plots of allelic model as instances) suggested no evidence of large publication bias during the pooling analysis mentioned above. In addition, we failed to detect the greatly changed values of ORs and 95% CIs through our leave-one-out sensitivity analysis (Fig. 5c for allelic model as an example).
The eQTL and sQTL analysis results
Finally, based on GTEx datasets, we analyzed the expression profile of the XPA gene in different tissues, and the correlation between the gene expression and rs1800975 SNP of XPA. As shown in Additional file 10: Fig. S9, the XPA gene is expressed in various tissues, such as the brain, colon, esophagus, lung or skin tissues, suggesting a low tissue specificity. Based on the “Significant Single-Tissue” eQTL data (Fig. 6), we observed the potential association between XPA gene expression and rs1800975 SNP, in the tissues of artery aorta (P-value = 1.8e−9), artery tibial (P-value = 1.55e−6), esophagus muscularis (P-value = 3.59e−9), muscle skeletal (P-value = 6.39e−12), but not the skin tissue of [“not sun exposed (suprapubic)”, P-value = 7.87e−1) or [“sun exposed (lower leg)”, P-value = 5.16e−1). The data of multi-tissue eQTL comparison also suggested that four tissues (artery aorta, artery tibial, esophagus muscularis, muscle skeletal) were predicted to have an eQTL effect (Fig. 7, all m-value = 1.00). Cross-tissue meta-analysis further showed a potential overall correlation between gene expression and rs1800975 SNP of XPA (Fig. 7, P-Value = 3.07e−50). In addition, our sQTL data further showed a potential association between rs1800975 SNP and the splicing changes of XPA gene in the thyroid tissue (Fig. 8).
Discussion
Although we observed a group of publications regarding the influence of XPA rs1800975 on the risk of certain specific cancers, such as lung cancer [69, 70], head and neck cancer [71], breast cancer [72], and digestive system cancer [73, 74], the evaluation strategies, study number and statistical power differed. We were interested in comprehensively exploring the impact of XPA rs1800975 on overall cancer susceptibility by pooling all currently available evidence. To date, there are only two reported meta-analyses from 2012 [12, 13] describing the association between XPA rs1800975 and susceptibility to overall cancer diseases. In the current study, we searched six online electronic databases, including PubMed, EMBASE, Cochrane, CNKI, WANFANG and VIP, with the last retrieval on April 8, 2020, to include a total of 71 case–control studies. Based on six genetic models (allelic, carrier, homozygotic, heterozygotic, dominant and recessive), a series of overall meta-analyses and subgroup analyses using the factors of race, control source and genotyping method, were used to scientifically assess the association between XPA rs1800975 polymorphism and the risk of cancer. Additionally, Begg’s test and Egger’s test, sensitivity analysis, FPRP analysis and TSA test were conducted.
In 2012, Ding et al. included a total of thirty-six case–control or case-cohort studies from twenty-eight publications to conduct a meta-analysis for the genetic effect of XPA rs1800975 on the susceptibility to overall cancer [13]. They did not detect a positive conclusion in the overall meta-analysis but a significant difference between controls and cases in the “lung cancer” subgroup analysis under the homozygotic and recessive models, the “Asian” subgroup in the dominant models, and the “skin cancer” subgroup in the homozygotic, heterozygotic, dominant and recessive models. In our updated meta-analysis, we excluded three publications in which the genotypic distribution of the control group was not in line with the HWE principle [75,76,77] and one publication related to oral premalignant lesions [78]. We also replaced one publication [79] with another one [67]. In addition, we added a total of twenty-eight publications for our new pooled analysis. In 2012, Liu et al. included twenty-four publications to conduct another meta-analysis and reported an increased colorectal cancer risk under the homozygotic and dominant models but a decreased susceptibility to lung cancer under the homozygotic and dominant models [12]. In the present study, we removed two publications owing to HWE [75, 77] and added another thirty new publications for our updated integrative analysis.
Our new findings showed a positive conclusion in the overall meta-analysis only under the carrier and recessive models, and in the “Caucasian” subgroup analysis under each model. We failed to detect a significant difference between cases and controls in the Asian population. The sample size contributes to the inconsistency with the data of Ding et al. [13].
Additionally, we detected a decreased lung cancer risk in cases under the GG vs. AA, AG vs. AA, AG + GG vs. AA models but an increased risk of colorectal cancer under the allelic, homozygotic, heterozygotic, dominant models, indicating the possible effect of the AG genotype of XPA rs1800975 on the susceptibility to colorectal cancer. These findings are partly in line with the conclusion of the above prior meta-analyses [12, 13]. Nevertheless, our data from FPRP analysis and another pooling analysis with only the population-based controls in the Caucasian population did not strongly support the protective role of the G allele within the XPA rs1800975 polymorphism in the risk of lung or colorectal cancer. Our data from the pooling analysis, FPRP analysis and TSA demonstrated a significant difference between skin cancer cases and negative controls under six genetic models, suggesting the contribution of the G allele within XPA rs1800975 to an enhanced susceptibility to skin cancer. Our eQTL and sQTL analysis data of GTEx showed that the XPA rs1800975 might not be associated with the gene expression or splicing changes of XPA in the skin tissue, suggesting the existence of other molecular mechanisms.
There are several strengths within our pooling analysis. No case–control study with poor quality was enrolled. We also excluded studies in which the genotypic contribution in the control group was not in Hardy-Weinberg equilibrium. In addition, both the absence of larger publication bias and the stability of pooling data were observed in all comparisons.
There are also several disadvantages during our analyses, which need to be discussed. First, fewer than ten case–control studies were enrolled in some comparisons, such as the subgroup meta-analysis of “breast cancer”, “gastric cancer”, “colorectal cancer”, “endometrial cancer”, “head and neck cancer”, and “skin cancer”. Therefore, several comparisons, such as subgroup analyses of “oral cancer” or “skin SCC”, were not carried out. In addition, high heterogeneity was present, and the “random-effect with DerSimonian and Laird method” was set in the overall meta-analyses under the allelic, homozygotic, heterozygotic, dominant and recessive models. There exists a decreased level of between-study heterogeneity in some subgroups of “Caucasian” (data not shown), indicating that ethnicity may be involved in the heterogeneity source.
After investigating the expression difference of XPA gene between tumor and adjacent normal tissues in TCGA project (Additional file 11: Fig. S10), we observed a higher expression level of XPA in the tissues of CHOL (Cholangiocarcinoma, P < 0.001) and LIHC (Liver hepatocellular carcinoma, P < 0.001), but a lower level in the tissues of BLCA (Bladder Urothelial Carcinoma), BRCA (Breast invasive carcinoma), KICH (Kidney Chromophobe), KIRC (Kidney renal clear cell carcinoma), KIRP (Kidney renal papillary cell carcinoma), LUAD (Lung adenocarcinoma), LUSC (Lung squamous cell carcinoma), READ (Rectum adenocarcinoma), THCA (Thyroid carcinoma), and UCEC (Uterine Corpus Endometrial Carcinoma) (all P < 0.05), compared with the corresponding control tissues. Apart from that, we predicted that the tissues of artery aorta, artery tibial, esophagus muscularis, muscle skeletal have an eQTL effect, while the thyroid tissue has a sQTL effect. Thus, it is meaningful to explore the potential genetic influence of all XPA genetic variants or the combined variants of XPA and other relevant genes (such as xeroderma pigmentosum group D, XPD) in the pathogenesis of the above tumors, arterial or muscular system-related diseases. The larger sample sizes are warranted, and the factors of age, sex, smoking, drinking, or therapy should be adjusted.
Conclusions
To summarize, our comprehensive integrative analysis data demonstrated statistical evidence on the association between the XPA rs1800975 A/G polymorphism and susceptibility to skin cancer, especially skin BCC, in the Caucasian population. The enrollment of more case–control studies following the HWE principle in diverse ethnicities will help researchers to further verify the potential genetic role of the XPA rs1800975 polymorphism in the risk of lung or colorectal cancer.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Abbreviations
- XPA:
-
Xeroderma pigmentosum group A
- OR:
-
Odd ratio
- FPRP:
-
False-positive report probability
- TSA:
-
Trial sequential analysis
- eQTL:
-
Expression quantitative trait loci
- sQTL:
-
Splicing quantitative trait loci
- BCC:
-
Basal cell carcinoma
- NER:
-
Nucleotide excision repair
- SNP:
-
Single nucleotide polymorphism
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- HWE:
-
Hardy–Weinberg equilibrium
- EMBASE:
-
Excerpta Medica Database
- CNKI:
-
China National Knowledge Infrastructure
- NOS:
-
Newcastle–Ottawa quality assessment Scale
- CI:
-
Confidence interval
- GTEx:
-
Genotype-Tissue Expression
- TPM:
-
Transcripts Per Million
- TIMER:
-
Tumor Immune Estimation Resource
- TCGA:
-
The Cancer Genome Atlas
- NES:
-
Normalized Effect Size
- CHOL:
-
Cholangiocarcinoma
- LIHC:
-
Liver hepatocellular carcinoma
- BLCA:
-
Bladder Urothelial Carcinoma
- BRCA:
-
Breast invasive carcinoma
- KICH:
-
Kidney Chromophobe
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- READ:
-
Rectum adenocarcinoma
- THCA:
-
Thyroid carcinoma
- UCEC:
-
Uterine Corpus Endometrial Carcinoma
- XPD:
-
Xeroderma pigmentosum group D
- BC:
-
Bladder cancer
- RCC:
-
Renal cell carcinoma
- SCC:
-
Squamous cell carcinoma
- LSCC:
-
Lung squamous cell carcinoma
- GCA:
-
Gastric cardiac adenocarcinoma
- LA:
-
Lung adenocarcinoma
- NSCLC:
-
Non-small cell lung cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- OSCC:
-
Oral squamous cell carcinoma
- ALL:
-
Acute lymphoblastic leukemia
- HCC:
-
Hepatocellular carcinoma
- PB:
-
Population-based control
- HB:
-
Hospital-based control
- PCR:
-
Polymerase chain reaction
- PCR-RFLP:
-
PCR-restriction fragment length polymorphism
- PCR-LDR:
-
PCR-ligase detection reaction
- MALDI-TOF-MS:
-
Matrix-assisted laser desorption/Ionization time of flight mass spectrometry
References
Sugitani N, Sivley RM, Perry KE, Capra JA, Chazin WJ. XPA: a key scaffold for human nucleotide excision repair. DNA Repair (Amst). 2016;44:123–35.
Fadda E. Role of the XPA protein in the NER pathway: a perspective on the function of structural disorder in macromolecular assembly. Comput Struct Biotechnol J. 2016;14:78–85.
Pradhan S, Sarma H, Kumar Mattaparthi VS. Investigation of the probable homo-dimer model of the Xeroderma pigmentosum complementation group A (XPA) protein to represent the DNA binding core. J Biomol Struct Dyn. 2019;37:3322–36.
Sugitani N, Shell SM, Soss SE, Chazin WJ. Redefining the DNA-binding domain of human XPA. J Am Chem Soc. 2014;136(31):10830–3.
Lawania S, Singh N, Behera D, Sharma S. Association of XPA polymorphisms towards lung cancer susceptibility and its predictive role in overall survival of North Indians. Mol Genet Genomic Med. 2018;56(4):375–96.
Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, Stangeland L, Phillips DH, Canzian F, Haugen A. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis. 2006;27(3):560–7.
Popanda O, Schattenberg T, Phong CT, Butkiewicz D, Risch A, Edler L, Kayser K, Dienemann H, Schulz V, Drings P, et al. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis. 2004;25(12):2433–41.
Butkiewicz D, Popanda O, Risch A, Edler L, Dienemann H, Schulz V, Kayser K, Drings P, Bartsch H, Schmezer P. Association between the risk for lung adenocarcinoma and a (-4) G-to-A polymorphism in the XPA gene. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2242–6.
Park JY, Park SH, Choi JE, Lee SY, Jeon HS, Cha SI, Kim CH, Park JH, Kam S, Park RW, et al. Polymorphisms of the DNA repair gene xeroderma pigmentosum group A and risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):993–7.
De Ruyck K, Szaumkessel M, De Rudder I, Dehoorne A, Vral A, Claes K, Velghe A, Van Meerbeeck J, Thierens H. Polymorphisms in base-excision repair and nucleotide-excision repair genes in relation to lung cancer risk. Mutat Res. 2007;631(2):101–10.
Sakoda LC, Loomis MM, Doherty JA, Julianto L, Barnett MJ, Neuhouser ML, Thornquist MD, Weiss NS, Goodman GE, Chen C. Germ line variation in nucleotide excision repair genes and lung cancer risk in smokers. Int J Mol Epidemiol Genet. 2012;3(1):1–17.
Liu J, Zhang Z, Cao XL, Lei DP, Wang ZQ, Jin T, Pan XL. XPA A23G polymorphism and susceptibility to cancer: a meta-analysis. Mol Biol Rep. 2012;39(6):6791–9.
Ding D, Zhang Y, Yu H, Guo Y, Jiang L, He X, Ma W, Zheng W. Genetic variation of XPA gene and risk of cancer: a systematic review and pooled analysis. Int J Cancer. 2012;131(2):488–96.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–42.
He J, Wang MY, Qiu LX, Zhu ML, Shi TY, Zhou XY, Sun MH, Yang YJ, Wang JC, Jin L, et al. Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Mol Carcinog. 2013;52(Suppl 1):E70–9.
Meng J, Wang S, Zhang M, Fan S, Zhang L, Liang C. TP73 G4C14-A4T14 polymorphism and cancer susceptibility: evidence from 36 case-control studies. Biosci Rep. 2018;38(6):1–14.
Liu X, Zhao Y, Li Y, Zhang J. Quantitative assessment of lncRNA H19 polymorphisms and cancer risk: a meta-analysis based on 48,166 subjects. Artif Cells Nanomed Biotechnol. 2020;48(1):15–27.
Dai Y, Song Z, Zhang J, Gao W. Comprehensive assessment of the association between XPC rs2228000 and cancer susceptibility based on 26835 cancer cases and 37069 controls. Biosci Rep. 2019;39(12):1–16.
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–10.
Han B, Eskin E. Interpreting meta-analyses of genome-wide association studies. PLoS Genet. 2012;8(3):e1002555.
Abbasi R, Ramroth H, Becher H, Dietz A, Schmezer P, Popanda O. Laryngeal cancer risk associated with smoking and alcohol consumption is modified by genetic polymorphisms in ERCC5, ERCC6 and RAD23B but not by polymorphisms in five other nucleotide excision repair genes. Int J Cancer. 2009;125(6):1431–9.
Akhmadishina LZ, Gilyazova IR, Kutlyeva LR, Korytina GF, Kochetova OV, Urmantsev MF, Izmailova SM, Izmailov AA, Kunsbaeva GB, Zagidullin AA, et al. DNA repair XPCC1 and XPD genes polymorphism as associated with the development of bladder cancer and renal cell carcinoma. Russian J Genetics. 2014;50(4):421–9.
Applebaum KM, Karagas MR, Hunter DJ, Catalano PJ, Byler SH, Morris S, Nelson HH. Polymorphisms in nucleotide excision repair genes, arsenic exposure, and non-melanoma skin cancer in New Hampshire. Environ Health Perspect. 2007;115(8):1231–6.
Bau DT, Tsai MH, Huang CY, Lee CC, Tseng HC, Lo YL, Tsai Y, Tsai FJ. Relationship between polymorphisms of nucleotide excision repair genes and oral cancer risk in Taiwan: evidence for modification of smoking habit. Chin J Physiol. 2007;50(6):294–300.
Chen L, Liu MM, Liu H, Lu D, Zhao XD, Yang XJ. ERCC1 and XRCC1 but not XPA single nucleotide polymorphisms correlate with response to chemotherapy in endometrial carcinoma. Onco Targets Ther. 2016;9:7019–28.
Crew KD, Gammon MD, Terry MB, Zhang FF, Zablotska LB, Agrawal M, Shen J, Long CM, Eng SM, Sagiv SK, et al. Polymorphisms in nucleotide excision repair genes, polycyclic aromatic hydrocarbon-DNA adducts, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2033–41.
Ding DP, Liu JX, Yu HL, Guo YB. Association of XPA polymorphism and the risk of breast cancer in women of Guangdong. Guangdong Med J. 2016;37(11):1651–3.
Ding P, Yang Y, Cheng L, Zhang X, Cheng L, Li C, Cai J. The relationship between seven common polymorphisms from five DNA repair genes and the risk for breast cancer in northern Chinese women. PLoS ONE. 2014;9(3):e92083.
Doherty JA, Weiss NS, Fish S, Fan W, Loomis MM, Sakoda LC, Rossing MA, Zhao LP, Chen C. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1873–82.
Dong Z, Guo W, Zhou R, Wan L, Li Y, Wang N, Kuang G, Wang S. Polymorphisms of the DNA repair gene XPA and XPC and its correlation with gastric cardiac adenocarcinoma in a high incidence population in North China. J Clin Gastroenterol. 2008;42(8):910–5.
Feng XX, Duan PF, Wang LB, Zhang JB, Lu ZX. Study on the relationship between polymorphisms of XPA gene and susceptibility of esophageal cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29(9):930–3.
Liang G. The Relationship between DNA repair genes, carcinogenic metabolism gene single nucleotide polymorphism and genetic susceptibility to lung cancer. Beijing: Beijing Union Med Univ; 2004. p. 28–34.
Ghanshela R, Banerjee BD, Siddarth M, Gupta S. DNA repair gene polymorphism (XPA and XPG) and risk of urinary bladder cancer in North-Indian population. Meta Gene. 2020;24:1–7.
Gil J, Ramsey D, Stembalska A, Karpinski P, Pesz KA, Laczmanska I, Leszczynski P, Grzebieniak Z, Sasiadek MM. The C/A polymorphism in intron 11 of the XPC gene plays a crucial role in the modulation of an individual’s susceptibility to sporadic colorectal cancer. Mol Biol Rep. 2012;39(1):527–34.
Guo W, Zhou RM, Wan LL, Wang N, Li Y, Zhang XJ, Dong XJ. Polymorphisms of the DNA repair gene xeroderma pigmentosum groups A and C and risk of esophageal squamous cell carcinoma in a population of high incidence region of North China. J Cancer Res Clin Oncol. 2008;134(2):263–70.
Hall J, Hashibe M, Boffetta P, Gaborieau V, Moullan N, Chabrier A, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. The association of sequence variants in DNA repair and cell cycle genes with cancers of the upper aerodigestive tract. Carcinogenesis. 2007;28(3):665–71.
Han W, Kim KY, Yang SJ, Noh DY, Kang D, Kwack K. SNP-SNP interactions between DNA repair genes were associated with breast cancer risk in a Korean population. Cancer. 2012;118(3):594–602.
Hansen RD, Sorensen M, Tjonneland A, Overvad K, Wallin H, Raaschou-Nielsen O, Vogel U. XPA A23G, XPC Lys939Gln, XPD Lys751Gln and XPD Asp312Asn polymorphisms, interactions with smoking, alcohol and dietary factors, and risk of colorectal cancer. Mutat Res. 2007;619(1–2):68–80.
Hsieh YY, Chang CC, Wang YK, Hsu KH, Chen CP, Hsu CM, Tsai FJ. Insulin-like growth factors II exon 9 and E-cadherin-Pml I but not myeloperoxidase promoter-463, urokinase-ApaL I nor xeroderma pigmentosum polymorphisms are associated with higher susceptibility to leiomyoma. Anticancer Res. 2010;30(6):2203–8.
Huang XX. Polymorphisms of XPA and XRCC3, environmental agent and susceptibilities to esophageal, cardia and non-cardia gastric cancer. Jiangxi: Fujian MedUniv; 2007. p. 22–36.
Jelonek K, Gdowicz-Klosok A, Pietrowska M, Borkowska M, Korfanty J, Rzeszowska-Wolny J, Widlak P. Association between single-nucleotide polymorphisms of selected genes involved in the response to DNA damage and risk of colon, head and neck, and breast cancers in a Polish population. J Appl Genet. 2010;51(3):343–52.
Joshi AD, Corral R, Siegmund KD, Haile RW, Le Marchand L, Martinez ME, Ahnen DJ, Sandler RS, Lance P, Stern MC. Red meat and poultry intake, polymorphisms in the nucleotide excision repair and mismatch repair pathways and colorectal cancer risk. Carcinogenesis. 2009;30(3):472–9.
Lawania S, Singh A, Sharma S, Singh N, Behera D. The multi-faceted high order polymorphic synergistic interactions among nucleotide excision repair genes increase the risk of lung cancer in North Indians. Mutat Res. 2019;816–818:111673.
Liu R. Study on expression characteristics of susceptibility genes and genetic susceptibility markers for esophageal carcinomas. Nanjing: Southeast University; 2007. p. 11–4.
Miller KL, Karagas MR, Kraft P, Hunter DJ, Catalano PJ, Byler SH, Nelson HH. XPA, haplotypes, and risk of basal and squamous cell carcinoma. Carcinogenesis. 2006;27(8):1670–5.
Palli D, Polidoro S, D’Errico M, Saieva C, Guarrera S, Calcagnile AS, Sera F, Allione A, Gemma S, Zanna I, et al. Polymorphic DNA repair and metabolic genes: a multigenic study on gastric cancer. Mutagenesis. 2010;25(6):569–75.
Pan J, Lin J, Izzo JG, Liu Y, Xing J, Huang M, Ajani JA, Wu X. Genetic susceptibility to esophageal cancer: the role of the nucleotide excision repair pathway. Carcinogenesis. 2009;30(5):785–92.
Paszkowska-Szczur K, Scott RJ, Serrano-Fernandez P, Mirecka A, Gapska P, Gorski B, Cybulski C, Maleszka R, Sulikowski M, Nagay L, et al. Xeroderma pigmentosum genes and melanoma risk. Int J Cancer. 2013;133(5):1094–100.
Pesz KA, Bieniek A, Gil J, Laczmanska I, Karpinski P, Makowska I, Trusewicz A, Sasiadek MM. Polymorphisms in nucleotide excision repair genes and basal cell carcinoma of the skin. Int J Dermatol. 2014;53(12):1474–7.
Qian B, Zhang H, Zhang L, Zhou X, Yu H, Chen K. Association of genetic polymorphisms in DNA repair pathway genes with non-small cell lung cancer risk. Lung Cancer. 2011;73(2):138–46.
Raaschou-Nielsen O, Sorensen M, Overvad K, Tjonneland A, Vogel U. Polymorphisms in nucleotide excision repair genes, smoking and intake of fruit and vegetables in relation to lung cancer. Lung Cancer. 2008;59(2):171–9.
Rafiq R, Bhat GA, Lone MM, Masood A, Dar NA. Potential risk of esophageal squamous cell carcinoma due to nucleotide excision repair XPA and XPC gene variants and their interaction among themselves and with environmental factors. Tumour Biol. 2016;37(8):10193–207.
Tang FF, Ouyang J, Xu Y, Zhou RF, Zhou M, Chen B, Zhang QG, Feng X, Zhang X, Chen MM, et al. The relationship between polymorphism of genes XPA, XPC, XPD, XRCC1 and susceptibility to acute lymphoblastic leukemia. Zhonghua Nei Ke Za Zhi. 2011;50(10):859–62.
Tao J, Zhuo ZJ, Su M, Yan L, He J, Zhang J. XPA gene polymorphisms and risk of neuroblastoma in Chinese children: a two-center case-control study. J Cancer. 2018;9(15):2751–6.
Vogel U, Overvad K, Wallin H, Tjonneland A, Nexo BA, Raaschou-Nielsen O. Combinations of polymorphisms in XPD, XPC and XPA in relation to risk of lung cancer. Cancer Lett. 2005;222(1):67–74.
Weiss JM, Weiss NS, Ulrich CM, Doherty JA, Voigt LF, Chen C. Interindividual variation in nucleotide excision repair genes and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2524–30.
Wu X, Zhao H, Wei Q, Amos CI, Zhang K, Guo Z, Qiao Y, Hong WK, Spitz MR. XPA polymorphism associated with reduced lung cancer risk and a modulating effect on nucleotide excision repair capacity. Carcinogenesis. 2003;24(3):505–9.
Xie WM. Association between nucleotide excision repair gene polymorphism and genetic susceptibility and clinical phenotype of hepatocellular carcinoma. Nanning: Guangxi Medical University; 2007. p. 30–40.
Zeng H, Kang MF. Relationship between XPA A23G and XPG His1104Asp gene polymorphisms and susceptibility to lung cancer. Guangdong Med J. 2013;34(3):413–6.
Zhang WC. Effects of genetic and environmental risk factors to esophageal cancer in Hunian population. Nanjing: Southeast University; 2006. p. 32–8.
Zhao Z, Zhang A, Zhao Y, Xiang J, Yu D, Liang Z, Xu C, Zhang Q, Li J, Duan P. The association of polymorphisms in nucleotide excision repair genes with ovarian cancer susceptibility. Biosci Rep. 2018;38(3):1–5.
Zhen Q. Relationship between XPA gene polymorphism and susceptibility to esophageal cancer in Henan Han population. Zhengzhou: Zhengzhou University; 2012. p. 18–24.
Zhu F, Peng QS, Wang H, Li Y, He BS, Zhu CB. Investigations on the association of single nucleotide polymorphisms of DNA repair XPD, XPA genes with the risk of breast cancer. Chin J Clin Lab Sci. 2015;33(7):498–546.
Zhu J, Fu W, Jia W, Xia H, Liu GC, He J. Association between NER pathway gene polymorphisms and wilms tumor risk. Mol Ther Nucleic Acids. 2018;12:854–60.
Zhu JF, Chen YJ, Zhou JM, Yu L, Huo X, Ma HX, Hu ZB, Chen HB. The single nucleotide polymorphism in the promotor of DNA repair gene XPA and in association with the risk of lung cancer. Tumor. 2005;25(3):246–9.
Zhu XL. Study on relationship between DNA repair genes polymorphism of hOGG1, XPC, XPA and esophageal squamous cell carcinoma susceptibility. Wuhan: Huazhong University of Science and Technology; 2008. p. 21–6.
Lou Y, Li R, Zhang Y, Zhong R, Pei J, Xiong L, Zhang X, Han B. XPA gene rs1800975 single nucleotide polymorphism and lung cancer risk: a meta-analysis. Tumour Biol. 2014;35(7):6607–17.
Liu X, Lin Q, Fu C, Liu C, Zhu F, Liu Z, Li S, Jiang L. Association between XPA gene rs1800975 polymorphism and susceptibility to lung cancer: a meta-analysis. Int J Cancer. 2016;12(2):448–58.
Wu L, Gao X, Ye D, Ding Y, Yang XI, Liu W. Association of the XPA A23G polymorphism with the risk of head and neck carcinomas: evidence from 5,491 subjects. Mol Clin Oncol. 2015;3(3):649–54.
Zhang Y, Guo Q, Yin X, Zhu X, Zhao L, Zhang Z, Wei R, Wang B, Li X. Association of XPA polymorphism with breast cancer risk: a meta-analysis. Medicine (Baltimore). 2018;97(26):e11276.
He L, Deng T, Luo H. XPA A23G polymorphism and risk of digestive system cancers: a meta-analysis. Onco Targets Ther. 2015;8:385–94.
Xu JL, Bai J, Jiao JF, Ding L, Lei L, Bai XP, Cheng YF. Meta-analysis on the association between xeroderma pigmentosum Group A A23G polymorphism and esophageal cancer in a Chinese population. J Cancer Res Ther. 2018;14(Supplement):S1173-s7.
Lin J, Pu X, Wang W, Matin S, Tannir NM, Wood CG, Wu X. Case-control analysis of nucleotide excision repair pathway and the risk of renal cell carcinoma. Carcinogenesis. 2008;29(11):2112–9.
Shen J, Desai M, Agrawal M, Kennedy DO, Senie RT, Santella RM, Terry MB. Polymorphisms in nucleotide excision repair genes and DNA repair capacity phenotype in sisters discordant for breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1614–9.
Sugimura T, Kumimoto H, Tohnai I, Fukui T, Matsuo K, Tsurusako S, Mitsudo K, Ueda M, Tajima K, Ishizaki K. Gene-environment interaction involved in oral carcinogenesis: molecular epidemiological study for metabolic and DNA repair gene polymorphisms. J Oral Pathol Med. 2006;35(1):11–8.
Wang Y, Spitz MR, Lee JJ, Huang M, Lippman SM, Wu X. Nucleotide excision repair pathway genes and oral premalignant lesions. Clin Cancer Res. 2007;13(12):3753–8.
Zhu J, Hu Z, Ma H, Huo X, Xu L, Zhou J, Shen H, Chen Y. Polymorphism of the DNA repair gene XPA and susceptibility to lung cancer. J Nanjing Med Univ. 2005;19(4):173–6.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MY and CY collected, extracted and analyzed the data, wrote the paper; MY, CY and KY performed quality assessment, FPRP, TSA, gene expression, eQTL, and sQTL analyses. MY and CY conceived and designed this study. All authors reviewed the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Search terms of six online databases. Table S2. The association between XPA rs1800975 and the risk of lung cancers in the Caucasian population. Table S3. The association between XPA rs1800975 and the risk of lung and colorectal cancers in the Caucasian population (only PB-based controls).
Additional file 2: Fig. S1.
Forest plot data of subgroup analysis by race (carrier model).
Additional file 3: Fig. S2.
Forest plot data of subgroup analysis by race (dominant model).
Additional file 4: Fig. S3.
Forest plot data of subgroup analysis by control source (allelic model).
Additional file 5: Fig. S4.
Forest plot data of subgroup analysis by control source (carrier model).
Additional file 6: Fig. S5.
Forest plot data of subgroup analysis by control source (dominant model).
Additional file 7: Fig. S6.
Forest plot data of subgroup analysis by cancer type (homozygotic model).
Additional file 8: Fig. S7.
Forest plot data of subgroup analysis by cancer type (heterozygotic model).
Additional file 9: Fig. S8.
Forest plot data of subgroup analysis by cancer type (dominant model).
Additional file 10: Fig. S9.
Violin plot of XPA expression profile across multiple tissues of GTEx project.
Additional file 11: Fig. S10.
Box plot of the expression difference of XPA gene between tumor and adjacent normal tissues across all TCGA tumors. ** P<0.01; *** P<0.001.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, M., Yu, C. & Yu, K. Association of human XPA rs1800975 polymorphism and cancer susceptibility: an integrative analysis of 71 case–control studies. Cancer Cell Int 20, 164 (2020). https://doi.org/10.1186/s12935-020-01244-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-020-01244-5