Abstract
Objective
In the pathogenesis of myeloproliferative neoplasms (MPN), inflammation plays an important role. However, it is unclear whether there is a causal link between inflammation and MPNs. We used a bidirectional, two-sample Mendelian randomization (MR) approach to investigate the causal relationship between systemic inflammatory cytokines and myeloproliferative neoplasms.
Methods
A genome-wide association study (GWAS) of 8293 European participants identified genetic instrumental variables for circulating cytokines and growth factors. Summary statistics of MPN were obtained from a GWAS including 1086 cases and 407,155 controls of European ancestry. The inverse-variance-weighted method was mainly used to compute odds ratios (OR) and 95% confidence intervals (Cl).
Results
Our results showed that higher Interleukin-2 receptor, alpha subunit (IL-2rα) levels, and higher Interferon gamma-induced protein 10 (IP-10) levels were associated with an increased risk of MPN (OR = 1.36,95%CI = 1.03–1.81, P = 0.032; OR = 1.55,95%CI = 1.09–2.22, P = 0.015; respectively).In addition, Genetically predicted MPN promotes expression of the inflammatory cytokines interleukin-10 (IL-10) (BETA = 0.033, 95% CI = 0.003 ~ 0.064, P = 0.032) and monokine induced by interferon-gamma (MIG) (BETA = 0.052, 95% CI = 0.002–0.102, P = 0.043) and, on activation, normal T cells express and secrete RANTES (BETA = 0.055, 95% CI = 0.0090.1, P = 0.018).
Conclusion
Our findings suggest that cytokines are essential to the pathophysiology of MPN. More research is required if these biomarkers can be used to prevent and treat MPN.
Similar content being viewed by others
Introduction
The chronic hematological malignancies known as myeloproliferative neoplasms (MPN), which include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF), advance at varying rates [1]. The incidence rates of PV, ET, and PMF are estimated to be 0.5 to 4.0, 1.1 to 2.0, and 0.3 to 2.0 per 100,000 people, respectively. It is reported that nearly 10–15% of patients with MPN progress to AML [2], more than 20% will develop thrombosis during the disease, and approximately 6.2% of newly diagnosed patients will suffer hemorrhage [3, 4]. The presence of these symptoms mentioned above raises the rate of disability and mortality in MPN patients [5] and imposes a huge economic burden on the family and society. The most common feature of MPN is hyperactivation of Janus kinase 2 (JAK2) signaling, which is caused by acquired mutations in JAK2, MPL, and CALR [6]. However, clinically used JAK2 inhibitors such as Ruxolitinib and Fedratinib have limited efficacy, high toxicity, and are prone to drug resistance [6,7,8]. Therefore, increased awareness of the pathogenic components may offer clues for halting the disease's course and creating novel treatments.
The chronic inflammatory environment is one of the typical features of myeloproliferative neoplasms, where inflammation is tightly intertwined with tumor clones, providing a permissive micro-environment for disease progression [9,10,11]. Inflammatory cytokines are essential immune mediators in the physiology and disease process of MPN and not only play a significant role in inflammatory pathology but are also inextricably linked to the development of the disease [9, 12]. GM-CSF, IL-1, IL-4, IL-5, IL-6, IL-10, IFN-2, MIP-1, IL-12, and TNF-α were shown to have higher cytokine levels in treatment-naive patients in all three MPN groups when compared to age-matched control participants, according to an observational study [9]. In addition, serum IL-2 and soluble IL-2 receptor alpha (sIL-2rα) increased as patients with MPNs progressed to advanced clinical stages [13], and serum IL-2, sIL-2rα, and IL-6 levels were positively correlated with bone marrow neovascularization, indicating that increased inflammatory responses may be connected to the course of MPN disease [14], suggesting that MPN patients may benefit from using cytokines as a tool for illness monitoring [15]. However, little is known about the mechanisms and duration of inflammation in MPNs [16]. The origins of the increased cytokine production in MPNs (alterations, others?) and whether inflammation may occur before the development of JAK2/CALR/MPL gene mutations are still up for dispute. Observational studies are prone to common biases such as reverse causality and residual confounding [17] and have limitations such as small sample sizes and short follow-up periods. These studies, however, only addressed a small subset of inflammatory cytokines and did not take into account how other physical factors can affect changes in inflammatory cytokine levels. Determining whether variations in inflammatory cytokines cause the development of MPN or whether MPN development influences the microenvironment and causes variations in inflammatory cytokines is crucial. Investigating the precise nature of the connection between inflammatory cytokines and MPN is crucial from a therapeutic standpoint given the lack of knowledge regarding the etiology of MPN.
To establish a link between inflammatory cytokines and MPN, we applied Mendelian randomization (MR).MR has the advantage of reducing confounding variables and measurement error, as well as addressing the limitations of traditional observational studies mentioned above. This approach can effectively avoid bias caused by reverse causality [18]. The greatest level of evidence hierarchy outside of randomized controlled trials is provided by MR, which uses genetic variation as an instrumental variable (IV), which has been a dependable tool for getting reliable estimates of the causal influence of numerous risk variables on health [19]. In the current investigation, we used a two-sample MR design to methodically evaluate the potential causal link between inflammatory cytokines and MPN risk. Additionally, reverse MR analysis was done to determine how MPN affected cytokines.
Methods
Study design
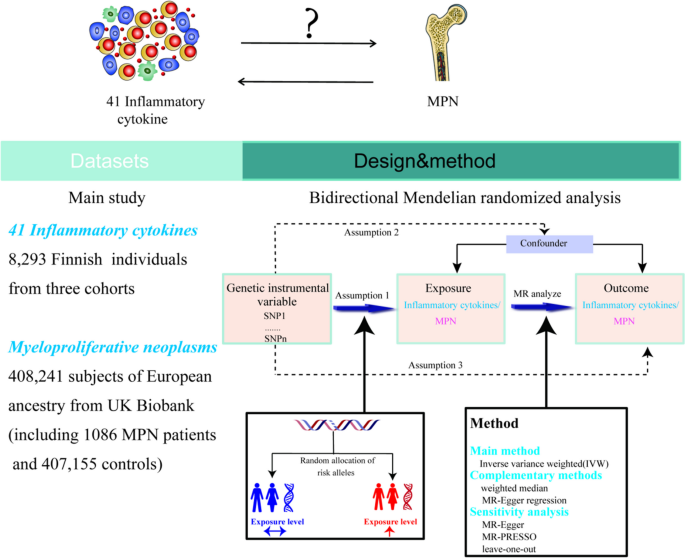
The Mendelian randomization design method uses publicly available datasets from extensive genome-wide association studies (GWAS) for risk factors and disease to examine whether exposure has a causal effect on disease emergence. As a genetic instrumental variable analysis, MR Uses single nucleotide polymorphisms (SNPs) as instrumental variables for the risk factor of interest. SNPS are randomly assigned at meiosis and are not subject to reverse causality bias, so the Mendelian randomization approach can overcome unmeasured confounders and lead to more reliable causal inferences [20].
To determine the relationship between inflammatory cytokine levels and the risk of MPN, we conducted a bidirectional Mendelian randomized trial. As the summary statistics from published research are made available to the public, the institutional review board did not need to approve our study's ethics further. In Supplementary Table 1, the features of the data used in this investigation are displayed.
Data sources and instruments
Cytokine
The data on inflammatory cytokines was obtained from a meta-analysis published in 2017 that summarized data from genome-wide association studies (GWAS) carried out with three Finnish cohorts (YFS and FINRISK, 1997 and 2002), totaling 8,293 Finns. The original publication has information about the cytokine assays, inclusion standards, etc. [21]. In MR analysis, P-values are used to measure whether there is an association between genetic variants and exposure factors. We set the P value to find the genetic variant loci associated with the trait. The independence of the selected instrumental variables was further ensured by removing all SNPs with linkage disequilibrium (LD) to avoid biased results (parameters kb = 250, r2 = 0.001). Calculate the F-statistic [using the formula: F = (N-2)*R2/(1-R2), N is the sample size]to assess the extent of weak instrument bias, F > 10 suggests that full instrumental SNPs are sufficiently strong to lessen any potential bias, while an F-statistic ≤ 10 implies weak instruments [22].Initially, we used P < 5 × 10–8 as a criterion to look for instrumental factors, and we discovered that the majority of cytokines had either no SNPs or only a few SNPs [3]. To get as many cytokines as possible into the study. Furthermore, we chose IVs using a permissive significance criterion (P < 5 × 10–6). Further, we use the parameter kb = 250, r2 = 0.001 to eliminate the linkage disequilibrium among variables. Supplementary Tables 2 and 3 provide extensive details on the features of the IVs.
Myeloproliferative neoplasms
The MPN Patients' data were obtained from publicly available GWAS data, which we downloaded from open GWAS (https://gwas.mrcieu.ac.uk/). The data come from UK Biobank, which is a cohort study conducted between 2006 and 2010. The study collected in-depth genetic and phenotypic data on approximately 500,000 people across the United Kingdom. The data we downloaded for analysis included 1086 MPN patients and 407,155 controls [23]. The original article describes the criteria and procedures for the data's quality control [23]. Erythrocytosis, primary thrombocythemia, myelofibrosis, chronic myeloid leukemia, and chronic myeloproliferative illness are all included in the UKBB's description of the MPN phenotype. Those who had polycythemia vera, essential thrombocythemia, myelofibrosis, chronic myeloid leukemia, or malignant mastocytosis were additionally labeled as cases if they had self-reported cancer, self-reported sickness code, or histology of cancer tumor code. In the MPN dataset, we screened SNPs as instrumental variables according to the following criteria: (P < 5 × 10–8, r2 = 0.001, kb = 250 kb). Five SNPs were kept as separate MPN IVs. Inverse MR analyses were performed using these SNPs to examine the genetic influence of MPN on the amount of cytokines.
Bioinformatics analysis
We use the data set from the GEO database for gene expression spectrum analysis (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi). The GSE103237 dataset is based on the GPL13667 platform and consists of 26 polycythemia vera, 24 essential thrombocythemia, and 15 normal bone marrow samples. The GSE136335 dataset is based on the GPL17586 platform consisting of 8 myelofibrosis patients and 6 normal bone marrow samples. We used the "Ggpubr" package to create violin plots to visualize the expression of IP-10, and IL-2ra in the three subtypes of MPN. The diagnostic value of IP-10 and IL-2ra was analyzed by the receiver operating characteristic (ROC) curve. The expression of genes was analyzed using the "pROC" package (v1.18.2), and genes with the area under the curve (AUC) > 0.7 were considered potential diagnostic markers.
Statistical analysis
We harmonized the effect of SNPs on inflammatory cytokines and MPNs before the Mendelian randomization analysis. Furthermore, we used the two-sample MR software package for MR analysis. The inverse variance weighted(IVW) methods were used to assess potential causality, while weighted median and MR-Egger regression methods were used as complementary methods for causality between inflammatory cytokines and MPN [24]. The MR-Egger method was used for multiple validity testing. We used Cochran's Q test to assess heterogeneity in IVW [25]. MR-PRESSO was used to detect the presence of outliers [26], and leave-one-out analyses were used to verify whether the causal effect depended on a single variant. We also performed instrumental strength tests using the F statistic, with F > 10 indicating sufficient strength [27]. Eventually, if the IVW method result is significant (P < 0.05), even if the findings of other techniques are not significant and there is no pleiotropy or heterogeneity discovered, it may be regarded as a good result, given that the beta values of the other methods are in the same direction [28].
Based on the number of cytokines, we used the Bonferroni approach to compensate for multiple comparisons and set statistical significance at a P-value < 1.22 × 10−3 (0.05/41) level. If a P-value was between 1.22 × 10−3 and 0.05, we considered suggestive evidence for a potential causal association. The majority of the work mentioned above was completed using the R analysis program (version 4.3.0), which was applied to the relevant R package, including Two-sample MR, data array, etc.
Results
Instrumental variables
Figure 1 offers a flowchart of the full-text logic. In the study of the effect of inflammatory factors on MPN, we screened instrumental variables according to the criteria of P < 5 × 10–6 and r2 < 0.001. A total of 354 SNPs linked to 41 cytokines were used for MR analysis after being harmonized with the outcome variable MPN (Supplementary Table 1).To analyze the effect of MPN on inflammatory factors, we used MPN as an exposure factor, By using such criteria (P < 5 × 10–8, r2 < 0.001), a total of five SNPs were obtained for subsequent analyses, and specific SNP information can be found in Supplementary Table 2. These SNPs' F statistics, which ranged from 20.77 to 781.85(Table 1), showed that the instrument was sufficiently reliable to rule out the possibility of a null relationship brought on by instrument bias.
Effect of inflammatory cytokines on MPN
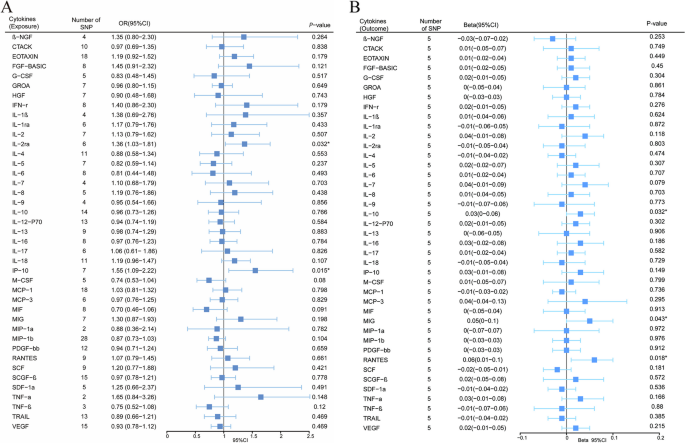
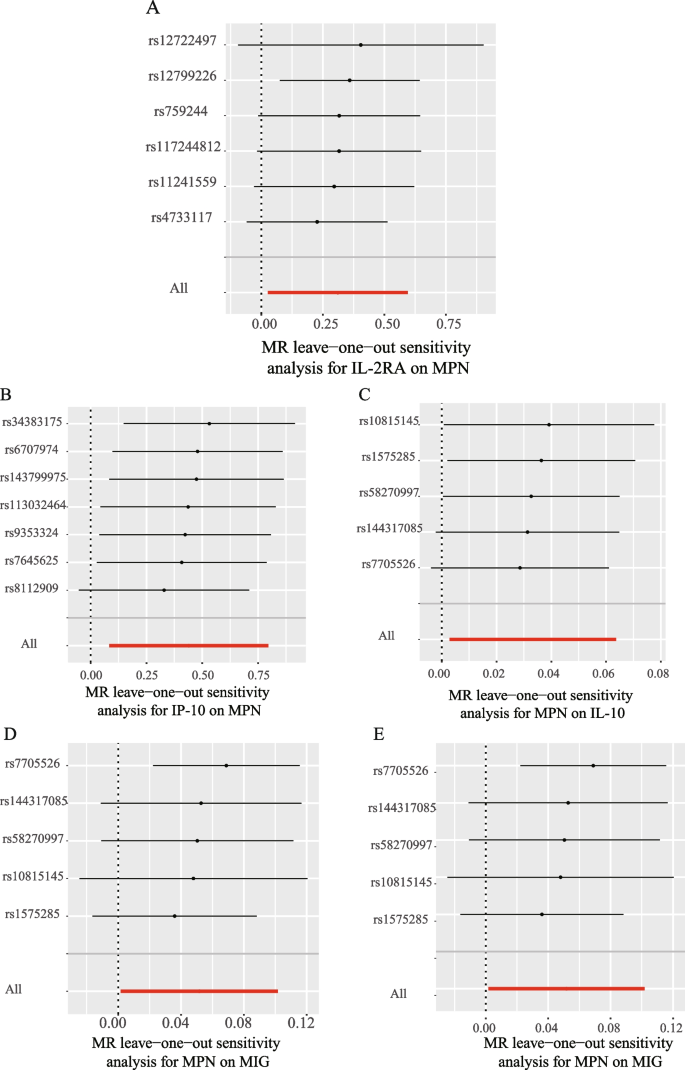
We explored the effects of 41 cytokines on MPN sequentially using a two-sample Mendelian randomization analysis (Supplementary Table 3). Only two cytokines (Interleukin-2 receptor, alpha subunit (IL − 2rα), and interferon gamma-induced protein 10 (IP-10) revealed suggestive associations with MPN risk after the Bonferroni correction. Genetically determined higher levels of circulating IL-2rα are suggestively positively associated with MPN risk [odds ratio (OR): 1.365,95% confidence interval (CI): 1.029–1.814, P = 0.032]. The other two complementary analytical methods Weighted median and MR Egger also obtained similar but not statistically significant results (Fig. 2A). Furthermore, Cochran's Q test did not reveal any heterogeneity (P = 0.268). A directional pleiotropy was also not discovered (MR egger-intercept = 0.021, P for MR egger-intercept = 0.710; P for MR PRESSO global test = 0.528) (Supplementary Table 5). Removing one SNP did not significantly change the results in the leave-one-out sensitivity analysis (Fig. 3A). Sensitivity analyses were conducted using the leave-one-out analyses to assess the reliability and stability of the results. The overall effect of the remaining instrumental variables was calculated by removing each SNP stepwise and observing whether the results changed after removing a single SNP. The results showed that removing individual SNPs did not significantly change the results of the exclusion sensitivity analyses (Fig. 3A). We did not find secondary phenotypes associated with SNPs that were used as instrumental variables on Pheno-Scanner's website.
Forest plot of Mendelian randomization analysis of the correlation between inflammatory cytokines and risk of myeloproliferative neoplasms (MR analysis method using IVW). A: Effects of 41 inflammatory cytokines on myeloproliferative tumors. B:Effects of myeloproliferative neoplasms on 41 inflammatory cytokines(*P < 0.05)
Leave-one-out analysis of bidirectional mendelian randomization in cytokines and MPN.The left side of the forest plot represents the SNPs for which the leave-one-out analysis was performed, and the short line parallel to the x-axis represents the 95% confidence interval for the OR/beta value of the MR analysis after excluding the corresponding SNPs. As shown in the figure, the overall error line does not change much after excluding each SNP, and all OR/beta values are on the 0 side, indicating that the results are reliable (A):Forest plots for the exposure of IL-2ra. B Forest plots for the exposure of IP-10. C Forest plots for the outcome of IL-10. D Forest plots for the outcome of MIG. E Forest plots for the outcome of RANTES
We also observed a suggestive association between genetically determined higher circulating IP-10 and a 55.3% increased risk of MPN (OR:1.553, 95% CI:1.088–2.216, P = 0.015), and it did not show heterogeneity (Cochrane Q test, P = 0.706)(Fig. 2A); nor did it show directional pleiotropy (MR egger-intercept = 0.085, P for MR egger-intercept = 0.305, P for MR PRESSO global test = 0.668) (Supplementary Table 5). Sensitivity analysis was conducted using leave-one-out studies, and the results showed that no individual study had any impact (Fig. 3B). We did not identify the SNP associated with other phenotypes on the Pheno-Scanner website, indicating that it does not increase the risk of MPN through the non-exposure pathway.
Effect of MPN on inflammatory cytokines
In analyzing the effect of MPN on inflammatory factors, We found a suggestive association between genetically predicted MPN and levels of the cytokines IL-10, MIG, and RANTES. Genetically predicted MPN were suggestively associated with levels of interleukin-10 (IL-10) (BETA = 0.033,95% CI = 0.003 ~ 0.064, P = 0.032) and Monokine induced by interferon-gamma (MIG) (BETA = 0.052,95% CI = 0.002–0.102, P = 0.043) and Regulated on activation, normal T Cell expressed and secreted (RANTES) (BETA = 0.055,95% CI = 0.009 − 0.1, P = 0.018) using IVW methods. It is worth paying attention to the fact that in the RANTES analysis, although the MR-egger results were not statistically significant, the direction of the MR-egger results was inconsistent with the IVW results, suggesting that the RANTES results may be unreliable (Fig. 2B). In these findings, there was no indication of pleiotropy or heterogeneity. Supplementary Tables 4 and 6 provides a summary of the abovementioned findings. The leave-one-out analysis, meanwhile, revealed that all SNPs contributed to consistent causal estimates. (Fig. 3C-E). The analysis mentioned above demonstrated the validity of the results.
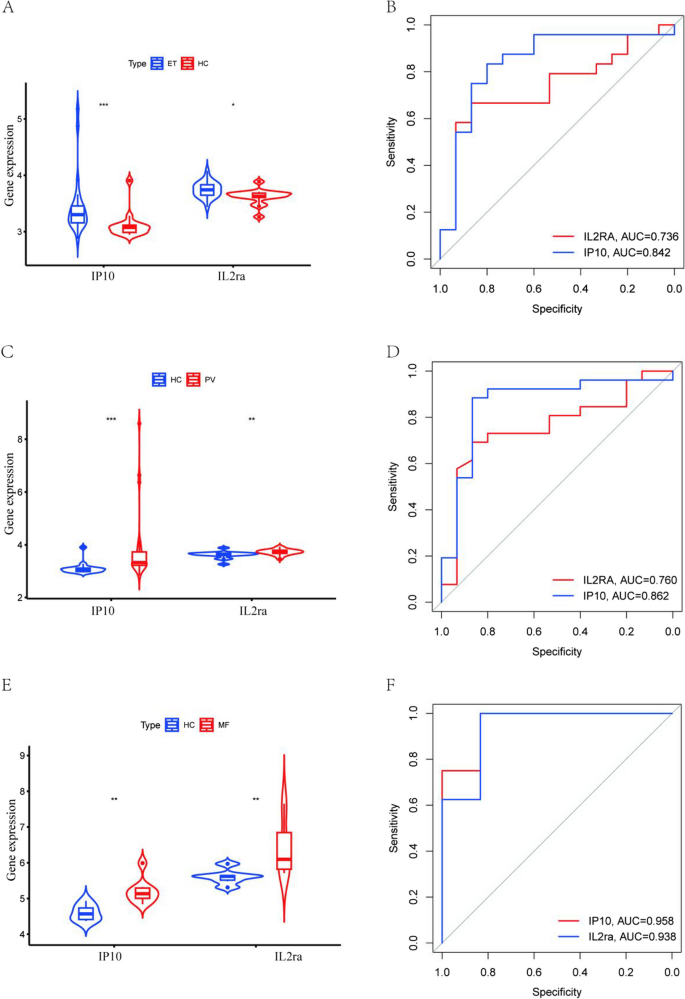
The expression of IP-10 and IL-2ra in various subtypes of MPN and their diagnostic value
By analysing the effect of inflammatory cytokines on MPN, we found that higher levels of IP-10, IL-2ra were associated with increased risk of MPN. However, due to data limitations, we did not have the opportunity to further analyse the effect of IP-10, IL-2ra on the risk of developing each subtype of MPN using the MR approach. Therefore, in order to investigate the expression of IP-10 and IL-2ra in each subtype of MPN and their diagnostic value, we analyzed their expression in healthy individuals and the three subtypes of MPN and their diagnostic value based on data from the GEO database, as shown in Fig. 4. Our results showed that IP-10 and IL-2ra were elevated in ET, PV, and MF compared with normal subjects. The highest diagnostic efficacy for MF was based on the expression of IP-10 and IL-2ra (IP10: AUC = 0.958, IL-2ra: AUC = 0.938); the areas under the ROC curves of IP-10 and IL-2ra in ET, PV, and MF were greater than 0.7, suggesting that IP-10 and IL-2ra may be used as potential diagnostic markers of MPN.
The GEO dataset analyses the expression of IP10 and IL2ra in each subtype of MPN and their diagnostic value.A: Expression of IP10 and IL2ra in ET and healthy donors; B: ROC curve of the prediction model based on IP10 and IL2ra to distinguish ET from healthy donors; C:IP10 and IL2ra expression in PV and healthy donors; D: ROC curve of the prediction model based on IP10 and IL2ra to distinguish PV from healthy donors; E:IP10 and IL2ra expression in MF and healthy donors; F: ROC curve of the prediction model based on IP10 and IL2ra to distinguish MF from healthy donors; ***P < 0.001, **P < 0.01,*P < 0.05,HC: Healthy donors
Discussion
Using publicly available pooled data from GWAS, we conducted a bidirectional two-sample MR analysis of the potential causative relationship between inflammatory cytokines and MPNs, and our study supported a causal association between inflammatory cytokines and MPNs. We found suggestive evidence that levels of the genetically predicted circulating cytokines IL-2rα, and IP-10 have a risk effect on MPNs. Reverse MR analysis found suggestive evidence of a positive causal effect of MPN on levels of the circulating cytokines IL-10, MIG, and RANTES. These findings passed sensitivity analyses and were not affected by heterogeneity or horizontal pleiotropy. To our knowledge, this investigation is anticipated to be the broadest and most thorough MR evaluation of links between genetically inflammatory cytokines and MPN risk to date.
The CXC chemokine family member interferon gamma-induced protein 10 (IP-10) is crucial for cell growth and proliferation [29].IP-10 combines with the CXCR3 receptor, being a key driver in cancer and autoimmune regulation [30]. Several observational studies have demonstrated the presence of aberrant IP-10 expression in MPN patients, especially in PMF and PV, where IP-10 expression is significantly elevated [31, 32]. Meanwhile, the serum level of IP-10 was also correlated with the disease progression of MPN [32]. Our MR analysis suggests that elevated IP-10 levels may contribute to MPN disease progression, which is consistent with results derived from observational studies. Previous basic research can explain our findings and the phenomena of observational studies in terms of pathogenesis.IP-10 expression is reported to be required for the activation of the JAK signaling pathway [33]and its level correlates with JAK2V617F status [9, 34]. Therefore, JAK inhibition can reduce downstream chemokine IP-10 production by disrupting T cell-induced macrophage activation [35]. However, stromal cells in the microenvironment can protect MPN clonal cells from JAK2 inhibitors by secreting IP-10, which can promote disease progression. These discoveries underscore the importance of researching IP-10 as a potential therapeutic target in the MPN tumor microenvironment and highlight the necessity of further studies on the exact mechanism of its role in MPN oncogenesis.
Our study also reveals a potential association between IL2rα and increased risk of MPN disease.IL2rα is an important component of IL-2R, a high-affinity receptor molecule highly expressed by activated T lymphocytes [36], and plays an important role in the regulation of T cell differentiation. Increasing IL-2rα expression on antigen-presenting cells (APCs) enhances the formation of memory T cells [37], and mutations in IL-2rα lead to decreased T cell function [38]. Accordingly, IL-2rα levels are associated with T-cell, B-cell, and immune system activation [36]. It has been demonstrated that conditions linked to cellular immune activation correlate with increased IL-2rα [39, 40]. Additionally, a few observational studies have shown a connection between IL2r and MPN. Katerina et al. found that serum levels of IL-2rα were significantly elevated in patients with MPN compared to normal individuals [14], which was confirmed by further studies, where IL2rα levels were correlated with overall survival in patients with MF in MPN [41], and levels of IL-2ra in patients with MPN were positively correlated with disease progression and bone marrow angiogenesis [42]. According to the findings of our MR investigation, elevated levels of IL-2rα in the circulatory system may accelerate the development of MPN disease. This finding is not only consistent with the results of observational studies but also compensates for the shortcomings of small sample sizes and potential confounders in the observational studies mentioned above and provides more reliable evidence for the association between IL2rα and MPN at the level of genetics, emphasizing the importance and necessity of further investigating the role ofIL2rα in the development of MPN disease.
Both our analyses and previous studies suggest that IP-10 and IL2rα may play an important role in MPN disease development. Considering the heterogeneity of the three subtypes of MPN, we sought to explore the effects of IP-10 and IL2rα on the disease risk of each subtype of MPN. Unfortunately, due to the limitation of the dataset, we had no way to further explore the relationship between IP-10 and IL2rα and different subtypes of MPN. Therefore, we initially analyzed the expression and diagnostic value of IP-10 and IL2rα in each subtype of MPN using the GEO database. We were surprised to find that IP-10 and IL2rα not only had elevated expression in the three subtypes of MPN compared to healthy individuals, but also had the potential to serve as independent biomarkers. This is consistent with our MR analysis that high expression of IP-10, IL2rα increases the risk of MPN disease. This greatly encourages our confidence in further exploring the role of IL2rα, IP-10 in MPN at a later stage.
Positive MR analysis has revealed the role of inflammatory cytokines, particularly IP-10 and IL-2rα, in MPN disease progression. Indeed, MPN cells can also release large amounts of pro-inflammatory products, which in turn cause genomic instability and drive clonal myeloproliferation [43, 44].To explore the effect of MPN disease on inflammatory cytokines, we performed a reverse MR analysis. The inverse MR analysis showed a potential positive correlation between genetically predicted MPN and the levels of cytokines IL-10, MIG, and RANTES, and that MPN could promote slightly increased levels of the above cytokines. Inverse MR analysis revealed a potential positive correlation between genetically predicted MPN and levels of the cytokines IL-10, MIG, and RANTES, with MPN promoting slightly elevated levels of the aforementioned cytokines, which is consistent with observational findings [9, 45,46,47]. Our review of the literature revealed that aberrantly expressed IL-10, MIG, and RANTES are all associated with premature atherosclerosis, a devastating consequence of chronic inflammation in the MPN [48,49,50].MIG binds to the receptor CXCR3 and not only participates in the recruitment of T cells to peripheral sites of inflammation [51]but also chemotactically recruits monocytes/macrophages to sites of inflammation. Activated inflammatory cells release pro-inflammatory factors to induce an inflammatory response [52], which promotes atherosclerosis. RANTES is one of the chemokines highly expressed upon platelet activation, and RANTES released by activated platelets facilitates the formation of atherosclerotic lesions by platelet-monocyte aggregation [53, 54], and RANTES also regulates local inflammatory processes and atherosclerosis progression by mediating CD4 + T-cell homing [55]. It is interesting to note that IL-10 appears to be a protective factor against atherosclerosis, a common clinical symptom of MPN, and that, as an anti-inflammatory cytokine, IL-10 can attenuate atherosclerotic lesions by preventing dilation of inflamed areas, decreasing the size of plaques, and other mechanisms [56]. Specifically, IL-10 attenuates atherosclerotic lesions by inhibiting macrophage activation, as well as inhibiting the expression of matrix metalloproteinases, proinflammatory cytokines, and cyclooxygenase-2 in lipid-loaded and activated macrophage foam cells [57, 58]. Therefore, it is necessary to investigate the correlation and mechanism between the elevated circulating levels of MPN-promoting inflammatory cytokines IL-10, MIG, and RANTES and the common clinical complications of MPN, and to provide the possibility of targeting the above cytokines to alleviate the clinical complications.
Our study has several advantages. (1) The link between inflammatory cytokines and MPN risk is explained for the first time in magnetic resonance research. (2) Unlike observational studies, our study minimized confounders and reverse causality, providing a reliable causal relationship between MPN and inflammatory cytokines. (3) Our research data were sourced from the openly available GWAS database, which houses a significant volume of original research data, and thus gives this study a solid guarantee.
There are also some limitations to our study. In the first place, all participants in the dataset we used were of European ethnicity, which limits our ability to generalise our findings to other ethnicities. It is well known that Mendelian randomisation investigates the effect of genotypic variation (exposure) on phenotype (outcome) from a genetic perspective. Bias caused by confounding variables or reverse causality is avoided [18].In reality, however, it is well known that the level of gene expression determines the unique characteristics of a cell, that differences in disease prevalence between populations are associated with the frequency of alleles that regulate polymorphisms, and that differences in allele frequencies between racial groups have highly significant phenotypic consequences [59].Significant differences in gene expression phenotypes have been reported for at least 25 percent of genes between Europeans and Asians, and specific genetic variants (allele frequencies) between populations are the main cause of these differences [59].Therefore, in Mendelian randomisation analyses, genetic differences in quantitative phenotypes between different ethnic groups may be functionally equally important. Environmental, genetic, dietary, and lifestyle factors in different racial groups may influence phenotypic results [60], so we think it is unavoidable that the results of MR may differ between races due to residual confounding and selection bias [61, 62].This is our limitation in this study. Therefore, whether elevated IP-10 and IL-2ra increase the risk of MPN prevalence in other populations requires specific analyses of gene expression variation for particular populations. Future studies will also need to enhance the analysis of gene expression variation between populations to improve understanding of the underlying genetics and population differences observed in complex genetic diseases. In the second, there are three subtypes of MPN, and due to the limitations of the GWAS dataset, we have not specifically stratified to explore the relationship between cytokines and the different subtypes of MPN. Finally, after Bonferroni correction, no cytokines showed statistically significant associations with MPN risk, and only two of them (IP-10, IL-2rα) showed suggestive associations.
In conclusion, our study suggests that elevated circulating levels of IP-10 and IL-2rα are associated with a high risk of MPN. Potential positive correlation between genetically predicted MPN and levels of the cytokines IL-10, MIG, and RANTES. Our results show that cytokines play a significant role in the pathophysiology of MPN. Further research is required on the potential use of these biomarkers for the prevention and treatment of MPN.
Availability of data and materials
The datasets analyzed during the current study are available in the FinnGen repository, More details in FinnGen are described at https://r9.finngen.fi.
References
Grinfeld J, et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N Engl J Med. 2018;379(15):1416–30.
Tefferi A. Myeloproliferative neoplasms: A decade of discoveries and treatment advances. Am J Hematol. 2016;91(1):50–8.
Rungjirajittranon T, et al. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer. 2019;19(1):184.
Abdul-Rahim AH, et al. National institutes of health stroke scale item profiles as predictor of patient outcome: external validation on independent trial data. Stroke. 2015;46(2):395–400.
Papageorgiou L, et al. Thrombotic and Hemorrhagic Issues Associated with Myeloproliferative Neoplasms. Clin Appl Thromb Hemost. 2022;28:10760296221097968.
Brkic S, Meyer SC. Challenges and Perspectives for Therapeutic Targeting of Myeloproliferative Neoplasms. Hemasphere. 2021;5(1):e516.
Vannucchi AM, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35.
Verstovsek S, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55.
Cacemiro MDC, et al. Philadelphia-negative myeloproliferative neoplasms as disorders marked by cytokine modulation. Hematol Transfus Cell Ther. 2018;40(2):120–31.
Braun LM, Zeiser R. Immunotherapy in Myeloproliferative Diseases. Cells. 2020;9(6):1559.
Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Mantovani A, et al. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50(4):778–95.
Nachbaur DM, et al. Serum levels of interleukin-6 in multiple myeloma and other hematological disorders: correlation with disease activity and other prognostic parameters. Ann Hematol. 1991;62(2–3):54–8.
Panteli KE, et al. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol. 2005;130(5):709–15.
Ramanathan G, Fleischman AG. The Microenvironment in Myeloproliferative Neoplasms. Hematol Oncol Clin North Am. 2021;35(2):205–16.
Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res. 2013;37(2):214–20.
Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98.
Davies N.M, Holmes M.V, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Sun Y, et al. Assessing the causal role of hypertension on left atrial and left ventricular structure and function: A two-sample Mendelian randomization study. Front Cardiovasc Med. 2022;9:1006380.
Ahola-Olli A, et al. Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am J Hum Genet. 2017;100(1):40–50.
Zhang Y, et al. Causal Association Between Tea Consumption and Kidney Function: A Mendelian Randomization Study. Front Nutr. 2022;9:801591.
Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Larsson SC, et al. Type 2 diabetes, glucose, insulin, BMI, and ischemic stroke subtypes: Mendelian randomization study. Neurology. 2017;89(5):454–60.
Bowden J, Hemani G, Davey Smith G. Invited Commentary: Detecting Individual and Global Horizontal Pleiotropy in Mendelian Randomization-A Job for the Humble Heterogeneity Statistic? Am J Epidemiol. 2018;187(12):2681–5.
Verbanck M, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52.
Chen X, et al. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med. 2020;9(23):9160–7.
Liu M, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22(3):121–30.
Karin N, Razon H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine. 2018;109:24–8.
Obro NF, et al. Longitudinal Cytokine Profiling Identifies GRO-alpha and EGF as Potential Biomarkers of Disease Progression in Essential Thrombocythemia. Hemasphere. 2020;4(3):e371.
Tefferi A, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63.
Schnoder TM, et al. Cell autonomous expression of CXCL-10 in JAK2V617F-mutated MPN. J Cancer Res Clin Oncol. 2017;143(5):807–20.
Allain-Maillet S, et al. Anti-Glucosylsphingosine Autoimmunity, JAK2V617F-Dependent Interleukin-1beta and JAK2V617F-Independent Cytokines in Myeloproliferative Neoplasms. Cancers (Basel). 2020;12(9):2446.
Nyirenda MH, et al. JAK inhibitors disrupt T cell-induced proinflammatory macrophage activation. RMD Open. 2023;9(1):e002671.
Waldmann TA. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986;232(4751):727–32.
Jones MC, et al. CD4 Effector TCR Avidity for Peptide on APC Determines the Level of Memory Generated. J Immunol. 2023;210(12):1950–61.
Picard C, Casanova JL. Inherited disorders of cytokines. Curr Opin Pediatr. 2004;16(6):648–58.
Semenzato G, et al. High serum levels of soluble interleukin 2 receptor in patients with B chronic lymphocytic leukemia. Blood. 1987;70(2):396–400.
Steis RG, et al. Serum soluble IL-2 receptor as a tumor marker in patients with hairy cell leukemia. Blood. 1988;71(5):1304–9.
Wang JC, Wang A. Plasma soluble interleukin-2 receptor in patients with primary myelofibrosis. Br J Haematol. 1994;86(2):380–2.
Bourantas KL, et al. Serum beta-2-microglobulin, TNF-alpha and interleukins in myeloproliferative disorders. Eur J Haematol. 1999;63(1):19–25.
Masselli E, et al. Protein kinase Cvarepsilon inhibition restores megakaryocytic differentiation of hematopoietic progenitors from primary myelofibrosis patients. Leukemia. 2015;29(11):2192–201.
Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58–63.
Pourcelot E, et al. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol. 2014;42(5):360–8.
Gangemi S, et al. Evaluation of interleukin-23 plasma levels in patients with polycythemia vera and essential thrombocythemia. Cell Immunol. 2012;278(1–2):91–4.
Kleppe M, et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015;5(3):316–31.
Li Q, et al. Activation of macrophage TBK1-HIF-1alpha-mediated IL-17/IL-10 signaling by hyperglycemia aggravates the complexity of coronary atherosclerosis: An in vivo and in vitro study. FASEB J. 2021;35(5):e21609.
Koenen RR, et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15(1):97–103.
Shi H, et al. CRISPR/Cas9 based blockade of IL-10 signaling impairs lipid and tissue homeostasis to accelerate atherosclerosis. Front Immunol. 2022;13:999470.
Park MK, et al. The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. J Immunol. 2002;169(3):1433–43.
Koper OM, et al. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27(6):849–56.
von Hundelshausen P, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103(13):1772–7.
Huo Y, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9(1):61–7.
Ley K. Role of the adaptive immune system in atherosclerosis. Biochem Soc Trans. 2020;48(5):2273–81.
Xu S, et al. The role of interleukin-10 family members in cardiovascular diseases. Int Immunopharmacol. 2021;94:107475.
Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(12):1876–90.
Han X, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost. 2015;113(3):505–12.
Spielman RS, et al. Common genetic variants account for differences in gene expression among ethnic groups. Nat Genet. 2007;39(2):226–31.
Zhao Y, et al. Associations between type 2 diabetes mellitus and chronic liver diseases: evidence from a Mendelian ranldomization study in Europeans and East Asians. Front Endocrinol (Lausanne). 2024;15:1338465.
Au Yeung SL, et al. Evaluating the role of non-alcoholic fatty liver disease in cardiovascular diseases and type 2 diabetes: a Mendelian randomization study in Europeans and East Asians. Int J Epidemiol. 2023;52(3):921–31.
Ford I, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol. 2011;40(6):1530–8.
Acknowledgements
We appreciate all investigators for sharing these data in our study.
Funding
This study was supported by Commissioned by the National Clinical Medical Research Center for Hematological Diseases (grant no. 2021WWA01) and the Construction of the clinical medical research center of the Gansu Science and Technology project (grant no. 21JR7RA435) and the Gansu Province Science and technology project natural science foundation (grant no. 21JR11RA104) and Lanzhou science and technology development plan project(grant no. 2020-ZD-99) and The Second Hospital of Lanzhou University "Cui-ying Science and Technology Innovation" Program (grant no. 2020QN-13).
Author information
Authors and Affiliations
Contributions
LSZ and LJL conceived, designed, and supervised the whole study. HX,HTZ performed the analyses. JB and YHL interpreted the results, and contributed in this work in study design, data interpretation, and manuscript writing.HX and HTZ wrote the manuscript. All authors provided critical comments and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent for participate
Not applicable.
Consent for publication
The authors have given consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiong, H., Zhang, H., Bai, J. et al. Associations of the circulating levels of cytokines with the risk of myeloproliferative neoplasms: a bidirectional mendelian-randomization study. BMC Cancer 24, 531 (2024). https://doi.org/10.1186/s12885-024-12301-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12301-x