Abstract
Background
The COVID-19 pandemic might have delayed cancer diagnosis and management. The aim of this systematic review was to compare the initial tumor stage of new cancer diagnoses before and after the pandemic.
Methods
We systematically reviewed articles that compared the tumor stage of new solid cancer diagnoses before and after the initial pandemic waves. We conducted a random-effects meta-analysis to compare the rate of metastatic tumors and the distribution of stages at diagnosis. Subgroup analyses were performed by primary tumor site and by country.
Results
From 2,013 studies published between January 2020 and April 2022, we included 58 studies with 109,996 patients. The rate of metastatic tumors was higher after the COVID-19 outbreak than before (pooled OR: 1.29 (95% CI, 1.06-1.57), I2: 89% (95% CI, 86-91)). For specific cancers, common ORs reached statistical significance for breast (OR: 1.51 (95% CI 1.07-2.12)) and gynecologic (OR: 1.51 (95% CI 1.04-2.18)) cancers, but not for other cancer types. According to countries, common OR (95% CI) reached statistical significance only for Italy: 1.55 (1.01-2.39) and Spain:1.14 (1.02-1.29). Rates were comparable for stage I-II versus III-IV in studies for which that information was available, and for stages I-II versus stage III in studies that did not include metastatic patients.
Conclusions
Despite inter-study heterogeneity, our meta-analysis showed a higher rate of metastatic tumors at diagnosis after the pandemic. The burden of social distancing policies might explain those results, as patients may have delayed seeking care.
Similar content being viewed by others
Background
In 2020, the COVID-19 pandemic disrupted healthcare systems worldwide. In cancer care, screening programs were suspended in many countries, and care strategies were sometimes adapted to avoid exposing patients to COVID-19 infection, and to reduce the burden on intensive care units. Patients may also have avoided consulting for fear of being contaminated. As a result, screening decreased by 40 to 50%, and cancer diagnoses fell by 27% in January-October 2020 compared to the pre-COVID-19 period [1, 2].
Although recovery plans have been implemented in many countries, it is possible that patients with new cancers whose initial care was delayed could present more advanced tumors, with poorer prognosis. Indeed, modeling studies have anticipated thousands of additional cancer-related deaths in the coming years due to delays in diagnosis and treatment, resulting in tens of thousands of total years of life lost compared with pre-pandemic setting [3].
The aim of this systematic review of the literature with meta-analysis was to compare the proportion of metastatic presentations, and the distribution of initial tumor stage at diagnosis, before and after the COVID-19 outbreak, for patients with solid cancers.
Materials and methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA 2020) and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines ([4, 5]).
Data sources, literature searches and eligibility criteria
We searched PubMed and Embase databases for English-language original articles published between January 2020 and April 2022 that included information on the impact of the COVID-19 pandemic on solid malignant tumor stage, using Medical Subject Headings (MeSH) terms and free words. The complete search equation is available in Supplementary materials, Appendix S1. We screened the list of retrieved articles by evaluating titles first, then abstracts, and finally full texts. At each step, two independent investigators evaluated each article. A third independent investigator settled disagreements. We included articles in English that compared cancer stages at diagnosis before versus after the Covid-19 outbreak, using any relevant cancer staging guideline. We included only studies of adult patients, with solid malignant tumors. We excluded reviews, editorials, posters, letters, and guidelines. A reminder list of inclusion and exclusion criteria are available in Appendix S2.
Data collection and risk of bias assessment
For each article, two independent investigators collected items of interest which are summarized in Appendix S3. In the event of a discrepancy, a third independent investigator settled the issue. We did not contact any study author. We classified primary tumor types as displayed in Appendix S4. We assessed the risk of bias of the included studies with the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
Data analysis
We used the rate of metastatic tumors for comparing cancer presentation before and after the initial COVID-19 pandemic waves. We calculated odds ratio (OR) and 95% confidence intervals (CI) for each study, and we then pooled these individual ORs using a random-effects meta-analysis. Subgroup analyses were performed according to the primary tumor site (when this information was available), and to the study country.
We conducted meta-analysis across all studies and at the subgroup level using the Mantel-Haenszel method. Because we expected heterogeneity between studies, we used the Hartung-Knapp method to calculate CIs on the main effect estimate, with a variance correction [6, 7]. We computed prediction intervals for exposure effect based on Hartung and Knapp’s method [8]. Results were graphically represented in forest plots. The extent of interstudy heterogeneity and subgroup differences were assessed with the Cochran I2 statistics and X2 tests, respectively. Between-study variance Tau2 was assessed using the Sidik and Jonkman’s approach and the Q-Profile method for Tau2’s CI [9, 10]. We applied a continuity correction of 0.5 in studies with zero events in one arm.
When articles mentioned missing data or unknown status for some patients, we ignored those patients. When studies overlapped, we only included the study with the broadest inclusion criteria [11]. In situ tumors were excluded from the analysis.
For analyses where more than ten studies could be included, we plotted funnel plots and conducted Thompson and Sharp’s arcsine test to assess the presence of small study effects [12, 13].
Using the same methods, we performed meta-analysis on the rate of stage I-II versus III-IV for the studies where this information was available. When studies mentioned ‘advanced stages’ with no more detailed information, we counted the ‘advanced’ patients as stage IV when a separate ‘locally advanced’ category was also available. These data were then included in the first analysis of metastatic vs non-metastatic status. If no separate ‘locally advanced’ category was available alongside ‘advanced’, we could not know if these ‘advanced’ patients were stage III or IV, and these data were included in the ‘stage I-II vs III-IV’ analysis.
Finally, for studies that did not include metastatic patients (e.g., studies that focused on patients undergoing surgery), we performed a separate meta-analysis comparing stage I-II versus stage III.
All analyses were conducted using R v.2.2.2 (The R project for statistical computing, www.r-project.org) and the meta package (v6.2.1.) [14]. No ethics committee approval and no patient consent were necessary because the study was restricted to publicly available data.
Results
Study characteristics
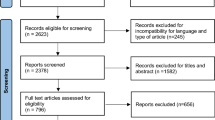
We identified 2,013 studies published between January 2020 and April 2022, and included 58 studies in our meta-analysis ([15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], Fig. 1, Supplementary Table S1). These articles covered Europe, Asia, North and South America (Supplementary Figure S1). The quality assessment of included studies is presented in Supplementary Table S2. For each study location, lockdowns and cancer screening postponement were summarized in Supplementary Table S3). Breast cancer was the most represented cancer type.
Forty-five studies (98,307 patients) compared metastatic stages IV versus non-metastatic stages I-II-III. Nine studies (7316 patients) compared stages I-II versus III-IV (some articles contributed to both the first and the second analyses) and six (4,373 patients) compared stages I-II versus III without including metastatic patients. The number of patients included per study ranged from 44 to 54,828 (Supplementary Table S1).
Two instances of overlapping studies were identified. In the case of two Dutch register-based studies, we kept the study that included all patients over the one that focused on screening and included only patients aged 50-74 [15, 73]. In the case of two Italian studies, one monocentric and one multicentric that included the previous center, we kept the multicentric study [16, 17].
Metastatic versus non-metastatic
In the 45 studies that contained information on metastatic stage shift, the OR (95% CI) on metastatic stage after vs before the COVID-19 outbreak reached 1.29 (1.06-1.57), indicating a higher probability of patients being metastatic after the outbreak (Fig. 2). Heterogeneity between studies was high, with a I2 of 89% (95% CI, 86-91) and ORs varying from 0.14 to 12.07. Funnel plot showed uneven distribution of small studies (Fig. 3), but the arcsine test was not significant (p = 0.25).
In subgroup analysis per country, results reached statistical significance for Italy (seven studies) and Spain (one study), with ORs of 1.55 (1.01-2.39) and 1.14 (1.02-1.29) respectively (Fig. 3). In other countries with more than one study, ORs were 1.65 (0.37-7.32) for Turkey, 1.14 (0.46-2.84) for the Netherlands, 1.88 (0.78-4.53) for the US, 1.48 (0.00-1,135.67) for France, 0.96 (0.26-3.53) for Germany, 1.36 (0.26-7.20) for Portugal, 0.91 (0.15-5.57) for China, 1.77 (0.76-4.10) for the United Kingdom, and 1.24 (0.75-2.04) for South Korea.
In subgroup analysis per location, the related OR reached statistical significance for breast and gynecologic cancers: 1.51 (1.07-2.12) and 1.51 (1.04-2.18) respectively (Fig. 4). ORs for other cancer types did not reach statistical significance: 0.79 (0.18-3.52) for lung cancer, 1.15 (0.89-1.49) for colorectal cancer, 1.45 (0.62-3.42) for other types of digestive cancers, 2.26 (0.51-10.05) for prostate cancer, 12.07 (0.57-253.68) for genito-urinary cancer (one study only), 2.49 (0.00-84,469.69) for melanomas, and 1.01 (0.59-1.75) for other types of cancers (X2 = 24.60, p<0.01) (Fig. 4a). The funnel plot for breast cancer (the only cancer type with more than ten studies) is available in Supplementary material (Figure S2). Arcsine test was non-significant (p = 0.76).
Localized versus advanced cancer (Stages I-II vs III-IV)
In the analysis of localized versus advanced cancer stages (i.e., stages I-II vs III-IV), the pooled OR was 1.48 (0.84-2.62) (Fig. 5). None of the subgroup analyses per location or per country reached statistical significance (Figs. 5 and 6). Heterogeneity was high, with I2 = 51% (0-77) and ORs varying between 0.13 and 10.67
Studies with no metastatic patients (Stages I-II vs III)
In the analysis of Stages I-II vs III, in studies that did not include metastatic patients, the pooled OR was 1.32 (0.92-1.89) (Figs. 7 and 8).
Discussion
We have reviewed published evidence on the impact of the Covid19 outbreak on cancer stages at diagnosis. The results of the main analysis (45 studies) showed an increased rate of metastatic stages at initial clinical presentation, for new solid cancer cases, after compared to before the COVID-19 outbreak. Subgroup analyses yielded significant results for breast and gynecologic cancers, and for Italy and Spain. Secondary analyses on Stages I-II vs III-IV (nine studies) and Stages I-II vs III (for the six studies that excluded metastatic patients) yielded non-significant results.
Based on these results, one may conclude that the pandemic has been associated with more severe forms of cancer at diagnosis. However, we noticed large variations between countries, as well as between tumor locations.
This heterogeneity is still present in more recent observational studies. For example, studies found significant stage shifts for melanomas in the US and Greece, for lung cancer in the UK, for breast cancer in Brazil and for genito-urinary cancers in Iran [74,75,76,77,78]. For colorectal cancer, stage shifts were absent in Canada and the US but noticeable in Italy and South Korea [79,80,81,82]. In a systematic review, Pararas et al. analyzed stage shifting for colorectal cancer. They noted a significant increase in the number of patients presenting with de novo metastatic neoplasms during the pandemic (OR 1.65, 95% CI 1.02–2.67) [83]. We found a positive but non-significant association between the Covid19 pandemic and de novo metastatic colorectal cancer, but our analysis included less patients for this location, which may explain the difference in findings.
In our study, Italy and Spain were associated with significant increase of de novo metastatic tumor stages. In the case of Italy, this might be due to the number of studies included (seven studies including 7,423 patients, versus five for the UK, six for the US and one to three for other countries). Interruptions to national screening programs could partly explain the excess of metastatic cases observed in Italy [84,85,86]. We also observed a significant increase in metastatic stages in Spain. However, we included only one Spanish study, limited to Malaga’s region, and more recent Spanish studies have obtained contrasting results [87, 88].
We found a significantly lower presence of metastatic stages at diagnosis after the COVID-19 outbreak in Taiwan and Canada. We included only one small study from each of these countries, so the results should be interpreted with caution. Taiwan drew on its experience of the 2003 SARS pandemic, and applied early policies of travel regulation, testing, and prevention, avoiding lockdowns and screening postponoments [89, 90]. We included a single Canadian study about lung cancer in Quebec. In the same province, Ramanakumar et al. did not find any significant difference in Stage IV for lung cancer before and after the pandemic [91].
We found significantly more metastatic stages at diagnosis after the Covid-19 outbreak for breast and gynecological cancers. In both cases, we included multiple large studies (15 studies with 23,409 patients for breast cancer, 5 studies with 6,270 patients for gynecological cancers). In both cases, interruptions of screening programs may have contributed to the result.
From a general point of view, our results suggest that cancer care disruptions such as national lockdowns and national screening programs postponement led to more severe cancer cases with more metastasis at diagnosis. Unfortunately, these findings only give weight to the dark projections obtained in modelling studies, which anticipate an increase in cancer-related deaths [92,93,94]. Lockdowns and interruptions to screening programs were probably only one factor contributing to the decrease in diagnoses. Patients feared Covid-19 infection, sometimes more than cancer [95, 96], which can explain the prolonged impact on care seeking behaviors.
We conducted a systematic review of the academic literature, with dual, blinded study selection and data extraction. We covered all cancer types and regions. We also analyzed studies per cancer type and per country, to account for the possibility of different impacts of the pandemic. We included a large number of studies, covering 109,996 patients over 19 countries. However, some limitations must be taken into consideration when considering our findings.
Many studies in our review were small and monocenter. Researchers in areas most affected by the pandemic may have been more prone to report observational data, generating a publication bias (although this was not detected in our analyses). Monocentric studies also cannot account for potential reconfigurations in care trajectories, with some hospitals attracting more cancer patients during the pandemic while others focused on Covid-19 care. Only a minority of studies were population-based, which is the only way to mitigate these issues. When analyzing studies by country, we could not account for variations between regions, including the level of restrictions imposed (e.g., between American states).
We also noticed methodological differences. The way pre- and post-Covid time periods were defined varied between studies. Some studies focused on a short period at the apex of the pandemic, when screening programs stopped, and their area was under lockdown. It is likely that only the most serious patients presented to hospital at these times, increasing the rate of advanced tumors while the absolute number of patients decreased. Other studies defined the COVID-19 period more broadly. Data sources were also heterogeneous in our sample. A few studies were based on registries, which normally guarantee good data completeness and reliability. Other studies mixed data sources, used EHRs or claims data. This may have affected both completeness and quality of the data.
Finally, we only included English sources, and focused on full articles. Data may also have been shared in other languages, and studies presented as conference abstracts may not have been published as full articles.
Despite these limitations, our results suggest that national cancer screening programs should be maintained in high-risk populations even during infectious outbreak waves. After the disruptions, platforms of rapid cancer diagnosis might compensate the interruptions of screening programs and clear diagnosis backlogs. The issue of how cancer care recovers from the pandemic would require population-based studies. Such studies are likely to be available only once registries have been updated, which may take some time [97]. These studies should also look at the evolution of patient survival, given the dark picture painted by modeling studies. Finally, the data we analyzed comes overwhelmingly from high-income countries. Analyzing outcomes in low- and middle-income countries is important to understand how their healthcare systems have worked to mitigate the pandemic impact.
Conclusion
The COVID-19 outbreak has affected cancer management around the world. This meta-analysis of 58 articles from 19 countries showed an increased rate of metastatic stages at initial clinical presentation for new solid cancer cases diagnosed after the COVID-19 outbreak, with variations between cancer types and between countries. Future studies on the long-term consequences of the pandemic should also assess the impact on patient survival.
Availability of data and materials
The data and the code that support the findings of this study are available on request from the corresponding author.
References
Teglia F, Angelini M, Astolfi L, Casolari G, Boffetta P. Global Association of COVID-19 Pandemic Measures With Cancer Screening: A Systematic Review and Meta-analysis. JAMA Oncol. 2022;8(9):1287–93.
Angelini M, Teglia F, Astolfi L, Casolari G, Boffetta P. Decrease of cancer diagnosis during COVID-19 pandemic: a systematic review and meta-analysis. Eur J Epidemiol. 2023;38(1):31–8.
Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–34.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156(8):787–8. https://doi.org/10.1001/jamasurg.2021.0522.
Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–89.
Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20(12):1771–82.
Partlett C, Riley RD. Random effects meta-analysis: Coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med. 2017;36(2):301–17.
Sidik K, Jonkman JN. Simple heterogeneity variance estimation for meta-analysis [Internet]. Appl Statist. 2005;54 Available from: https://academic.oup.com/jrsssc/article/54/2/367/7112945
Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26(1):37–52.
Senn SJ. Overstating the evidence: double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;13(9):10.
Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27(5):746–63.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Eijkelboom AH, de Munck L, Lobbes MBI, van Gils CH, Wesseling J, Westenend PJ, et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med (Baltim). 2021;151:106602.
Vanni G, Pellicciaro M, Materazzo M, Pedini D, Portarena I, Buonomo C, et al. Advanced stages and increased need for adjuvant treatments in breast cancer patients: The effect of the one-year covid-19 pandemic. Anticancer Res. 2021;41(5):2689–96.
Vanni G, Tazzioli G, Pellicciaro M, Materazzo M, Paolo O, Cattadori F, et al. Delay in breast cancer treatments during the first COVID-19 lockdown. a multicentric analysis of 432 patients. Anticancer Res. 2020;40(12):7119–25.
Kaltofen T, Hagemann F, Harbeck N, Wuerstlein R, Kost BP, Burges A, et al. Changes in gynecologic and breast cancer diagnoses during the first wave of the COVID-19 pandemic: analysis from a tertiary academic gyneco-oncological center in Germany. Arch Gynecol Obstet. 2021; https://doi.org/10.1007/s00404-021-06211-7.
Knoll K, Reiser E, Leitner K, Kögl J, Ebner C, Marth C, et al. The impact of COVID-19 pandemic on the rate of newly diagnosed gynecological and breast cancers: a tertiary center perspective. Arch Gynecol Obstet. 2021; https://doi.org/10.1007/s00404-021-06259-5.
Lim JH, Lee WY, Yun SH, Kim HC, Cho YB, Huh JW, et al. Has the COVID-19 pandemic caused upshifting in colorectal cancer stage? Ann Coloproctol. 2021;37(4):253–8.
Davis CH, Ho J, Greco SH, Koshenkov VP, Vidri RJ, Farma JM, et al. COVID-19 is Affecting the Presentation and Treatment of Melanoma Patients in the Northeastern United States. Ann Surg Oncol. 2021; https://doi.org/10.1245/s10434-021-11086-8.
Bonadio RC, Messias AP, Moreira OA, Leis LV, Orsi BZ, Testa L, et al. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience. 2021;15
Caldarella C, Cocciolillo F, Taralli S, Lorusso M, Scolozzi V, Pizzuto DA, et al. The impact of the COVID-19 pandemic on oncological disease extent at FDG PET/CT staging: the ONCOVIPET study. Eur J Nucl Med Mol Imaging. 2021;
Kuzuu K, Misawa N, Ashikari K, Kessoku T, Kato S, Hosono K, et al. Gastrointestinal Cancer Stage at Diagnosis before and during the COVID-19 Pandemic in Japan. JAMA Netw Open. 2021;
Ruiz-Medina S, Gil S, Jimenez B, Rodriguez-Brazzarola P, Diaz-Redondo T, Cazorla M, et al. Significant decrease in annual cancer diagnoses in spain during the covid-19 pandemic: A real-data study. Cancers (Basel). 2021;13(13)
Lucidi D, Valerini S, Federici G, Miglio M, Cantaffa C, Alicandri-Ciufelli M. Head and Neck Cancer During Covid-19 Pandemic: Was there a Diagnostic Delay? Indian Journal of Otolaryngology and Head and Neck. Surgery. 2022;
Dauti Işıklar A, Deniz C, Soyder A, Güldoğan N, Yılmaz E, Başaran G. How Do Breast Cancer Patients Present Following COVID-19 Early Peak in a Breast Cancer Center in Turkey? Eur J Breast Health. 2021;17(3):253–7.
Nossiter J, Morris M, Parry MG, Sujenthiran A, Cathcart P, van der Meulen J, et al. Impact of the COVID-19 pandemic on the diagnosis and treatment of men with prostate cancer. BJU Int. 2022;130(2):262–70.
Cai M, Wang GG, Wu Y, Wang Z, Wang GG, Tao K. Study of the gastrointestinal tumor progression during the COVID-19 epidemic in Wuhan. British J Surg. 2020;107(11):e502–3.
Hawrot K, Shulman LN, Bleiweiss IJ, Wilkie EJ, Frosch ZAK, Jankowitz RC, et al. Time to Treatment Initiation for Breast Cancer During the 2020 COVID-19 Pandemic. JCO Oncol Pract. 2021;17(9):534–40.
Kuusk T, Cullen D, Neves JB, Campain N, Barod R, Boleti E, et al. Impact of the first surge of the COVID-19 pandemic on a tertiary referral centre for kidney cancer. BJU Int. 2021;128(6):752–8.
Pepe P, Pepe L, Pennisi M, Fraggetta F. Prostate cancer diagnosis and management during one year of the COVID-19 pandemic. Anticancer Res. 2021;41(6):3127–30.
Brito M, Laranjo A, Sabino J, Oliveira C, Mocanu I, Fonseca J. Digestive Oncology in the COVID-19 Pandemic Era. GE Port J Gastroenterol. 2021 Mar 22 579(5):1–8.
Purushotham A, Roberts G, Haire K, Dodkins J, Harvey-Jones E, Han L, et al. The impact of national non-pharmaceutical interventions ('lockdowns’) on the presentation of cancer patients. Ecancermedicalscience. 2021;15
Riju J, Tirkey AJ, Mathew M, Chamania G, Babu M, Patil S, et al. Analysis of Early Impact of COVID-19 on Presentation and Management of Oral Cancers – an Experience from a Tertiary Care Hospital in South India. Indian J Surg Oncol. 2021;12:242–9.
Heimes D, Müller LK, Schellin A, Naujokat H, Graetz C, Schwendicke F, et al. Consequences of the COVID-19 pandemic and governmental containment policies on the detection and therapy of oral malignant lesions—a retrospective, multicenter cohort study from germany. Cancers (Basel). 2021;13(12)
Morais S, Antunes L, Rodrigues J, Fontes F, Bento MJ, Lunet N. The impact of the COVID-19 pandemic on the short-term survival of patients with cancer in Northern Portugal. Int J Cancer. 2021;149(2):287–96.
Stevens MN, Patro A, Rahman B, Gao Y, Liu D, Cmelak A, et al. Impact of COVID-19 on presentation, staging, and treatment of head and neck mucosal squamous cell carcinoma. Am J Otolaryngol Head Neck Med Surg. 2022;43(1)
Kasymjanova G, Anwar A, Cohen V, Sultanem K, Pepe C, Sakr L, et al. The impact of COVID-19 on the diagnosis and treatment of lung cancer at a canadian academic center: A retrospective chart review. Current Oncol. 2021;28(6):4247–55.
Murri D, Botti C, Bassano E, Fornaciari M, Crocetta FM, Ghidini A. Reduction in healthcare services during the COVID-19 pandemic: Patient screening based on symptoms is an effective strategy for avoiding delayed laryngeal cancer diagnosis. Am J Otolaryngol. 2021;42(6):103162.
Goenka L, Anandaradje A, Nakka T, Kayal S, Dubashi B, Chaturvedula L, et al. The “collateral damage” of the war on COVID-19: impact of the pandemic on the care of epithelial ovarian cancer. Med Oncol. 2021;38(11)
Guven DC, Sahin TK, Yildirim HC, Cesmeci E, Incesu FGG, Tahillioglu Y, et al. Newly diagnosed cancer and the COVID-19 pandemic: tumour stage migration and higher early mortality. BMJ Support Palliat Care. 2021; https://doi.org/10.1136/bmjspcare-2021-003301.
Bertolaccini L, Ciani O, Prisciandaro E, Sedda G, Spaggiari L. Lung cancer stage distribution from before COVID-19 through 18 months of the pandemic: the experience of a large-volume oncological referral centre. Eur J Surg Oncol. 2021;48(2):470–1.
Oderda M, Soria F, Rosi F, Calleris G, Mazzoli S, Giordano A, et al. COVID-19 pandemic impact on uro-oncological disease outcomes at an Italian tertiary referral center. World J Urol. 2021; https://doi.org/10.1007/s00345-021-03842-y.
Ikemura M, Tomishima K, Ushio M, Takahashi S, Yamagata W, Takasaki Y, et al. Impact of the coronavirus disease-2019 pandemic on pancreaticobiliary disease detection and treatment. J Clin Med. 2021;10(18)
Zhang Y, Li J, Li ZK, Yang X, Bai J, Liu L, et al. Impact of Coronavirus Disease 2019 on Clinical Characteristics in Patients With Lung Cancer: A Large Single-Centre Retrospective Study. Front Oncol. 2021;16:11.
Zhang D, Fu Y, Zhou L, Liang N, Wang T, Del Rio P, et al. Thyroid surgery during coronavirus-19 pandemic phases I, II and III: lessons learned in China, South Korea, Iran and Italy. J Endocrinol Invest. 2021;44(5):1065–73.
Borsky K, Shah K, Cunnick G, Tsang-Wright F. Pattern of breast cancer presentation during the COVID-19 pandemic: results from a cohort study in the UK. Future Oncol. 2022;18(4):437–43.
Wai KC, Xu MJ, Lee RH, El-Sayed IH, George JR, Heaton CM, et al. Head and neck surgery during the coronavirus-19 pandemic: The University of California San Francisco experience. Head Neck. 2021;43(2):622–9.
Chou CP, Lin HS. Delayed breast cancer detection in an asian country (Taiwan) with low covid-19 incidence. Cancer Manag Res. 2021;13:5899–906.
Toss A, Isca C, Venturelli M, Nasso C, Ficarra G, Bellelli V, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6(2)
Meerwein CM, Stadler TM, Balermpas P, Soyka MB, Holzmann D. Diagnostic pathway and stage migration of sinonasal malignancies in the era of the COVID-19 pandemic. Laryngoscope Investig Otolaryngol. 2021;6(5):904–10.
İlgün AS, Özmen V. The Impact of the COVID-19 Pandemic on Breast Cancer Patients. Eur J Breast Health. 2022;18(1):85–90.
Miyawaki Y, Sato H, Lee S, Fujita S, Oya S, Sugita H, et al. Impact of the coronavirus disease 2019 pandemic on first-visit patients with oesophageal cancer in the first infection wave in Saitama prefecture near Tokyo: a single-centre retrospective study. Jpn J Clin Oncol. 2022;52(5):456–65.
Szewczyk M, Pazdrowski J, Golusiński P, Pazdrowski P, Więckowska B, Golusiński W. The impact of the COVID-19 pandemic on the management of head and neck cancer patients at a tertiary care institution in Poland. Współczesna Onkologia. 2021;25(4):264–9.
Bogani G, Scambia G, Cimmino C, Fanfani F, Costantini B, Loverro M, et al. Characteristics and patterns of care of endometrial cancer before and during COVID-19 pandemic. J Gynecol Oncol. 2022;33(1):e10.
Choi JY, Park IJ, Lee HG, Cho E, Il KY, Kim CW, et al. Impact of the COVID-19 pandemic on surgical treatment patterns for colorectal cancer in a tertiary medical facility in Korea. Cancers (Basel). 2021;13(9)
Dolan DP, Swanson SJ, Lee DN, Polhemus E, Kucukak S, Wiener DC, et al. Esophagectomy for Esophageal Cancer Performed During the Early Phase of the COVID-19 Pandemic. Semin Thorac Cardiovasc Surg. 2021;34(3):1075–80.
Kempf E, Priou S, Lamé G, Daniel C, Bellamine A, Sommacale D, et al. Impact of two waves of Sars-Cov2 outbreak on the number, clinical presentation, care trajectories and survival of patients newly referred for a colorectal cancer: A French multicentric cohort study from a large group of university hospitals. Int J Cancer. 2022;150(10):1609–18.
Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J, et al. Impact of the COVID-19 Pandemic on the Diagnosis and Surgery of Breast Cancer: A Multi-Institutional Study. J Breast Cancer. 2021;24(6):491–503.
Schoonbeek RC, de Jel DVC, van Dijk BAC, Willems SM, Bloemena E, Hoebers FJP, et al. Fewer head and neck cancer diagnoses and faster treatment initiation during COVID-19 in 2020: A nationwide population-based analysis: Impact of COVID-19 on head and neck cancer. Radiother Oncol. 2022;167:42–8.
Davies JM, Spencer A, Macdonald S, Dobson L, Haydock E, Burton H, et al. Cervical cancer and COVID—an assessment of the initial effect of the pandemic and subsequent projection of impact for women in England: A cohort study. BJOG. 2022;129(7):1133–9.
Elibol E, Koçak Ö, Sancak M, Arslan B, Gül F, Babademez MA. The effect of COVID-19 pandemic on laryngeal cancer in a tertiary referral center. Eur Arch Oto-Rhino-Laryngol. 2022; https://doi.org/10.1007/s00405-022-07268-z.
Tang A, Neeman E, Vuong B, Arasu VA, Liu R, Kuehner GE, et al. Care in the time of COVID-19: impact on the diagnosis and treatment of breast cancer in a large, integrated health care system. Breast Cancer Res Treat. 2022; https://doi.org/10.1136/bmjspcare-2021-003301.
Park JY, Lee YJ, Kim T, Lee CY, Il KH, Kim JH, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer. 2020;20(1).
Shinkwin M, Silva L, Vogel I, Reeves N, Cornish J, Horwood J, et al. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Disease. 2021;23(8):2014–9.
Thompson JA, Lubek JE, Amin N, Joy R, Dyalram D, Ord RA, et al. Impact of the Novel Coronavirus 2019 (COVID-19) Pandemic on Head and Neck Cancer Care. Otolaryngol- Head Neck Surg (United States). 2022;166(1):93–100.
Lee T, Cheng DZ, Foo FJ, Sivarajah SS, Ho LML, Aw D, et al. Did the COVID-19 lockdown result in a delay of colorectal cancer presentation and outcomes? A single centre review. Langenbecks Arch Surg. 2022 Jan; https://doi.org/10.1007/s00423-022-02448-1.
Simão D, Sardinha M, Reis AF, Spencer AS, Luz R, Oliveira S. What Has Changed During the COVID-19 Pandemic? - The Effect on an Academic Breast Department in Portugal. Eur J Breast Health. 2022;18(1):74–8.
Kiong KL, Diaz EMEM, Gross ND, Diaz EMEM, Hanna EY. The impact of COVID-19 on head and neck cancer diagnosis and disease extent. Head Neck. 2021;43(6):1890–7.
Linck PA, Garnier C, Depetiteville MP, MacGrogan G, Mathoulin-Pélissier S, Quénel-Tueux N, et al. Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre. Eur Radiol. 2021; https://doi.org/10.1007/s00330-021-08264-3.
Romics L, Doughty J, Stallard S, Mansell J, Blackhall V, Lannigan A, et al. A prospective cohort study of the safety of breast cancer surgery during COVID-19 pandemic in the West of Scotland. Breast. 2021;55(55):1–6.
Eijkelboom AH, de Munck L, Vrancken Peeters MJTFD, Broeders MJM, Strobbe LJA, Bos MEMM, et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J Hematol Oncol. 2021;14(1)
Fanning JE, Kalsi S, Krag DN. Impact of the COVID-19 pandemic on melanoma diagnosis and presentation: a `review. Int J Dermatol. 2023; https://doi.org/10.1111/ijd.16684.
Hasegawa N, Rusakevich A, Bernicker E, Teh BS, Schefler A. Comparison of Tumor Size and Gene Expression at Presentation in Uveal Melanoma Patients before and during the COVID-19 Pandemic. Ocul Oncol Pathol. 2022;8(3):156–60.
Seretis K, Bounas N, Gaitanis G, Bassukas I. A Meta-Analysis on the Impact of the COVID-19 Pandemic on Cutaneous Melanoma Diagnosis in Europe. Cancers. 2022;14
Jaitly J, Mavilakandy A, Naeem M, Reddy RV, Goodman L, Johnson N, et al. Lung cancer recovery focus post pandemic: an income-deprived area paradigm. Clin Med (Lond). 2023;23(1):38–44.
Taheri D, Jahanshahi F, Khajavi A, Kafi F, Pouramini A, Farsani RM, et al. The Impact of Covid-19 Pandemic on Genitourinary Cancers Stage and Grade. Clin Genitourin Cancer. 2023;21(1):84–90.
Castonguay M, El Sayed R, Richard C, Vachon MF, Nassabein R, Charpentier D, et al. COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience. Current Oncology. 2022;29(5):3282–90.
Elamin D, Ozgur I, Steele SR, Khorana AA, Jia X, Gorgun E. Impact of COVID-19 pandemic on treatment of colorectal cancer patients. Am J Surg. 2023;225(5):934–6.
Frazzoni L, Dajti E, Silviu Ungureanu B. The impact of the COVID-19 pandemic on colorectal and gastric cancer diagnosis, disease stage and mortality [Internet]. Available from: www.cbs.gov.il
Park YY, Lee J, Lee K, yong, Oh ST. Short-Term Impact of Temporary Shutdown of a University-Affiliated Hospital on Patients With Colorectal Cancer During the Coronavirus Disease 2019 Pandemic. J Korean Med Sci. 2022;37(21)
Pararas N, Pikouli A, Papaconstantinou D, Bagias G, Nastos C, Pikoulis A, et al. Colorectal Surgery in the COVID-19 Era: A Systematic Review and Meta-Analysis. Cancers. 2022;14
Battisti F, Falini P, Gorini G, de Bianchi PS, Armaroli P, Giubilato P, et al. Cancer screening programmes in Italy during the COVID-19 pandemic: an update of a nationwide survey on activity volumes and delayed diagnoses. Ann Ist Super Sanita. 2022;58(1):16–24.
Mentrasti G, Cantini L, Zichi C, D’Ostilio N, Gelsomino F, Martinelli E, et al. Alarming Drop in Early Stage Colorectal Cancer Diagnoses After COVID-19 Outbreak: A Real-World Analysis from the Italian COVID-DELAY Study. Oncologist. 2022;27(9):e723–30.
Mentrasti G, Cantini L, Vici P, D’Ostilio N, La Verde N, Chiari R, et al. Rising incidence of late stage breast cancer after COVID-19 outbreak. Real-world data from the Italian COVID-DELAY study. Breast. 2022;65:164–71.
Garrido-Cantero G, Longo F, Hernández-González J, Pueyo Á, Fernández-Aparicio T, Dorado JF, et al. Impact of the COVID-19 Pandemic on Cancer Diagnosis in Madrid (Spain) Based on the RTMAD Tumor Registry (2019–2021). Cancers. 2023 Mar;15(6):1753.
Bosch G, Posso M, Louro J, Roman M, Porta M, Castells X, et al. Impact of the COVID-19 pandemic on breast cancer screening indicators in a Spanish population-based program: a cohort study. Elife. 2022;11:77434.
Tsai HY, Chang YL, Shen CT, Chung WS, Tsai HJ, Chen FM. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast. 2020;1(54):52–5.
Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: Big Data Analytics, New Technology, and Proactive Testing. JAMA – J Am Med Assoc. 2020;323:1341–2.
Ramanakumar AV, Annie B, Frederic L, Christine B, Cathy R, Jean L. Evaluating the impact of COVID-19 on cancer declarations in Quebec, Canada. Cancer Med. 2022;12(5):6260–9.
Duffy SW, Seedat F, Kearins O, Press M, Walton J, Myles J, et al. The projected impact of the COVID-19 lockdown on breast cancer deaths in England due to the cessation of population screening: a national estimation. Br J Cancer. 2022;126(9):1355–61.
Yong JH, Mainprize JG, Yaffe MJ, Ruan Y, Poirier AE, Coldman A, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28(2):100–7.
Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez de la Jara J, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol. 2021;22(10):1427–37.
Momenimovahed Z, Salehiniya H, Hadavandsiri F, Allahqoli L, Günther V, Alkatout I. Psychological Distress Among Cancer Patients During COVID-19 Pandemic in the World: A Systematic Review. Front Psychol. 2021;12:682154.
Guven DC, Sahin TK, Aktepe OH, Yildirim HC, Aksoy S, Kilickap S. Perspectives, Knowledge, and Fears of Cancer Patients About COVID-19. Front Oncol. 2020;28:10.
Jansen L, Schröder CC, Emrich K, Holleczek B, Pritzkuleit R, Brenner H. Disclosing progress in cancer survival with less delay. Int J Cancer. 2020;147(3):838–46.
Acknowledgements
* CRAB: Cancer Research Application on Big Data
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
Simon Marty had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SM, EK, GC, GL, SP, CT. Acquisition of data: SM, EK, GC, GL, EG, SP. Analysis and interpretation of data: SM, EK, GC, GL, CT. Drafting of the manuscript: SM, EK, GC, GL. Critical revision of the manuscript for important intellectual content: SM, EK, GC, GL, CT, SP, EG. Statistical analysis: GL. Administrative, technical, or material support: EG, GL, EK. Supervision: EK, GC, GL, CT. Other: None
Corresponding author
Ethics declarations
Ethic approval and consent to participate
Not applicable.
Competing interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Location of included studies. Figure S2. Funnel plot for subgroup metastatic vs non-metastatic analysis on breast cancer. Table S1. Study characteristics. Table S2. Study quality assessment60. Table S3. Synthesis of first national lockdowns and cancer screening disruptions. Appendix S1. Pubmed and EMBASE search equations. Appendix S2. Inclusion and exclusion criteria for article selection. Appendix S3. List of data elements we extracted from included articles. Appendix S4. Classification of primary cancer types.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marty, S., Lamé, G., Guével, E. et al. Impact of the Sars-Cov-2 outbreak on the initial clinical presentation of new solid cancer diagnoses: a systematic review and meta-analysis. BMC Cancer 24, 143 (2024). https://doi.org/10.1186/s12885-023-11795-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11795-1