Abstract
Background
Cancer survivors are at risk of developing second and subsequent primary cancers, referred to as multiple primary cancers (MPCs). It is not clear whether the risk of MPCs has increased over recent decades, but increasing use of radiological imaging and potentially harmful effects of certain cancer treatments raise this possibility. A systematic review was undertaken to assess whether there has been a temporal change in the risk of developing MPCs.
Methods
A systematic search to identify population-based studies of MPCs was performed in Medline/PubMed and Embase databases from inception to August 2016. Included studies were those reporting risk of MPCs for all sites combined following a first cancer at any site or a specific site, using standard incidence ratios (SIRs) or equivalent, and with analysis stratified by calendar years.
Results
We identified 28 articles eligible for inclusion, comprising 26 population-based studies and two monographs. MPC incidence was reported in nearly 6.5 million cancer survivors. For all first cancer sites combined, a higher rate of MPCs was reported in more recent than earlier calendar periods in four of the six relevant studies. The SIRs ranged from 1.14 for a first cancer diagnosis in the early 1980s to 1.21–1.46 in the late 1990s in the USA and Australia. Two studies from Italy and France showed no significant difference in SIRs across time periods 1978–2010 and 1989–2004. The remaining 22 studies reported various temporal trends in the risk of developing MPCs after a first cancer at a specific site, but most showed little change.
Conclusion
Overall, the risk of developing MPCs appears to have increased since the 1980s when considering studies of all primary cancer sites combined from the USA and Australia but not from Europe. With the introduction of more routine nuclear medical imaging over the last 15 years, more studies are needed to confirm recent trends of MPC risk in adult cancer survivors.
Similar content being viewed by others
Background
Survival for most cancers has increased steadily over the last three decades, mainly due to increased detection of early-stage cancers and advances in cancer treatment [1, 2]. This has been a global phenomenon and has led to a growing number of cancer survivors worldwide [3, 4]. Increasing attention has been given to the long-term outcomes of cancer survivors including the risk of developing new primary cancers [5, 6]. In a seminal report from the USA, up to 10 % of cancer survivors were diagnosed with a second or higher-order primary cancers during a 27-year period 1973 to 2000 [7]. A higher rate of new cancers was observed among cancer survivors with a first cancer diagnosed in more recent (between 1995 and 2000) than in earlier time periods (1973–79).
Two or more primary cancers occurring in the same individual that are neither extensions, recurrences nor metastases of each other are defined as Multiple Primary Cancers (MPCs) [8]. Factors associated with any change in the risk of developing MPCs might include increased use of diagnostic imaging and adverse cancer treatment effects. The past 30 years has seen a large increase in the use of diagnostic imaging, particularly radiologic medicine examinations such as diagnostic X-rays and computed tomography (CT) scanning [9–11]. Medical radiation exposure to the USA population has increased approximately 600 % since the 1980s [12]. In addition, cancer survivors tend to receive more frequent radiologic imaging than the general population due to follow-up care after primary treatment [13–15]. The rising use of various imaging modalities might be expected, therefore, to increase the possibility of incidental findings of new cancers during a routine follow-up examination and/or may increase the future risk of cancer due to the radiation exposure [16].
Some MPCs may also be treatment-related [17, 18]. Patients treated with radiotherapy and some specific chemotherapeutics can experience a number of significant late effects. One of the most serious potential long-term side effects is the development of MPCs [19–21]. The risk of developing MPCs is increased among survivors treated with radiotherapy, alkylating agents, anthracyclines and epipodophyllotoxins [3, 21–23]. A mutation in a susceptibility gene may also promote two or more cancers in an individual [22–24]. However, genetic risk factors for MPCs would not be expected to change over recent decades, unless they interact with other risk factors that demonstrate temporal trends.
In order to better understand temporal trends in the risk of developing MPCs, we performed a systematic review of the scientific literature to determine whether the risk of MPCs has increased over recent decades.
Methods
Scope of the review
We conducted a systematic literature search to identify studies describing adult cancer survivors with the diagnosis of MPCs. The review was focused on the following question: has there been an increase in the risk of developing MPCs over time?
Search strategy and selection criteria
We used two approaches to conduct the systematic search in two phases (Table 1). The original review was conducted in PubMed and Embase databases for eligible articles published prior to 1st March 2015. The update was conducted to August 2016. The MeSH terms related to “multiple primary cancers” and “second cancers” and related subcategories were used in separate searches: Neoplasms/Multiple Primary, Neoplasms/Second Primary and epidemiology/prevention and control. A number of key words (“multiple primary cancer* or malignanc* or tumo*”, “population-based” and “time period* or interval* or calendar years”) were also used and combined in different databases.
Following the Preferred Reporting items for Systematic reviews and Meta-analyses (PRISMA) statement [25, 26], eligibility criteria for included studies were as follows: (i) Type of studies: published population-based studies and reports published in English; (ii) Types of patients: adult cancer survivors (≥19 years) who were diagnosed with a first primary cancer (index cancer); (iii) Types of outcomes measures: adult cancer survivors (≥19 years) who developed a second or higher-order primary cancer (all sites combined). Studies of cancer survivors who developed MPCs at a specific site and studies based on autopsy cases were excluded because we were interested in the overall MPC risk among adult cancer survivors. Studies of MPCs in patients undergoing specific therapies or by treatment periods were also excluded given we were interested in all factors that affected the trends in MPC risk rather than treatment effects only.
Data extraction and analysis
Titles and abstracts of identified articles were assessed against the inclusion criteria by one author (YY). The full text of potentially relevant studies and the reference lists of included studies were read to identify further original articles. Two authors (YY and AV) developed an extraction sheet to record first author’s name, publication year, source of data, the number of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria met, site of first primary cancer, study period and follow-up, study size, study population (definition and inclusion criteria), definition of MPCs, calendar year of first cancer diagnosis, and the standardised incidence ratios (SIRs) or relative risks (RRs) and 95 % confidence intervals (95 % CIs) for MPCs by time periods. Typically, SIRs were derived from the observed number of MPCs divided by the expected number (O/E), with the expected number calculated from age-, sex- and calendar year- specific incidence rates in the general population [7, 27]. Alternatively, RRs were calculated as the risk of MPCs occurring in one time period compared with a reference period [28].
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria were used to assess the strengths, weaknesses, and generalizability of included studies [29]. The STROBE statement was developed to help readers when critically appraising published articles. Two authors (YY and AV) used a modified checklist of items for cohort studies to assess the number of criteria met in each study. We evaluated the coding rules of MPCs (i.e. Surveillance, Epidemiology, and End Results (SEER) [30] or International Association of Cancer Registries (IACR) and the International Agency for Research on Cancer (IARC) (IARC/IACR) [8] coding rules for MPCs) applied in each study as the diagnostic criteria in the STROBE checklist (Additional file 1).
Results
Literature search
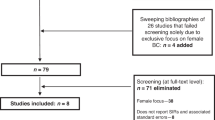
The defined search criteria identified 1832 relevant articles and four were added through manual review of references. Of the 225 articles assessed as eligible for full-text review, 23 articles met the inclusion criteria and were included in the narrative synthesis. After combining five eligible articles in the updated search, 28 studies were included in the final analysis comprising 26 population-based studies and two monographs (Fig. 1).
Study characteristics
All 28 included studies were population-based, published between 1987 and 2015, presenting data from Europe, North America, Australia and Japan (Table 2). 26 were peer-reviewed publications, reporting on more than 2.8 million survivors of adult cancer over the period of 1943 to 2012 [31–56], with 178,091 MPCs identified. Four of them reported the risk of developing MPCs following first cancer with all sites combined [45, 46, 48, 52]. Others focused on the risk of MPCs following first cancer at a specific site. The remaining were two monographs from the USA and Italy. One was a SEER monograph that used data from nine cancer registries in the USA, reported on more than 2 million cancer survivors during the follow-up period from 1973 to 2000, and a total of 185,407 MPCs were observed [7]. The other was a monograph of the Italian Association of Cancer Registries (AIRTUM), using data from 38 general and five specialised cancer registries in Italy, that reported on more than 1.6 million cancer survivors during the period of 1976–2010 with 85,399 MPCs identified [57].
The coding rules to define MPCs varied across studies. Seven studies and the SEER monograph used incidence and follow-up data from SEER program registries, and employed SEER coding rules [7, 39–44, 53]. Eight studies and the Italian monograph used coding rules proposed by the IARC/IACR [37, 38, 45, 47, 48, 50, 52, 56, 57]. While it may be difficult to directly compare risk estimates derived using different coding rules, comparisons of temporal trends will be valid if the rules used to define MPCs are consistent over time within a single study population [58].
Study quality
The number of STROBE criteria met in all population-based studies ranged from 18 to 28 of 30 items in total, with 21 studies meeting at least 25 criteria. Included studies had various objectives, data sources, study sizes and STROBE criteria met, but they all reported the risk of MPCs over different time periods.
Risk of MPCs following first primary cancer with all sites combined
Six studies reported temporal trends in MPC risk among survivors of adult cancer with all first cancer sites combined. Four of them observed an increasing trend in MPC risk from earlier to more recent periods. The SEER monograph reported a 14 % higher risk of developing MPCs than would be expected in the general SEER population during the 25 years of follow-up, with a total of 185,407 observed MPCs compared with 162,602 expected (SIR, 1.14; 95 % CI, 1.14 to 1.15). There was an increasing trend of SIRs rising from 1.12 with periods of first cancer diagnosis during 1973–79 to 1.21 during 1995–2000 [7]. Three large population-based studies from Australia, Finland and Japan (Australia and Japan including more than 200,000 cancer survivors, Finland including 470,000 cancer survivors) also showed an increase in the risk of developing MPCs across the whole study period when all first cancer sites are combined. In Australia, the SIRs grew from 1.14 with periods of first diagnosis in 1982–1986 to 1.46 in 1997–2001 [46]. In Finland, the SIRs increased from 1.09 in the 1950s to 1.14 in the 1980s [52]. In Japan, the relative risk increased from 1.00 in 1966–1971 (reference) to 2.89 in 1984–1986 [45]. However, two studies from Italy and France showed no significant difference in SIRs across different time periods. The Italian monograph reported SIRs of 1.10 in 1978–1987, 1.08 in 1988–1997 and 1.10 in 1998–2010, with a 10 % higher risk of developing MPCs than expected across the entire study period 1976–2010 (SIR, 1.10; 95%CI, 1.09 to 1.10) [57]. The French study was a large population-based study using data from 10 French population-based cancer registries, with a first cancer diagnosis between 1989 and 2004 [48].
Risk of MPCs following first primary cancers at specific sites
The risk of MPCs following first cancers at specific sites did not differ significantly across calendar periods of first cancer diagnosis in 14 studies [33, 35, 36, 38–41, 43, 44, 47, 49–51, 55]. Six studies reported an increasing temporal trend in MPC risk [32, 34, 42, 53, 54, 56], whilst two studies reported a decreasing trend in MPC risk after breast cancer during the study period 1943–2000 [31, 37]. There were a total of three studies on breast cancer as the first cancer.
MPCs following leukaemia, lymphoma and myeloma
Eight studies assessed the risk of MPCs following first cancers of the hematopoietic and lymphoid system [32, 34–36, 40, 43, 51, 54]. The risk of MPCs did not reveal any particular trends with respect to variations in SIRs over time in either of two major leukaemia (chronic myeloid leukaemia, chronic lymphocytic leukaemia) [35, 36] or the uncommon hairy cell leukaemia [40, 51]. These studies compared the risk of MPCs before and after a time point around 1990 when novel therapeutics such as interferon, fludarabine and other nucleoside analogues were introduced. Three studies with first cancers of Hodgkin or non-Hodgkin lymphoma reported an increasing trend in the risk of MPCs over time [32, 34, 54]. The risk of MPCs increased from 1.2 in the 1960s to 1.6 in the 1970s among 3139 cases of Hodgkin’s disease [32]. For MPCs following non-Hodgkin lymphoma (NHL), the risk was higher in the time interval of 2000–2006 (RR = 1.00, reference) than in 1980–1984 (RR = 0.65; 95%CI: 0.59-0.72) in more than 60,000 registered cases from three Nordic countries [34]. A similar pattern was also confirmed in France. The risk of MPCs was 1.37 (95%CI: 1.08-1.74) times higher for NHL diagnoses in 2000–2004 than in the 1989–1994 reference category [54]. For MPCs following multiple myeloma, there was no significant change in the risk before and after 2000. However, the overall risk of MPCs was also not significant (SIR = 0.98; 95 % CI: 0.94–1.02) [43].
MPCs following breast and ovarian cancer
No statistically significant change or decreasing trends in MPCs risk was observed in three breast cancer studies, two from multicentre studies and one from a single cancer registry [31, 37, 38]. One large multicentre study used data on 525,527 breast cancer survivors from 13 population-based cancer registries from Europe, Australia, Canada and Singapore covering the study period 1943–2000. The risk of MPCs decreased from 1.32 (95%CI 1.30-1.35) before 1975 to 1.18 (95%CI 1.14-1.22) after 1991 [37]. Another multicentre study encompassing four Scandinavian cancer registries reported data on more than 300,000 one-year survivors of breast cancer during a similar period, 1943–2002. The risk of MPCs was lower after 1980 than before (SIR = 1.09 and 1.19, respectively). However, second haematological cancers were excluded from the analysis [31]. No significant change in trend was observed in a study from a single cancer registry in Spain during the period of 1985–2007 (SIR 1.37, 95%CI 1.16-1.58 in 1985–1995 and SIR 1.41, 95%CI 1.18-1.64 in 1996–2007) [38]. The risk of MPCs following ovarian cancer increased from 1.1 in 1961–69 to 1.2 in 1970–80 (no 95%CI provided), using data from a single cancer registry in the UK. However, the observed numbers were mostly too small to obtain reliable estimates [32].
MPCs following thyroid cancer
In the USA, the risk of MPCs was higher in patients diagnosed with a first thyroid cancer during 2004–2008 (SIR 1.45, 95%CI 1.28-1.62) than in 1973–1983 (SIR 1.02, 95%CI 0.97-1.07) [42]. In Korea, however, the risk only reached statistical significance for a first thyroid cancer diagnosis between 2003 and 2007 (SIR 1.09, 95%CI 1.03-1.15), with smaller increases in other periods (SIR = 0.92 in 1993–1997, 1.05 in 1998–2002 and 1.06 in 2008–2010) [49].
MPCs following prostate cancer
Interestingly, the risk of MPCs following a first prostate cancer was significantly lower than expected in two relevant studies from the USA and Korea [33, 55]. The value of SIRs was consistently 0.7 from 1974 to 1994 using data from SEER (USA) cancer registries [33] and ranged from 0.6 to 0.7 during 1993–2011 using data from a nationwide hospital-based cancer registry in Korea [55].
MPCs following first cancers at other sites
From the early 1970s to the mid-2000s, the temporal trends in the risk of MPCs did not change significantly with a first cancer diagnosis of malignant meningioma, oesophageal cancer and ocular melanoma [39, 44, 47], but varied for first cancer at head and neck [50]. The risk of MPCs following testicular cancer remained consistent and lower than expected from 1961 to 1980. Although the SIRs for first cancer of Merkel Cell carcinoma increased from 1.09 (95%CI 0.83-1.70) in 1986–1994 to 1.37 (95%CI 1.05-1.76) in 1995–2002, the increase was not statistically significant [41]. For MPCs following colorectal cancer and bladder cancer, both risks increased with the year of first cancer diagnosis. The SIRs increased significantly from 1.53 (95%CI 1.37-1.71) in 1989–1992 to 2.02 (95%CI 1.79-2.27) in 2001–2004 for first bladder cancer [56]. For first cancer at colon and rectum, the SIRs were higher in 2002–2012 (1.25 for colon, 1.16 for rectum) than in 1992–2001 (1.08 for colon, 1.00 for rectum) [53].
Discussion
To our knowledge, this is the first systematic review focusing on the temporal trends in the risk of MPCs. There was an increasing time trend in the risk of developing MPCs in the USA and Australia when all first cancer sites were combined. Risk increased from 1.12 to 1.14 following a first cancer diagnosis in the early 1980s, to 1.21-1.46 in the late 1990s. In European countries, the risk remained similar during 1978–2010 for Italian cancer survivors and showed no significant change between 1989 and 2004 for French cancer survivors. Three potential explanations are postulated for the increasing trends in the USA and Australia: 1) increased detection of MPCs, both intended and incidental; 2) increased radiation exposure and 3) changed cancer treatments. The trends in the risk of developing MPCs varied by site of first cancer from 1943 to 2012, but mostly there was little change.
Increased detection
Increasing risk of MPCs might be a result of increased detection arising from the introduction of cancer screening programs for the early detection of cancers in the community; and incidental findings arising from the increased use of sensitive imaging tests in the routine clinical follow-up of cancer survivors. Early detection and incidental findings are not necessarily of benefit, as they can lead to “overdiagnosis”. Overdiagnosis is the detection of a “cancer” that would not cause symptoms or death in a patient’s lifetime [59].
Higher MPCs risk was observed in studies from the USA and Australia in the late 1990s [7, 46]. These findings coincide with the introduction of national cancer screening programs for cervical cancer and breast cancer in Australia in the early 1990s [60]. In the USA, the use of cancer screening for cervical cancer, breast cancer, colorectal cancer and prostate cancer has increased since 1987, particularly for breast cancer [61–63]. As well as providing benefits, screening has led to the overdiagnosis of non-progressive or low progressing cancers [64–67].
Cancer survivors are considered at increased risk for future cancers, and screening has been specifically recommended for survivors of some cancer types [68]. A meta-analysis of 20 studies demonstrated that cancer survivors tended to receive more frequent screening for new primary cancers, especially for breast, cervical, colorectal and prostate cancer than non-cancer controls [69]. This may thus differentially lead to increased detection among cancer survivors compared with the general population.
Another activity leading to a higher rate of MPCs may be the increased use and improvements in diagnostic imaging in recent time periods. The use of diagnostic imaging, especially computed tomography (CT) scans, has increased dramatically worldwide since 1980, particularly in the USA, Australia and Japan [9, 11, 70]. Cancer survivors routinely undergo CT scanning and other imaging procedures during follow-up, the detection of a new primary cancer, therefore, becomes more likely than in the general population with no prior cancer diagnosis [71]. Some of these incidental findings might be clinically unimportant, leading to overdiagnosis [10, 59, 72].
Increased radiation exposure during the follow-up after a first cancer diagnosis
Radiation exposure due to monitoring may of itself lead to harmful effects [73]. This possibility is consistent with our finding that the highest risk of MPCs, from the late 1990s, has occurred since radiation exposure has increased enormously [9]. Since the 1980s, the average annual per-capita effective dose from medical radiological procedures doubled worldwide [9, 11]. In the USA, the average annual per capita dose for medical procedures increased almost six-fold from 0.5 mSv in 1980 to 3.0 mSv in 2006 [9], with X-rays and CT scan the two most common imaging procedures leading to radiation exposure [11].
The lifetime risk of cancer attributable to diagnostic X-rays is estimated to vary between 0.6 and 1.8 % across 15 countries, including the USA and Australia, between 1991 and 1996 [74]. In Japan, the attributable risk has been estimated at 3.2 %, but the Japanese results are confounded by the impact of background radiation following the atomic bombings in World War II [74].
CT scanning has been widely used since the 1990s and delivers a much higher radiation dose than diagnostic X-rays [75]. In Australia, the cancer risk in children and adolescents who underwent CT scanning between 1985 and 2005 was found to be 24 % higher than in their peers who did not undergo CT scanning [16]. The impact on lifetime risk could not be ascertained given that cancer excess was still occurring at the end of the study. Increased risk of cancer due to CT scanning is not, however, limited to children. Adults are also at increased risk of cancer from the radiation exposure [76]. Imaging-based evaluation, especially CT scans, is the preferred modality to assess response to treatment, and for routine surveillance of most cancer survivors [77]. Therefore, increased risk of MPCs could be partly attributable to cumulative radiation exposure due to recurrent CT scans [78].
Changed cancer treatments
Studies of MPCs consequent to a primary cancer at a specific site can help us understand the impact of treatment changes on trends of MPC risk. No statistically significant change in risk of MPC was observed in most specific site studies, including after the 1990s when many newer (and improved) treatments or management strategies were introduced [33, 35, 36, 38–41, 43, 44, 51, 55]. A decrease in risk was observed in two of three breast cancer studies, which may reflect the improvement in radiation techniques [31, 37]. On the other hand, an increase in MPC risk was observed for a first non-Hodgkin lymphoma diagnosis in the mid-2000s than in the early 1990s, which may suggest an adverse treatment effect with increased use of nucleoside analogues (e.g. Fludarabine) at the end of the 1990s [34, 54]. The increase in the risk of MPCs following thyroid cancer might occur as a consequence of aggressive radiation treatment [42]. This is particularly concerning in regard to the more recent increased detection and treatment of microcarcinoma [79], largely considered to encompass overdiagnosis and overtreatment [72].
Together these findings suggest that most treatments have not impacted the baseline risk of MPCs in persons recently diagnosed with a primary cancer (post-1990), with the exceptions of breast cancer (reduction), non-Hodgkin lymphoma and thyroid cancer (increase).
Other factors to be considered
Changing patterns in lifestyle or environmental risk factors may also affect the value of SIRs over time. Temporal trends in population exposure to carcinogenic factors such as alcohol and tobacco consumption, differed across countries [48]. Any differences over time in exposure in the reference population compared with the population of cancer survivors could contribute to changing SIRs.
Limitations
Several factors limit the interpretation of our findings. First, studies of all first cancer sites combined from the USA and Australia did not cover the last 15 years. This period is of particular relevance given the introduction of nuclear medicine imaging, for example, PET/CT scanning. Second, cancer survivor profile (age at first cancer diagnosis, site of first cancer) may have varied according to year of first cancer diagnosis and these factors may contribute to changing SIRs [48]. For example, cancer survivor profiles have changed with the implementation of cancer screening programs. Third, variation in screening guidelines, follow-up care, management and treatment for cancer across countries potentially affect the generalizability of the results. Fourth, some included studies did not clearly specify the coding rules or definition of MPCs, which may result in misclassification and less reliable estimation of the observed number of MPCs. Fifth, different timeframes of the study cohorts may limit the ability to evaluate long-term effects of changing patterns in medical surveillance and treatment of the primary cancer. Some studies published before 2000 lack sufficient data to compare SIRs pre-1990s and post-1990s when treatment improved [32, 33, 45, 52]. Sixth, articles in languages other than English were excluded, which could lead to language bias. Last, although we defined the age of the study population as adults, some studies, mostly those using SEER cancer registries, reported across all ages [7, 32, 34, 36, 39–45, 47, 49, 52, 54, 57]. However, childhood cancer survivors accounted for a limited proportion of all cancer survivors (no more than 2 % in SEER cancer registries), and the types of cancer developed in children were limited [7], which should minimise any impact on the overall results.
Conclusion
The overall risk of developing MPCs appears to have increased in the USA and Australia from the 1980s to 2000 when all first cancer sites are combined. Increased detection due to the more frequent use of diagnostic imaging and increased medical radiation exposure may be potential explanations. Studies from Italy and France, however, showed no significant change in MPC risk in patients diagnosed with a first cancer after 2000. Given the implementation of new cancer screening programs and the growth of nuclear medical imaging (e.g. PET/CT scanning) since 2000, continued long-term surveillance is needed from non-European as well as European countries. Future studies are needed to assess the extent of overdiagnosis and overtreatment among cancer survivors relative to the general population, and thus to assess whether there is potential for optimising follow-up strategies.
Abbreviations
- CI:
-
Confidence Interval
- IARC/IACR:
-
International Agency for Research on Cancer/International Association of Cancer Registries
- MPCs:
-
Multiple Primary Cancers
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RR:
-
Relative Risk
- SEER:
-
Surveillance, Epidemiology, and End Results
- SIR:
-
Standardised Incidence Ratio
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):23–34.
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71.
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, Bannon F, Ahn JV, Johnson CJ, Bonaventure A. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010.
Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16(3):566–71.
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37.
Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr (eds). New malignancies among cancer survivors. SEER cancer registries, 1973–2000. NIH Publ. No. 05-5302. Bethesda: National Cancer Institute; 2006.
Working GR. International rules for multiple primary cancers (ICD-0 third edition). Eur J Cancer Prev. 2005;14(4):307.
Mettler Jr FA, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG. Radiologic and Nuclear Medicine Studies in the United States and Worldwide: Frequency, Radiation Dose, and Comparison with Other Radiation Sources—1950–2007 1. Radiology. 2009;253(2):520–31.
Miglioretti DL, Smith-Bindman R. Overuse of computed tomography and associated risks. Am Fam Physician. 2011;83(11):1252–4.
United Nations. Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation: sources. Vol. 1. United Nations Publications; 2000.
Linet MS, Slovis TL, Miller DL, Kleinerman R, Lee C, Rajaraman P, Berrington de Gonzalez A. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62(2):75–100.
Colt HG, Murgu SD, Korst RJ, Slatore CG, Unger M, Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e437S–454S.
Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–5.
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–70.
Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360.
Lorigan P, Califano R, Faivre-Finn C, Howell A, Thatcher N. Lung cancer after treatment for breast cancer. Lancet Oncol. 2010;11(12):1184–92.
Lorigan P, Radford J, Howell A, Thatcher N. Lung cancer after treatment for Hodgkin’s lymphoma: a systematic review. Lancet Oncol. 2005;6(10):773–9.
Pui C-H, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–68.
Pui C-H, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, Mirro Jr J, Kalwinsky DK, Dahl GV, Murphy SB. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N Engl J Med. 1989;321(3):136–42.
Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2020–6.
Neugut AI, Robinson E, Meadows AT: Multiple primary cancers: Lippincott Williams & Wilkins; 1999.
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734–45.
Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. Int J Cancer J Int du cancer. 2014;135(8):1756–63.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;15(4):W-65–94.
Schoenberg BS, Myers MH. Statistical methods for studying multiple primary malignant neoplasms. Cancer. 1977;40(S4):1892–8.
Breslow N, Day N. IARC Scientific Publications No. 82: Statistical Methods in Cancer Research Vol. II: The Design and Analysis of Cohort Studies. International Agency for Research on Cancer: Lyon, France 1987.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England). 2007;370(9596):1453–7.
Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards B. The 2007 Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute. Surveillance, Epidemiology and End Results Program. 2007.
Brown LM, Chen BE, Pfeiffer RM, Schairer C, Hall P, Storm H, Pukkala E, Langmark F, Kaijser M, Andersson M, et al. Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat. 2007;106(3):439–51.
Coleman M, Bell C, Fraser P. Second primary malignancy after Hodgkin’s disease, ovarian cancer and cancer of the testis: a population-based cohort study. Br J Cancer. 1987;56(3):349.
Levi F, Randimbison L, Te VC, Erler G, La Vecchia C. Second primary tumors after prostate carcinoma. Cancer. 1999;86(8):1567–70.
Lorenzo Bermejo J, Pukkala E, Johannesen TB, Sundquist J, Hemminki K. Age-time risk patterns of solid cancers in 60 901 non-Hodgkin lymphoma survivors from Finland, Norway and Sweden. Br J Haematol. 2014;164(5):675–83.
Rebora P, Czene K, Antolini L, Gambacorti Passerini C, Reilly M, Valsecchi MG. Are chronic myeloid leukemia patients more at risk for second malignancies? A population-based study. Am J Epidemiol. 2010;172(9):1028–33.
Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121(1):151–6.
Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, Andersen A, Brewster DH, Pukkala E, McBride ML, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118(9):2285–92.
Molina-Montes E, Pollan M, Payer T, Molina E, Davila-Arias C, Sanchez MJ. Risk of second primary cancer among women with breast cancer: A population-based study in Granada (Spain). Gynecol Oncol. 2013;130(2):340–5.
Bao X, Cao L, Piao H, Xie L. Treatment-related secondary cancer in malignant meningiomas: A population-based study. J Cancer Res Clin Oncol. 2014;140(4):583–8.
Hisada M, Chen BE, Jaffe ES, Travis LB. Second cancer incidence and cause-specific mortality among 3104 patients with hairy cell leukemia: a population-based study. J Natl Cancer Inst. 2007;99(3):215–22.
Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomark Prev. 2006;15(8):1545–9.
Kim C, Bi X, Pan D, Chen Y, Carling T, Ma S, Udelsman R, Zhang Y. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid. 2013;23(5):575–82.
Razavi P, Rand KA, Cozen W, Chanan-Khan A, Usmani S, Ailawadhi S. Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J. 2013, 3(6).
Zhu G, Chen Y, Zhu Z, Lu L, Bi X, Deng Q, et al. Risk of second primary cancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25(6):505–11.
Tsukuma H, Fujimoto I, Hanai A, Hiyama T, Kitagawa T, Kinoshita N. Incidence of second primary cancers in Osaka residents, Japan, with special reference to cumulative and relative risks. Jpn J Cancer Res. 1994;85(4):339–45.
Youlden DR, Baade PD: The relative risk of second primary cancers in Queensland, Australia: A retrospective cohort study. BMC cancer. 2011, 11.
Scélo G, Boffetta P, Autier P, Hemminki K, Pukkala E, Olsen JH, et al. Associations between ocular melanoma and other primary cancers: an international population-based study. Int J Cancer. 2007;120(1):152–9.
Jegu J, Colonna M, Daubisse-Marliac L, Tretarre B, Ganry O, Guizard AV, Bara S, Troussard X, Bouvier V, Woronoff AS, et al. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer. 2014;14:94.
Cho YY, Lim J, Oh CM, Ryu J, Jung KW, Chung JH, Won YJ, Kim SW. Elevated risks of subsequent primary malignancies in patients with thyroid cancer: a nationwide, population-based study in Korea. Cancer. 2015;121(2):259–68.
Jegu J, Binder-Foucard F, Borel C, Velten M. Trends over three decades of the risk of second primary cancer among patients with head and neck cancer. Oral Oncol. 2013;49(1):9–14.
Federico M, Zinzani PL, Frassoldati A, Vinceti M, Modè A, Annino L, Chisesi T, Pagnucco G, Invernizzi R, Spriano M. Risk of second cancer in patients with hairy cell leukemia: long-term follow-up. J Clin Oncol. 2002;20(3):638–46.
Sankila R, Pukkala E, Teppo L. Risk of subsequent malignant neoplasms among 470,000 cancer patients in Finland, 1953–1991. Int J Cancer. 1995;60(4):464–70.
Guan X, Jin Y, Chen Y, Jiang Z, Liu Z, Zhao Z, Yan P, Wang G, Wang X. The Incidence characteristics of second primary malignancy after diagnosis of primary colon and rectal cancer: a population based study. PLoS One. 2015;10(11):e0143067.
Rossi C, Jégu J, Mounier M, Dandoit M, Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard A-V, Bara S. Risk assessment of second primary cancer according to histological subtype of non-Hodgkin lymphoma. Leuk Lymphoma. 2015;56(10):2876–82.
Joung JY, Lim J, Oh C-M, Jung K-W, Cho H, Kim SH, Seo HK, Park WS, Chung J, Lee KH. Risk of second primary cancer among prostate cancer patients in Korea: a population-based cohort study. PLoS One. 2015;10(10):e0140693.
Muller J, Grosclaude P, Lapôtre‐Ledoux B, Woronoff AS, Guizard AV, Bara S, Colonna M, Troussard X, Bouvier V, Trétarre B: Trends in the risk of second primary cancer among bladder cancer survivors: a population‐based cohort of 10 047 patients. BJU international 2015.
AIRTUM Working Group. Italian cancer figures, report 2013: Multiple tumours. Epidemiol Prev. 2013;37(4–5 Suppl 1):1.
Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary rules on population-based cancer survival. Cancer Causes Control. 2013;24(6):1231–42.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13.
Australian Institute of Health and Welfare. Cancer in Australia: an overview 2014. Cancer series no. 90. Cat. no. CAN 88. Canberra: AIHW; 2014.
Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–13.
Smith RA, Cokkinides V, von Eschenbach AC, Levin B, Cohen C, Runowicz CD, Sener S, Saslow D, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2002;52(1):8–22.
Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States. Cancer. 2003;97(6):1528–40.
Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–92.
Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, Chiles C, Black WC, Aberle DR. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–74.
Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–29.
Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005.
Vogel VG. Identifying and screening patients at risk of second cancers. Cancer Epidemiol Biomark Prev. 2006;15(11):2027–32.
Corkum M, Hayden JA, Kephart G, Urquhart R, Schlievert C, Porter G. Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7(3):455–63.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84.
Kattlove H, Winn RJ. Ongoing care of patients after primary treatment for their cancer. CA Cancer J Clin. 2003;53(3):172–96.
Morgan DJ, Dhruva SS, Wright SM, Korenstein D. Update on medical practices that should be questioned in 2015. JAMA Intern Med. 2015;175(12):1960–4.
UNSCEAR S. Effects of Ionizing Radiation: 2000 Report to the General Assembly, with Scientific Annexes, Vol. II: Effects. United Nations, New York. 2000
de Gonzalez AB, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363(9406):345–51.
Smith-Bindman R, Lipson J, Marcus R, Kim K-P, Mahesh M, Gould R, de González AB, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–86.
Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102(21):1628–36.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–16.
Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, Khorasani R. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults 1. Radiology. 2009;251(1):175–84.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7.
Acknowledgements
We would like to thank the librarians at the University of Tasmania for their advice on the search strategies for the electronic databases.
Funding
This study was not funded by any outside source.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors’ contribution
The first author (YY) conducted the search strategy, the data extraction, the narrative analysis and drafted the paper. The senior author (AV) created the scope of the review, defined the inclusion and exclusion criteria with YY and evaluated the quality of the included studies with YY. AV and AN edited all drafts of the paper. KW gave additional advice on the interpretation of the results. All authors approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
STROBE Statement—Checklist of items that should be included in reports of cohort studies. (DOC 87 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ye, Y., Neil, A.L., Wills, K.E. et al. Temporal trends in the risk of developing multiple primary cancers: a systematic review. BMC Cancer 16, 849 (2016). https://doi.org/10.1186/s12885-016-2876-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2876-y