Abstract
Objective
To compare the efficacy and safety of TACE combined with Donafenib and Toripalimab versus TACE combined with Sorafenib in the treatment of unresectable hepatocellular carcinoma (HCC), aiming to guide personalized treatment strategies for HCC and improve patient prognosis.
Materials and methods
A retrospective analysis was conducted on the clinical data of 169 patients with unresectable advanced-stage HCC who underwent treatment at the Interventional Department of Wuhan Union Hospital from January 2020 to December 2022. Based on the patients’ treatment strategies, they were divided into two groups: TACE + Donafenib + Toripalimab group (N = 81) and TACE + Sorafenib group (N = 88). The primary endpoints were objective response rate (ORR), disease control rate (DCR), overall survival (OS), and progression-free survival (PFS) of the two groups’ tumors. The secondary endpoint was the occurrence of treatment-related adverse events in the two groups of patients.
Results
The TACE + Donafenib + Toripalimab group showed higher ORR and DCR compared to the TACE + Sorafenib group (66.7% vs. 38.6%, 82.6% vs. 68.2%, P < 0.05). The TACE + Donafenib + Toripalimab group also demonstrated longer median progression-free survival (mPFS) (10.9 months vs. 7.0 months, P < 0.001) and median overall survival (mOS) (19.6 months vs. 10.9 months, P < 0.001) compared to the TACE + Sorafenib group. When comparing the two groups, the TACE + Sorafenib group had a higher incidence of grade 3–4 hypertension (14.8% vs. 4.9%, P = 0.041), higher incidence of diarrhea (all grades) (18.2% vs. 7.4%, P = 0.042), and higher incidence of hand-foot syndrome (all grades) (26.1% vs. 12.3%, P = 0.032).
Conclusion
TACE combined with Donafenib and Toripalimab demonstrates superior efficacy and safety in treating unresectable HCC patients. This combination therapy may serve as a feasible option to improve the prognosis of unresectable HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular Carcinoma (HCC) is one of the most prevalent malignant tumors worldwide and represents the primary type of liver malignancy [1]. According to the World Health Organization (WHO), as of 2020, HCC ranked sixth in terms of incidence and third in terms of mortality among all cancers globally [2]. In certain high-risk regions such as China and Southeast Asia, the incidence and mortality rates of HCC are even higher [3, 4]. The major risk factors for HCC include viral infections, dietary habits, alcohol consumption, obesity, medication, and exposure to toxins [5,6,7]. Due to its asymptomatic early stage and rapid progression, most patients are diagnosed at an advanced stage when surgical resection is not feasible [8, 9]. Despite the challenges associated with HCC treatment, there has been some progress in recent years. Transarterial chemoembolization (TACE) is one of the commonly used approaches for the treatment of unresectable HCC [10]. Particularly for patients classified as BCLC B stage, TACE significantly improves survival outcomes [11]. Some BCLC C stage patients also benefit from TACE in terms of survival [12, 13]. TACE delivers chemotherapy drugs directly to the tumor region via the hepatic artery and combines them with embolic agents to inhibit tumor growth [14]. However, the efficacy of single TACE treatment is limited in terms of long-term survival and disease control. Consequently, researchers have begun exploring the possibility of combining TACE with other treatment modalities to improve patient prognosis [15, 16]. In this regard, molecular targeted therapy and immunotherapy have attracted widespread attention as novel treatment strategies for HCC [17, 18]. Commonly used molecular targeted drugs include sorafenib, lenvatinib, regorafenib, apatinib, and donafenib [19]. Donafenib, a multitarget tyrosine kinase inhibitor, exerts antitumor effects by inhibiting tumor cell proliferation and angiogenesis [20]. Early clinical trials have shown that donafenib significantly prolongs progression-free survival and overall survival in patients with unresectable HCC [20]. Toripalimab, an immune checkpoint inhibitor, activates the patient’s immune system by blocking the PD-1 and PD-L1 signaling pathways, enhancing the ability to attack tumors [21]. Although clinical research on tislelizumab in HCC is still in the early stages, some studies have shown its potential in tumor control and improvement of patient prognosis [22]. For unresectable HCC patients, the combination of TACE with donafenib and toripalimab has attracted researchers’ interest. The theoretical basis for this comprehensive treatment strategy is to directly target the tumor with TACE while utilizing the molecular targeting and immune-modulating effects of donafenib and toripalimab to enhance treatment efficacy. In comparison, TACE combination with sorafenib is another commonly used treatment approach [23]. Sorafenib, another multitarget tyrosine kinase inhibitor, exerts its effects through mechanisms such as inhibiting tumor cell proliferation, anti-angiogenesis, and immune modulation [24]. Sorafenib has been widely used in the treatment of unresectable HCC patients and has shown certain efficacy. However, there is limited research data directly comparing the effectiveness of TACE combination with donafenib and toripalimab versus TACE combination with sorafenib in treating unresectable HCC. Therefore, this study aims to evaluate the efficacy and safety of these two treatment approaches by retrospectively analyzing clinical data from two patient groups. We expect that the results of this study will provide further evidence for the treatment selection of unresectable HCC patients. By comparing the clinical outcomes and safety profiles of TACE combination with donafenib and toripalimab versus TACE combination with sorafenib, we will gain a better understanding of the advantages and limitations of these treatment approaches in improving patient survival, controlling tumor progression, and minimizing adverse events. This is of significant importance in guiding personalized treatment strategies for HCC and promoting patient prognosis.
Materials and methods
General information
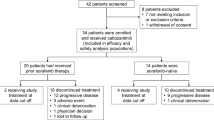
Clinical data of 169 patients with unresectable advanced-stage hepatocellular carcinoma (HCC) who received treatment at the Department of Interventional Radiology, Tongji Medical College, Huazhong University of Science and Technology, Union Hospital, from January 2020 to December 2022 were collected. Inclusion criteria were as follows: (1) Age between 18 and 70 years; (2) Diagnosis of HCC confirmed by pathological and radiological examinations [25]; (3) No previous treatment for liver cancer, including radiation therapy, chemotherapy, targeted therapy, or immunotherapy; (4) Child-Pugh liver function classification of A-B, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; (5) White blood cell count ≥ 3.0 G/L, platelet count ≥ 50 G/L, international normalized ratio (INR) ≤ 1.5; (6) Complete clinical follow-up data. Exclusion criteria were as follows: (1) Presence of primary or metastatic cancer in other sites; (2) Severe abnormalities in cardiac, pulmonary, renal, hematological, neurological, or coagulation functions; (3) Tumor volume > 70% of liver volume; (4) Allergy to iodinated contrast agents, donafenib, sorafenib, or toripalimab. Based on the treatment strategy, the patients were divided into two groups: TACE + Donafenib + Toripalimab group (N = 81) and TACE + Sorafenib group (N = 88). Baseline data were collected, including gender, age, etiology of liver cirrhosis, preoperative Child-Pugh liver function classification, ECOG performance status, BCLC tumor staging, pre-treatment total bilirubin, ALT, AST, white blood cell count, red blood cell count, and platelet count.
Methods
TACE Procedure [26]
The patient was positioned supine, and the groin area was sterilized with iodine solution. Local anesthesia was administered at the puncture site using 2% lidocaine, and a 5 F vascular sheath was inserted using the Seldinger technique. A 5 F Yashiro catheter was advanced into the celiac trunk and superior mesenteric artery (or other collateral arteries if necessary) for angiography to identify the tumor-feeding arteries. Then, a 2.7 F microcatheter was selectively inserted into the tumor-feeding artery of the HCC, and a mixture of iodized oil and epirubicin was injected to form an emulsion. Finally, 300–500 μm gelatin sponge particles were injected for embolization, and the embolization endpoint was defined as the stasis of forward blood flow in the tumor-feeding artery. If an arterioportal or arteriovenous shunt was found during intraoperative angiography, the microcatheter was selectively advanced to the shunt site, and polyvinyl alcohol (PVA) particles were used to embolize and occlude the shunt before proceeding with subsequent chemoembolization. After the treatment, the catheter was removed, and the puncture site was compressed and bandaged.
Materials and drugs used for TACE included: 5 F vascular sheath (TERUMO5F-10CM, Terumo, Japan), 0.035 inch guidewire (RFGA35153M, Terumo, Japan), 5 F Yashiro catheter (Terumo, Japan), 2.7 F microcatheter (Terumo, Japan), epirubicin (GYZZ H19990280, Zhejiang Hisun Pharmaceutical Co., Ltd.), iodized oil (GYZZ H20163348, Jiangsu Hengrui Medicine Co., Ltd.).
Usage of Donafenib and Toripalimab
Donafenib: 200 mg, orally, twice daily.
Toripalimab: 240 mg, intravenous infusion, every 3 weeks.
Usage of Sorafenib
Dosage: 400 mg, orally, twice daily.
Patients underwent contrast-enhanced CT or MRI follow-up every 4–6 weeks, and the decision for further TACE treatment was based on the follow-up results.
Observation indicators
Primary endpoints:
-
(1)
Evaluation of tumor response after treatment in both groups using the mRECIST criteria [27], including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
-
(2)
Objective response rate (ORR) and disease control rate (DCR) of the tumors in both groups.
-
(3)
Overall survival (OS) and progression-free survival (PFS) in both groups.
Secondary endpoints:
-
(1)
Changes in liver function and blood routine before treatment and three months after treatment in both groups.
-
(2)
Incidence of treatment-related adverse events in both groups.
Statistical methods
Statistical analysis was performed using SPSS 24.0 software. Categorical data were presented as frequencies (percentages), and intergroup differences were assessed using the chi-square test, including Pearson Chi-Square and Fisher’s Exact Test. Continuous data were presented as mean ± standard deviation, and intergroup differences were analyzed using the t-test. OS and PFS were displayed using Kaplan-Meier curves, and the comparison of OS and PFS between the two groups was performed using the Log-Rank test. A p-value of < 0.05 was considered statistically significant.
Results
Comparison of baseline characteristics between the two groups (see Table 1)
There were no significant statistical differences (P > 0.05) in terms of gender, age, etiology of liver cirrhosis, preoperative liver function based on Child-Pugh classification, ECOG score, BCLC stage of the tumor, pre-treatment total bilirubin, ALT, AST, white blood cell count, red blood cell count, and platelet count between the two groups.
Comparison of blood parameters at three months after treatment in the two groups (see Table 2).
There were no significant differences (P > 0.05) in terms of total bilirubin, ALT, AST, white blood cell count, red blood cell count, and platelet count at three months after treatment between the two groups.
Evaluation of tumor response after treatment in the two groups (see Table 3)
The proportion of patients achieving complete response (CR) and partial response (PR) after treatment was higher in the TACE + Donafenib + Toripalimab group compared to the TACE + Sorafenib group, and the proportion of patients with progressive disease (PD) was lower in the TACE + Donafenib + Toripalimab group compared to the TACE + Sorafenib group (P = 0.002). The objective response rate (ORR) and disease control rate (DCR) after treatment were both higher in the TACE + Donafenib + Toripalimab group compared to the TACE + Sorafenib group (P < 0.05).
Comparison of overall survival (OS) and progression-free survival (PFS) in the two groups (see Table 4).
The median PFS was longer in the TACE + Donafenib + Toripalimab group compared to the TACE + Sorafenib group (10.9 months vs. 7.0 months), and the difference was statistically significant (P < 0.001, Fig. 1). The median OS was longer in the TACE + Donafenib + Toripalimab group compared to the TACE + Sorafenib group (19.6 months vs. 10.9 months), and the difference was statistically significant (P < 0.001, Fig. 2).
Incidence of treatment-related adverse events in the two groups (see Table 5)
Compared to the TACE + Donafenib + Toripalimab group, the TACE + Sorafenib group had a higher incidence of grade 3–4 hypertension (14.8% vs. 4.9%, P = 0.041), a higher incidence of diarrhea (all grades) (18.2% vs. 7.4%, P = 0.042), and a higher incidence of hand-foot syndrome (all grades) (26.1% vs. 12.3%, P = 0.032). There were no statistically significant differences (P > 0.05) between the two groups in terms of abdominal pain, fever, vomiting, rash, fatigue, anorexia, and gastrointestinal hemorrhage.
Discussion
Hepatocellular carcinoma (HCC) is one of the most common primary malignant tumors of the liver, and its incidence and mortality rates are increasing. Due to the highly malignant and complex nature of HCC, its treatment has been a focus of clinical research. Transarterial chemoembolization (TACE) has become a commonly used method for treating unresectable HCC. On one hand, the chemotherapeutic drugs injected through the catheter can induce tumor cell apoptosis and inhibit tumor cell proliferation. At the same time, embolizing the tumor-feeding arteries can lead to ischemia, hypoxia, and necrosis of the tumor tissue [28]. The theoretical basis of TACE treatment is that the normal liver tissue has a dual blood supply, with the portal vein as the main supply. Therefore, performing arterial chemoembolization of liver tumors has minimal impact on normal liver tissue [29]. Jinpeng Li et al. [30] reported on 172 HCC patients who underwent TACE treatment, and the results showed that the objective response rate (ORR) at 2, 4, and 6 months after treatment was 78.7%, 71.6%, and 63.2%, respectively, and the disease control rate (DCR) was 95.3%, 92.1%, and 85.9%, respectively. However, TACE embolization can cause tumor tissue ischemia and hypoxia, leading to upregulation of hypoxia-inducible factor-1 alpha (HIF-1α) and subsequent upregulation of levels of VEGF, FGF, and other factors, which may contribute to tumor recurrence and metastasis [31, 32]. Adriana Sergio et al. [33] reported that when TACE is not completely effective, it may induce a significant neovascularization response, such as increased levels of VEGF and b-FGF after treatment, which can impact patient survival.
Donafenib can simultaneously inhibit the activity of various receptor tyrosine kinases, such as VEGFR and PDGFR, and directly inhibit various Raf kinases, as well as downstream Raf/MEK/ERK signaling pathways. It inhibits tumor cell proliferation and tumor angiogenesis, exerting a dual inhibitory and multi-target blocking anti-tumor effect [34]. Therefore, the combination of TACE and donafenib has a synergistic effect in terms of mechanisms. Shukui Qin et al. [35] reported on 668 HCC patients who were randomly assigned to the donafenib group (328 cases) and the sorafenib treatment group (331 cases), and the median overall survival (mOS) in the donafenib treatment group was significantly longer than that in the sorafenib treatment group (FAS; 12.1 vs. 10.3 months; hazard ratio, 0.831; P = 0.0245).
TACE combined with immune checkpoint inhibitors is a combination strategy used for comprehensive treatment of hepatocellular carcinoma (HCC), and its mechanisms and principles are as follows [36]: TACE blocks the blood supply from the hepatic artery, which not only directly kills tumor cells but also releases tumor-associated antigens (TAAs) and inflammatory mediators, promoting activation of the immune system. Ahmed Montasser et al. [37] reported on 82 HCC cases that underwent surgical treatment, with 32 cases receiving prior TACE and 50 cases not receiving TACE. The study results showed increased expression of PD-1 and PD-L1 in HCC after TACE treatment. The mechanism of immune checkpoint inhibitors [38, 39]: Immune checkpoint inhibitors such as PD-1 inhibitors and CTLA-4 inhibitors can block the signaling of immune checkpoint receptors on the surface of liver cancer cells and their ligands, restoring the activation and attacking capability of immune cells. These inhibitors can relieve immune suppression and enhance the recognition and killing of liver cancer cells by T cells and natural killer (NK) cells. By combining TACE and immune checkpoint inhibitors, a synergistic effect of local treatment and systemic immune activation can be achieved. The release of tumor antigens by TACE can stimulate immune cell responses, while immune checkpoint inhibitors can eliminate immune suppression and enhance the activity of immune cells. This combination treatment strategy helps promote antigen-specific immune responses against hepatocellular carcinoma and improve treatment outcomes. Brett Marinelli et al. [40] reported that patients receiving TACE combined with immune therapy had longer median progression-free survival (mPFS) and median overall survival (mOS) compared to those receiving single-agent immune therapy, with values of 8.8 months vs. 3.7 months (p < 0.01) and 35.1 months vs. 16.6 months (p = 0.12), respectively. Caihua Zhu et al. [41] reported on 20 patients with advanced-stage liver cancer who received neoadjuvant TACE combined with PD-1 inhibitor bridging to surgical treatment. The objective response rate (ORR) of neoadjuvant treatment was 75.0%, and the disease control rate (DCR) was 100.0%. Fourteen patients (70.0%) achieved successful downstaging (converted to CNLC Stage I).
Toripalimab is a fully human monoclonal antibody against the PD-1 receptor [42], which can block the binding of PD-1 on T lymphocytes to PD-L1 on tumor cells, relieving immune suppression of tumor cells and allowing immune cells to exert anti-tumor immune effects and kill tumor cells [43]. ZhiCheng Lai et al. [44] reported on 36 patients with advanced HCC who received lenvatinib, toripalimab, and FOLFOX-HAIC, with a median progression-free survival (mPFS) of 10.4 months and median overall survival (mOS) of 17.9 months. Wei-Feng Qu et al. [45] reported on 51 unresectable HCC patients, with 30 patients receiving triple combination therapy (t-CT: lenvatinib, TACE, plus toripalimab) and 20 patients receiving dual combination therapy (d-CT: lenvatinib plus TACE). Compared to d-CT, t-CT had higher objective response rate (ORR) (76.7% vs. 47.6%, P = 0.042) and disease control rate (DCR) (90.0% vs. 57.1%, P = 0.042).
Currently, many scholars have utilized transcatheter arterial chemoembolization (TACE) combined with molecular targeted therapy and immune checkpoint inhibitors for the treatment of unresectable hepatocellular carcinoma (uHCC), achieving significant therapeutic efficacy. Shuping Qu et al. [46] reported a study involving 110 uHCC patients, among whom 56 patients received combination therapy (TACE Combined With Lenvatinib Plus PD-1 Inhibitors) and 54 patients received TACE alone. Compared to the TACE group, the combination therapy group demonstrated a higher objective response rate (67.9% vs. 29.6%, p < 0.001), longer median progression-free survival (11.9 months vs. 6.9 months, p = 0.003), and longer median overall survival (23.9 months vs. 15.3 months, p < 0.001). Mingyue Cai et al. [47] reported a study involving 81 patients with advanced HCC, among whom 41 received TACE combined with lenvatinib plus PD-1 inhibitor and 40 received TACE combined with lenvatinib. Compared to the TACE combined with lenvatinib group, the TACE combined with lenvatinib plus PD-1 inhibitor group exhibited extended median overall survival (16.9 months vs. 12.1 months, p = 0.009), prolonged median progression-free survival (7.3 months vs. 4.0 months, p = 0.002), higher objective response rate (56.1% vs. 32.5%, p = 0.033), and higher disease control rate (85.4% vs. 62.5%, p = 0.019). Yan-Jun Xiang et al. [48] reported that in patients with BCLC stage B HCC, TACE combined with PD-1 inhibitors and lenvatinib treatment significantly improved clinical outcomes compared to TACE combined with PD-1 inhibitors alone, while maintaining controllable safety.
However, currently, there are multiple options for molecular targeted drugs and immune checkpoint inhibitors used in the treatment of hepatocellular carcinoma, and whether different drug combination regimens can achieve favorable therapeutic effects, as well as which combination regimen can provide greater survival benefits for HCC patients, are directions that many scholars have been exploring. This study found that the TACE + Donafenib + Toripalimab group had longer median progression-free survival and overall survival than the TACE + Sorafenib group, with statistical differences (p < 0.001).
In addition, we observed that the TACE combined with Donafenib and Toripalimab group showed advantages in terms of objective response rate (ORR) and disease control rate (DCR) (66.7% vs. 38.6%, 82.6% vs. 68.2%, p < 0.05). This suggests that this combination therapy regimen can better control tumor growth and progression, thereby improving treatment response and disease control level in patients. The results of this study indicate that patients in the TACE combined with Sorafenib group had relatively less improvement in terms of survival. Sorafenib, as a targeted therapy drug, has been widely used in the treatment of HCC. However, its efficacy as a single agent is not satisfactory, which is consistent with the results of some clinical studies. Shou Wu Lee et al. [49] reported a study involving 53 BCLC stage C HCC patients who received TACE combined with Sorafenib treatment, with a median time to progression (mTTP) of 6.42 months and median overall survival (mOS) of 11.21 months. Yashwant Patidar et al. [50] reported a study involving 31 advanced HCC patients treated with TACE combined with Sorafenib, with a disease control rate (DCR) of 44.9%, mTTP of 4.6 months, and mOS of 10.1 months. Therefore, our results further support the effectiveness of TACE combined with Donafenib and Toripalimab as a treatment strategy for unresectable HCC.
Regarding safety assessment, we observed that patients in the TACE combined with Donafenib and Toripalimab group had lower incidence rates of high-grade hypertension, diarrhea (all grades), and hand-foot syndrome (all grades) compared to the TACE combined with Sorafenib group. The incidence rates and severity of other common adverse events were comparable between the two groups. This indicates that while providing patients with more treatment options, the combination therapy regimen did not significantly increase adverse effects. The reason for this may be that donafenib is a deuterated derivative of sorafenib, which enhances its stability and reduces susceptibility to hepatic drug-metabolizing enzymes, leading to increased plasma exposure and decreased formation of toxic metabolites [51, 52]. The improved pharmacokinetic profile of donafenib may explain its improved safety characteristics compared to sorafenib, resulting in a lower incidence of adverse reactions. Shukui Qin et al. [35] reported that patients receiving donafenib treatment had a significantly lower incidence of grade 3 or higher drug-related adverse events compared to sorafenib (38% vs. 50%; p = 0.0018). Jingrui Liu et al. [53] reported a study involving 27 eligible advanced HCC patients treated with oral donafenib. They were randomly divided into 200 mg and 300 mg bid groups, and both groups showed good safety and tolerability of donafenib, with most adverse events being grade 1 or grade 2.
It is important to note that this study has several limitations. Firstly, due to its retrospective design, there may be selection bias and limitations in data acquisition. Secondly, this study is single-center, which may limit the generalizability and external validity of the results. Future studies could employ a multicenter, prospective design to further validate our findings.
Conclusion
The TACE combined with Donafenib and Toripalimab group showed higher ORR and DCR, as well as longer PFS and OS compared to the TACE combined with Sorafenib group. The TACE combined with Donafenib and Toripalimab group did not increase the incidence of treatment-related adverse events. In conclusion, the results of this study suggest that the combination of TACE with Donafenib and Toripalimab demonstrates good efficacy and safety in the treatment of unresectable HCC patients. This combination therapy regimen may be a feasible option to improve the prognosis of unresectable HCC patients. However, further research is still needed to comprehensively evaluate its efficacy and safety and optimize treatment strategies for personalized HCC therapy.
Availability of data and materials
The datasets used during the current study are available from the corresponding author upon request.
References
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1–61. https://doi.org/10.1016/bs.acr.2020.10.001. Epub 2020 Nov 28. PMID: 33579421; PMCID: PMC8796122.
Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15(11):1295–307. https://doi.org/10.1080/17474124.2021.1991792. Epub 2021 Oct 22. PMID: 34624198.
Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48(1):7–17. https://doi.org/10.1007/s15010-019-01345-y. Epub 2019 Jul 25. PMID: 31347138.
Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci. 2019;64(4):910–7. https://doi.org/10.1007/s10620-019-05537-2. PMID: 30835028.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y. Epub 2019 Aug 22. PMID: 31439937; PMCID: PMC6813818.
Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477-491.e1. https://doi.org/10.1053/j.gastro.2018.08.065. Epub 2018 Oct 24. PMID: 30367835; PMCID: PMC6340716.
Budny A, Kozłowski P, Kamińska M, Jankiewicz M, Kolak A, Budny B, Budny W, Niemunis-Sawicka J, Szczypiór G, Kurniawka B, Burdan F. Epidemiologia i czynniki ryzyka rozwoju raka wątrobowokomórkowego [Epidemiology and risk factors of hepatocellular carcinoma]. Pol Merkur Lekarski. 2017;43(255):133–9 Polish. PMID: 28987047.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2. Epub 2018 Jan 5. PMID: 29307467.
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907. https://doi.org/10.1038/s12276-020-00527-1. Epub 2020 Dec 2. PMID: 33268834; PMCID: PMC8080814.
Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21)8165. https://doi.org/10.3390/ijms21218165 PMID: 33142892; PMCID: PMC7662786.
Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic Liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–35. https://doi.org/10.3748/wjg.v21.i36.10327. PMID: 26420959; PMCID: PMC4579879.
Zhao GS, Liu S, Liu Y, Li C, Wang RY, Bian J, Zhang YW, Zhou J, Lin YJ, Wu J. Assessment of efficacy and prognostic factors by Gelfoam for DEB-TACE in unresectable large hepatocellular carcinoma with portal vein tumor thrombus: a multi-center retrospective study. Expert Rev Gastroenterol Hepatol. 2022;16(7):673–80. https://doi.org/10.1080/17474124.2022.2091545. Epub 2022 Jun 21. PMID: 35709813.
Miyayama S, Arai Y, Matsui O. Transarterial chemoembolization for hepatocellular carcinoma with vascular invasion. Br J Radiol. 2022;95(1138):20211316. https://doi.org/10.1259/bjr.20211316. Epub 2022 Feb 10. PMID: 35143258; PMCID: PMC9815726.
Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, White S, Rilling W, Gamblin TC. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). 2017;19(8):659–66. https://doi.org/10.1016/j.hpb.2017.04.016. Epub 2017 May 25. PMID: 28552299.
Brown ZJ, Hewitt DB, Pawlik TM. Combination therapies plus transarterial chemoembolization in hepatocellular carcinoma: a snapshot of clinical trial progress. Expert Opin Investig Drugs. 2022;31(4):379–91. https://doi.org/10.1080/13543784.2022.2008355. Epub 2021 Nov 25. PMID: 34788184.
Feng J, Yang JH, Li JH, Jin XS, Sun Q. Transcatheter arterial chemoembolisation combined with radiofrequency ablation on hepatocellular carcinoma and levels of relevant markers. J Coll Physicians Surg Pak. 2020;30(3):259–62. https://doi.org/10.29271/jcpsp.2020.03.259. PMID: 32169132.
Han Z, Yang F, Zhang Y, Wang J, Ni Q, Zhu H, Zhou X, Gao H, Lu J. Prognostic efficacy and prognostic factors of TACE plus TKI with ICIs for the treatment of unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1029951 . https://doi.org/10.3389/fonc.2022.1029951PMID: 36591442; PMCID: PMC9798199.
Xie D, Sun Q, Wang X, Zhou J, Fan J, Ren Z, Gao Q. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. 2021;9(8):652–652. https://doi.org/10.21037/atm-20-7037. PMID: 33987350; PMCID: PMC8106062.
Zhu XD, Tang ZY, Sun HC. Targeting angiogenesis for liver cancer: past, present, and future. Genes Dis. 2020;7(3):328–35. https://doi.org/10.1016/j.gendis.2020.03.010. PMID: 32884987; PMCID: PMC7452391.
Chen R, Ielasi L, di Carlo A, Tovoli F. Donafenib in hepatocellular carcinoma. Drugs Today (Barc). 2023;59(2):83–90. https://doi.org/10.1358/dot.2023.59.2.3507751. PMID: 36811408.
Zhang L, Hao B, Geng Z, Geng Q. Toripalimab: the First Domestic Anti-Tumor PD-1 Antibody in China. Front Immunol. 2022;12:730666. https://doi.org/10.3389/fimmu.2021.730666. PMID: 35095833; PMCID: PMC8789657.
Chen J, Hu X, Li Q, Dai W, Cheng X, Huang W, Yu W, Chen M, Guo Y, Yuan G. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. 2020;8(18):1187–1187. https://doi.org/10.21037/atm-20-6063. PMID: 33241036; PMCID: PMC7576044.
Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y, TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–501. https://doi.org/10.1136/gutjnl-2019-318934. Epub 2019 Dec 4. PMID: 31801872; PMCID: PMC7398460.
Zhang X, Wang K, Wang M, Yang G, Ye X, Wu M, Cheng S. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–27. https://doi.org/10.18632/oncotarget.15075. PMID: 28177886; PMCID: PMC5438741.
Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–9. https://doi.org/10.1053/j.semdp.2016.12.011. Epub 2016 Dec 20. PMID: 28108047.
Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–63. https://doi.org/10.21037/hbsn-20-480. PMID: 32832496; PMCID: PMC7423548.
Sato Y, Watanabe H, Sone M, Onaya H, Sakamoto N, Osuga K, Takahashi M, Arai Y, Japan Interventional Radiology in Oncology Study Group-JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118(1):16–22. https://doi.org/10.3109/03009734.2012.729104. Epub 2012 Nov 20. PMID: 23167460; PMCID: PMC3572665.
Melchiorre F, Patella F, Pescatori L, Pesapane F, Fumarola E, Biondetti P, Brambillasca P, Monaco C, Ierardi AM, Franceschelli G, Carrafiello G. DEB-TACE: a standard review. Future Oncol. 2018;14(28):2969–84. https://doi.org/10.2217/fon-2018-0136. Epub 2018 Jul 10. PMID: 29987957.
Maleux G, van Malenstein H, Vandecaveye V, Heye S, Vaninbroukx J, Nevens F, Verslype C. Transcatheter chemoembolization of unresectable hepatocellular carcinoma: current knowledge and future directions. Dig Dis. 2009;27(2):157–63. https://doi.org/10.1159/000218348. Epub 2009 Jun 22. PMID: 19546554.
Li J, Wang N, Shi C, Liu Q, Song J, Ye X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J Cancer Res Ther. 2021;17(3):733–9. https://doi.org/10.4103/jcrt.JCRT_1848_20. PMID: 34269307.
Petrillo M, Patella F, Pesapane F, Suter MB, Ierardi AM, Angileri SA, Floridi C, de Filippo M, Carrafiello G. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 2018;14(28):2957–67. https://doi.org/10.2217/fon-2017-0739. Epub 2018 May 1 PMID: 29712486.
Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with Hepatocellular Carcinoma. J Clin Med Res. 2016;8(4):297–302. https://doi.org/10.14740/jocmr2496w. Epub 2016 Feb 27. PMID: 26985249; PMCID: PMC4780492.
Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–21. https://doi.org/10.1111/j.1572-0241.2007.01712.x. Epub 2008 Jan 2. PMID: 18177453.
Keam SJ, Duggan S. Donafenib: first approval. Drugs. 2021;81(16):1915–20. https://doi.org/10.1007/s40265-021-01603-0. PMID: 34591285.
Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. 2021;39(27):3002–11. https://doi.org/10.1200/JCO.21.00163. Epub 2021 Jun 29. PMID: 34185551; PMCID: PMC8445562.
Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021 9(9):e003311. https://doi.org/10.1136/jitc-2021-003311. PMID: 34593621; PMCID: PMC8487214.
Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, Paradis V. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79(1):36–46. https://doi.org/10.1111/his.14317. Epub 2021 Mar 28. PMID: 33326644.
Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel). 2021;9(5):532. https://doi.org/10.3390/vaccines9050532. PMID: 34065489; PMCID: PMC8160723.
Waidmann O. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther. 2018;18(8):905–10. https://doi.org/10.1080/14712598.2018.1499722. Epub 2018 Jul 20. PMID: 29995439.
Marinelli B, Kim E, D'Alessio A, Cedillo M, Sinha I, Debnath N, Kudo M, Nishida N, Saeed A, Hildebrand H, Kaseb AO, Abugabal YI, Pillai A, Huang YH, Khan U, Muzaffar M, Naqash AR, Patel R, Fischman A, Bishay V, Bettinger D, Sung M, Ang C, Schwartz M, Pinato DJ, Marron T. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022 Jun;10(6):e004205. https://doi.org/10.1136/jitc-2021-004205. PMID: 35710293; PMCID: PMC9204420.
Zhu C, Dai B, Zhan H, Deng R. Neoadjuvant transarterial chemoembolization (TACE) plus PD-1 inhibitor bridging to tumor resection in intermediate-stage hepatocellular carcinoma patients. Ir J Med Sci. 2022. https://doi.org/10.1007/s11845-022-03131-6. Epub ahead of print. PMID: 35996068.
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, Li J, Shi YR, Jin F, Xu R, Pan J, Qu S, Li P, Hu C, Liu YC, Jiang Y, He X, Wang HM, Lim WT, Liao W, He X, Chen X, Liu Z, Yuan X, Li Q, Lin X, Jing S, Chen Y, Lu Y, Hsieh CY, Yang MH, Yen CJ, Samol J, Feng H, Yao S, Keegan P, Xu RH. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536–43. https://doi.org/10.1038/s41591-021-01444-0. Epub 2021 Aug 2. Erratum in: Nat Med.28(1):214. PMID: 34341578.
Keam SJ. Toripalimab: first global approval. Drugs. 2019;79(5):573–8. https://doi.org/10.1007/s40265-019-01076-2. (PMID: 30805896).
Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, Li Q, Xu L, Zhang Y, Wei W, Chen M, Kan A, Shi M. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. https://doi.org/10.1016/j.ejca.2022.07.005. Epub 2022 Aug 15. PMID: 35981413.
Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu XT, Zhang ZH, Zhang X, Huang A, Tang M, Tian MX, Jiang XF, Huang R, Tao CY, Fang Y, Gao J, Wu XL, Zhou J, Fan J, Liu WR, Shi YH. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open. 2022;6(5):zrac114. https://doi.org/10.1093/bjsopen/zrac114. PMID: 36125345; PMCID: PMC9499852.
Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, Hu L, Zhang J, Wang R, Wei L, Wu D. Efficacy and Safety of TACE Combined With Lenvatinib Plus PD-1 Inhibitors Compared With TACE Alone for Unresectable Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Front Oncol. 2022;12:874473. https://doi.org/10.3389/fonc.2022.874473. PMID: 35530353; PMCID: PMC9068979.
Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhou J, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. https://doi.org/10.3389/fimmu.2022.848387. PMID: 35300325; PMCID: PMC8921060
Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, Feng JK, Bo MH, Qin YY, Zheng YT, Shan YF, Zhou LP, Zhai J, Cheng SQ. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52(8):721–9. https://doi.org/10.1111/hepr.13773. Epub 2022 May 22. PMID: 35536197.
Lee SW, Lee TY, Peng YC, Yang SS, Yeh HZ, Chang CS. The therapeutic benefits of combined sorafenib and transarterial chemoembolization for advanced hepatocellular carcinoma. J Dig Dis. 2020;21(5):287–92. https://doi.org/10.1111/1751-2980.12866. Epub 2020 Jun 4 PMID: 32315498.
Patidar Y, Chandel K, Condati NK, Srinivasan SV, Mukund A, Sarin SK. Transarterial Chemoembolization (TACE) combined with sorafenib versus TACE in patients with BCLC Stage C Hepatocellular carcinoma - a retrospective study. J Clin Exp Hepatol. 2022;12(3):745–54. https://doi.org/10.1016/j.jceh.2021.12.009. Epub 2021 Dec 21. PMID: 35677519; PMCID: PMC9168730.
Li X, Qiu M, Wang S, Zhu H, Feng B, Zheng L. A phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85(3):593–604. https://doi.org/10.1007/s00280-020-04031-1. Epub 2020 Feb 1. PMID: 32008115.
Russak EM, Bednarczyk EM. Impact of deuterium substitution on the pharmacokinetics of pharmaceuticals. Ann Pharmacother. 2019;53(2):211–6. https://doi.org/10.1177/1060028018797110. Epub 2018 Aug 23 PMID: 30136594.
Liu J, Li X, Zhang H, Chen G, Chen H, Hu Y, Niu J, Ding Y. Safety, pharmacokinetics and efficacy of donafenib in treating advanced hepatocellular carcinoma: report from a phase 1b trial. Pharmazie. 2019;74(11):688–93. https://doi.org/10.1691/ph.2019.9626. PMID: 31739839.
Acknowledgements
We Thank Pro. Fan Yang for her help in manuscript revising. Thank Dr. Huimin Liang for his help in data collection. Thanks to Dr. Xin Li for his help in reference.
Funding
No funding is provided in this study.
Authors’ contributions.
Haohao Lu contributed to the conception and design of the work, the acquisition, analysis of data, as well as manuscript writing. Bin Liang contributed to the design of the work. Xiangwen Xia contributed to the acquisition, analysis of data. Chuansheng Zheng contributed to analysis, interpretation of data,and manuscript writing.
Author information
Authors and Affiliations
Contributions
Haohao Lu contributed to the conception and design of the work, the acquisition, analysis of data, as well as manuscript writing. Bin Liang contributed to the design of the work. Xiangwen Xia contributed to the acquisition, analysis of data. Chuansheng Zheng contributed to analysis, interpretation of data,and manuscript writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The medical ethics committee at Union Hospital, Tongji Medical College, Huazhong University of science and technology, Wuhan, Hubei Province approved the retrospective study. The requirement for informed consent was waived by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of science and technology due to the retrospective nature of the study. During follow-up, we informed patients about the study and they agreed to use their data. We confirmed that all methods were performed in accordance with the relevant guidelines and Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, H., Liang, B., Xia, X. et al. Efficacy and safety analysis of TACE + Donafenib + Toripalimab versus TACE + Sorafenib in the treatment of unresectable hepatocellular carcinoma: a retrospective study. BMC Cancer 23, 1033 (2023). https://doi.org/10.1186/s12885-023-11535-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11535-5