Abstract
Background
Patients with advanced pancreatic cancer have a poor prognosis and high burden of cancer-related symptoms. It is necessary to assess the trade-off of clinical benefits and possible harms of treatments with anticancer drugs (TAD). This systematic review aims to compare the effectiveness of TAD versus supportive care or no treatment, considering all patient-important outcomes.
Methods
We searched PubMed, Embase, Cochrane Library, and Epistemonikos. Two reviewers performed selection, data extraction and risk of bias assessment. We assessed certainty of the evidence using the GRADE approach.
Results
We included 14 randomised controlled trials. Chemotherapy may result in a slight increase in overall survival (MD: 2.97 months (95%CI 1.23, 4.70)) and fewer hospital days (MD: -6.7 (-8.3, -5.1)), however, the evidence is very uncertain about its effect on symptoms, quality of life, functional status, and adverse events. Targeted/biological therapy may result in little to no difference in overall survival and a slight increment in progression-free survival (HR: 0.83 (95%CI 0.63, 1.10)), but probably results in more adverse events (RR: 5.54 (95%CI 1.24, 23.97)). The evidence is very uncertain about the effect of immunotherapy in overall survival and functional status.
Conclusions
The evidence is very uncertain about whether the benefits of using treatment with anticancer drugs outweigh their risks for patients with advanced pancreatic cancer. This uncertainty is further highlighted when considering immunotherapy or a second line of chemotherapy and thus, best supportive care would be an appropriate alternative. Future studies should assess their impact on all patient-important outcomes to inform patients in setting their goals of care.
Similar content being viewed by others
Background
Pancreatic cancer (PC) has the highest incidence-to-mortality ratio of any solid tumour, accounting for almost as many deaths as new cases in 2020 [1]. Most patients are diagnosed at an incurable, advanced stage, either regional (28%) or distant (48%) [2, 3]. The overall prognosis of these patients is very poor, with 1- and 5-year survival rates of 59% and 14% for regional stage, and of 21% and 3% for distant stage at diagnosis [2].
PC patients present a high burden of cancer-related symptoms, including pain, weight loss, fatigue, depression, and anxiety, which tend to increase closer to the end-of-life (EoL) period [4, 5]. Therefore, for patients with advanced PC and a poor prognosis, it seems coherent that treatment goals should mostly prioritise the improvement on quality of life [6].
The most widely used approach for advanced PC is treatment with anticancer drugs (TAD). Currently, guidelines recommend chemotherapy as first line therapy for locally advanced and metastatic PC in patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0, 1 or 2 [7,8,9]. However, these treatments are usually suggested on the basis of a few weeks to months survival advantage, but do not explicitly assess and report the impact these treatments may have in terms of improving symptom burden, toxicity, functional status, and quality of life near death. More recently, other TADs are being evaluated, mainly immunotherapy, —due to promising results in other malignancies— [10, 11] and targeted/biological therapies —due to the advances in molecular characterization of PC [12].
However, through previous stages of our broad evidence synthesis project [13] we found that previous systematic reviews (SRs) that compared TAD with supportive care (SC) or no treatment in patients with advanced PC did not find conclusive evidence to support one option over the other. Moreover, we found that there were relevant primary studies that were not included in those SRs [14]. Therefore, we think it is still reasonable to compare TADs with SC or no treatment to assess the trade-off of clinical benefits and possible harms and in consequence, we aimed to conduct a new SR, following strict methodological guidelines, to assess the efficacy of TAD versus SC or no treatment in people with advanced PC, considering all patient-important outcomes.
Methods
We conducted a SR adhering the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [15]. The protocol for this study was prospectively registered and is publicly available in Open Science Framework [16].
This study is the last part of a three-stage comprehensive evidence synthesis project (ASTAC project) [16]. Briefly, we first conducted an overview of SRs [13] and then a scoping review and evidence map of primary and secondary studies assessing the effectiveness of TAD versus SC/no treatment for advanced PC [14].
Eligibility criteria
We used the PICO framework to determine our research question and guide our eligibility criteria [17]. The clinical question was: Are TADs (chemotherapy, immunotherapy or targeted/biological therapies) more effective compared to SC/no treatment in patients with advanced PC?
Types of studies
We included randomised clinical trials (RCTs) and excluded quasi-experimental studies, observational studies, and reviews. For studies with more than two arms, we included those of interest only if the authors provided the necessary disaggregated data.
Types of participants
We considered studies including adults with primary or recurrent advanced PC. We considered PC as advanced when in stage III or IV, or when considered as such by the study authors. Authors may refer to this as incurable, secondary, metastatic, terminal, or progressive cancer. We excluded neuroendocrine neoplasms.
Types of interventions
For the experimental arm, we considered any TAD (chemotherapy, immunotherapy, or targeted/biological therapies), either monotherapy or in combination, whether individual or combined, with or without SC. We excluded trials that evaluated surgery or radiotherapy without TADs, intraperitoneal chemotherapy, and studies that consider chemotherapy only as adjuvant/neoadjuvant therapy or maintenance therapy.
For the control arm, we considered any supportive treatment, administered with the purpose of symptomatic or palliative control. This comprehends either usual treatment, SC, or best supportive care (BSC) [18, 19]. We also included trials that did not explicitly define the intervention, or using with placebo. We excluded studies if the control arm included any type of TAD or treatments with non-palliative intention (e.g., radiotherapy or surgery with curative intention).
Types of outcomes
We considered studies that reported any of the following outcomes.
-
1.
Primary outcomes: overall survival (OS), quality of life (QoL), progression-free survival (PFS), functional status (FS), and toxicity measured as moderate or severe adverse events
-
2.
Secondary outcomes: symptoms related to the disease, admissions to hospital or long-term centre, or emergency consultations, and quality of death (QoD) measured as admissions to hospital at the EoL (last 30 days of life), palliative care provided during the last year and/or place of death.
Search methods for identification of studies
We identified potentially eligible references through a search strategy conducted for the first two stages of the broad evidence synthesis project [16], which involved a literature search in MEDLINE (access via PubMed), Embase (access via Ovid), the Cochrane Database of Systematic Reviews, CENTRAL, and Epistemonikos from inception onwards. We updated this search on April 2022, using the same search strings for each electronic database, but only focused on PC (Additional file 1). We also searched in clinicaltrials.gov to identify protocols of potentially eligible studies. In addition to electronic database searches, we asked experts in the field for relevant studies. We conducted a forward and backward citation search starting from the included studies, using citationchaser software [20,21,22]. We did not apply language restrictions.
Selection of studies
Two reviewers independently screened the results of the search, first by title and abstract and in a second stage by full text. A third reviewer solved any disagreement in both stages. For this process, we used Rayyan, a web-based software platform [23].
Data extraction and risk of bias assessment
Two reviewers independently extracted data using a sheet that was previously piloted. We solved disagreements through discussion. We extracted the following characteristics of the included RCTs: year of publication, country, number of arms, study phase, number of randomised and analysed participants, characteristics of included participants, of interventions and comparisons, outcomes assessed, results, conflicts of interest, and funding.
Two reviewers independently assessed the risk of bias at outcome level using the Cochrane Risk of Bias 2 (RoB2) tool [24]. We solved disagreements by consensus or a third reviewer.
Data synthesis and analysis
We calculated mean difference (MD) for continuous outcomes and risk ratios (RR) for dichotomous outcomes, with their respective 95% confidence interval. When the number of events was zero in a treatment arm, we followed Cochrane Handbook guidance [25]. For survival outcomes we also used hazard ratios (HR) extracted from the published data [26].
When a study included multiple arms, we only considered comparisons relevant for our review, and when two comparisons (e.g., drug A versus placebo and drug B versus placebo) were combined in the same study, we followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions, to avoid double-counting [27].
Data synthesis
We performed a meta-analysis using a random effects model for outcomes where studies were reasonably homogeneous (both clinically and methodologically), using ReviewManager (RevMan) 5.4. For other cases, we report results descriptively.
For each intervention, we grouped studies and considered all possible interventions in the control group (i.e., placebo, SC, BSC, usual treatments and others) as one unique comparator. We also performed subgroup analyses according to the lines of therapy [28].
We were not able to conduct the other pre-planned subgroup analysis [16] due to lack of information. Due to the small number of studies included in the meta-analyses, we were unable to analyse publication bias through funnel plots.
Assessment of heterogeneity
We assessed heterogeneity both visually and using I2 through Software Review Manager 5.4. Cut-off values for I2 are not absolutes. An I2 below 40% might not be important and between 50%-90% may represent substantial heterogeneity. We considered the heterogeneity assessment for purposes of the estimation of certainty of evidence.
Assessment of certainty of evidence
We assessed the certainty of the evidence according to GRADE guidance [29] and made a Summary of Findings (SoF) table for all outcomes. We classified the certainty of the evidence for each outcome as high, moderate, low, or very low. We initially rated the certainty for each outcome as high, since data comes from RCT, and we lowered in presence of important bias, indirectness, inconsistency, imprecision, or suspicion of publication bias.
Results
Description of studies
Results of the search
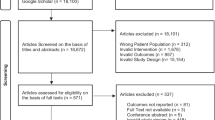
Through the previous stages of our broad evidence synthesis project [14], we identified 43 studies, with 59 references, that included participants with advanced PC and were included in our scoping review and evidence map. We identified 337 additional references through our search update. After removing duplicates, we screened 286 references by title and abstract, and finally, 6 full-text articles for inclusion. We identified one reference corresponding to the protocol registration of an RCT already included in the previous stages of our project. Therefore, we included a total of 14 RCTs, with 21 references, to answer our review question.
See Fig. 1 for the PRISMA flow chart. See additional file 2 for the reasons for exclusion of 44 reports.
Included Studies
See Table 1 for characteristics of the included studies and Table 2 for baseline characteristics of the included participants. See additional file 3 for the studies’ patient eligibility criteria and intervention details.
We included 14 RCTs assessing the effects of TAD vs either BSC, SC, placebo, or no treatment in patients with advanced PC [30,31,32,33, 35, 36, 38, 39, 41,42,43,44,45, 47]. The year of publication ranged from 1979 to 2014, with only two studies published in the last 10 years [45, 47]. Eight (57.1%) studies did not specify the sources of funding and nine (64.2%) did not report conflicts of interest.
Chemotherapy versus No-TAD
We included 11 studies for this comparison [30,31,32,33, 35, 36, 38, 39, 41,42,43]. The number of participants included in each study ranged from 31 to 303, totalling 903 participants.
The studies included participants with unresectable advanced PC [30, 32, 33, 35, 36, 39, 41,42,43], “non-curable PC” [31], or only metastatic PC [38]. Six studies provided baseline performance status (PS) details: two included participants with Karnofsky PS (KPS) > 70 [38, 39], two with WHO PS 0 to 2 [32, 41], and two with WHO PS 0 to 3 [35, 43] (Table 2). Three studies only provided this information in their eligibility criteria [31, 36, 42], and two studies did not inform baseline PS [30, 33].
Nine studies assessed 5-FU-based regimes [30,31,32,33, 35, 39, 41,42,43], one study assessed gemcitabine [36], and one glufosfamide [38]. Six studies evaluated the intervention as first line [30,31,32,33, 35, 36], two as second [38, 39] and three as non-specified lines of therapy [41,42,43]. For the control arms, seven studies described it as either BSC [31, 38, 39, 42] or SC [32, 33, 41]. Both groups received palliative surgery in three studies [30, 36, 43] and palliative radiotherapy in one study [35].
Immunotherapy versus No-TAD
We included two studies for this comparison [44, 45]. Results of the study by Oortgiesen et al. were only available as an abstract [44]. Each study included 154 participants, with unresectable advanced PC [44] or with advanced PC unwilling or unsuitable to receive chemotherapy [45].
One study planned to include participants with KPS ≥ 60, however, due to a screening error, it included one participant with KPS 30 at baseline in the control arm [45] and the other did not inform participants’ baseline PS [44] (Table 2).
One study tested a polyclonal antibody stimulator (PAS) vaccination, which elicits a specific and high-affinity anti-gastrin antibody and did not specify the line of therapy [44], while the other tested an anti-gastrin immunogen (G17DT) as first-line therapy [45]. In both studies, the control arm received a matching placebo.
Targeted/biological therapy versus No-TAD
We included one study for this comparison, which included 207 participants with unresectable locally advanced or metastatic PC. All participants had an ECOG PS 0 to 2 (Table 2). The study evaluated erlotinib without specifying the line of therapy and compared it to a matching placebo [47].
Risk of bias of included studies
We judged only one study to have a low risk of bias for all outcomes [47]. We judged four studies to have some concerns about risk of bias for all outcomes, mainly due to lack of information about allocation concealment and a pre-planned analysis [30, 32, 36, 44]. We judged four studies to have a high risk of bias for all outcomes, mainly due to deviations from intended interventions and issues arising from the randomisation process [31, 33, 38, 45]. Lastly, we judged five studies to have some concerns of risk of bias for survival outcomes, mainly due to lack of information about allocation concealment: however, for outcomes such as symptoms related to the disease, toxicity, and functional status, we judged them as having a high risk of bias, mainly due to concerns about differences in the measurement of the outcomes between groups [35, 39, 41,42,43] (See additional file 4 for the risk of bias per outcome in all the included studies).
Effects of interventions
See Tables 3, 4 and 5 and appendices 5 and 6 for a summary of the results and certainty of evidence per outcome.
Chemotherapy versus No-TAD
Eight studies reported a longer median or mean OS in the group that received chemotherapy [31,32,33, 35, 38, 39, 41, 42], while two reported a longer OS in the no-TAD group [30, 36]. Only three studies provided sufficient data for meta-analysis for OS as a continuous outcome and showed that chemotherapy may slightly increase OS (MD: 2.97 months (95%CI 1.23, 4.70); low certainty) [32, 39, 42] (Additional file 5.1).
Six studies provided sufficient data to calculate HR for OS, with 647 participants analysed. The pooled analysis showed chemotherapy may have little to no effect on OS (HR: 0.92 (95%CI 0.74, 1.16); very low certainty) (Additional file 5.1). Pooled analysis of seven studies showed that chemotherapy resulted in an absolute mortality risk reduction of 253 per 1,000 patients at 6-months (RR: 0.67 (95%CI 0.48, 0.94); very low certainty) and of 135 per 1,000 patients at 12-months (RR: 0.86 (95%CI: 0.76, 0.98; very low certainty)(Additional file 5.2). Lastly, only one study assessed PFS and reported results in favour of chemotherapy, however inconclusive (HR: 0.76 (95%CI 0.57, 1.05); very low certainty) [38].
QoL was assessed by only two studies. Glimelius et al. reported results in favour of chemotherapy over a 4-month period [31], while Xinopoulos et al. found that in a 6-month period the no-TAD group had better results [36]. Two studies assessed participants’ FS and reported results in favour of chemotherapy [42, 43]. Additionally, seven studies reported toxicity [32, 35, 36, 38, 39, 41, 42], but only four did so in both groups allowing for comparison (Additional file 5.3). Ciuleanu et al. reported a higher occurrence of toxicities in the chemotherapy group [38], while the other three reported no significant differences between groups for each adverse event [35, 39, 42]. Lastly, four studies assessed symptoms related to the disease using different scales to measure pain [33, 38], gastrointestinal symptoms [33], depression and anxiety [41], and improvement of overall symptomatology [43] and reported contradicting results (Additional file 5.4). Overall, the evidence is very uncertain about the effect of chemotherapy on QoL, FS, toxicity and symptoms related to the disease.
Only one study assessed the number of hospital days and reported a lower mean in the control group (99.3 versus 115.7 days). However, after adjusting per month of survival, the chemotherapy group presented fewer hospital days (MD: -6.7 (-8.3, -5.1); low certainty) [42].
We did not find studies that assessed QoD in both groups. Only one study assessed an aspect of QoD in the chemotherapy group and reported that 15 out of 16 participants in the intervention group did not receive gemcitabine in the last 2–3 weeks before death when their condition was very poor. The other participant had previously requested treatment discontinuation [36].
Immunotherapy versus No-TAD
The two studies assessing immunotherapy reported results for OS in favour of the intervention, with a median of 22 to 66 days of advantage [44, 45], with only one reporting a HR of 0.75 (95%CI 0.51, 1.10); very low certainty. Only one study assessed FS and reported longer time to deterioration of KPS (below 60) in the immunotherapy group [45]. Overall, the evidence is very uncertain about the effect of immunotherapy in OS and FS.
We did not find studies that assessed or adequately reported the following outcomes: PFS, QoL, toxicity, symptoms related to the disease, admissions to hospital, and QoD.
Targeted/biological therapy versus No-TAD
The only study addressing this comparison reported that erlotinib may result in little to no difference in OS (HR: 1.04 (95%CI 0.77,1.39); low certainty) and it may increase PFS (HR: 0.83 (95%CI 0.63, 1.10); low certainty). However, it probably results in an increase in treatment related serious adverse events (RR. 5.54 (95%CI 1.24, 23.97); moderate certainty) (Additional file 5.3) [47].
We did not find studies that assessed or adequately reported the following outcomes: QoL, FS, symptoms related to the disease, admissions to hospital and QoD.
Discussion
Our SR identified, evaluated, and summarised the evidence of a total of 14 RCTs that assessed the efficacy of TADs versus SC or no treatment for patients with advanced PC. We identified very few RCTs that compared TADs, either chemotherapy (n = 11), immunotherapy (n = 2), or targeted/biological therapies (n = 1) versus no oncological treatment (using as comparator usual supportive care or placebo or no treatment), with most assessing only first-line therapies. Besides the sparsity in the number of trials, there are several serious drawbacks to consider in order to interpret the results correctly. First, the overall certainty of evidence was low or very low, due to many concerns of risk of bias, inconsistency in the results, and imprecision of the effect estimates. Additionally, most studies lack transparency regarding potential conflicts of interest. Given all these limitations, it is remarkable that there are only two RCTs conducted in the last ten years, since this is still a critical question with an important level of uncertainty.
Second, outcomes different from survival are seldom measured or reported —even when they are undeniably relevant in this context. Patients with advanced PC often present a substantial symptom burden (including pain, tiredness and lack of energy) which affects their functionality, daily life activities [49], and ultimately constitutes one of the main reasons for TADs discontinuation [50]. Moreover, a recent RCT confirmed that the integration of routine symptom monitoring during cancer treatment improves survival outcomes [51]. Unfortunately, the included RCTs in our study did not systematically assess potential changes in symptoms control or quality of life throughout the course of treatments.
Third, we could not include all the data provided by the primary studies to assess toxicity either, due to several reasons. In some cases, authors provided data only for the experimental arm, with no information given for the control group. Others did not assess the adverse events grouped by severity, reporting the results extensively disaggregated. Finally, in some reports it was unclear whether the unit of analysis were the patients or the events (e.g. if two adverse events in one patient were counted as “2” or “1”). We collected data only from those primary studies whose reports were appropriate for our predefined methods, but there is a large room for improvement when reporting toxicity form RCTs.
Fourth, almost all studies included participants with a PS from 0 to 2, which is consistent with the population considered in guidelines’ recommendations for the use of TAD for advanced PC [7, 9, 52]. As we did not find studies assessing this comparison for patients with PS 3 or higher, this lack of evidence related to more advanced patients would be an additional argument for rigorously assessing the performance status of all PC patients in clinical practice to avoid treating those with a poorer prognosis and therefore, with increasing chances to produce them more harms than benefits. This is why there is a progressive recognition about the importance of a valid evaluation of patients’ experience through patient-reported outcome (PRO) instruments. Instruments such as the EORTC QLQ-C30/PAN26 have been deemed highly relevant by patients since it measures most aspects of their experience with the disease and treatment [49]. While it is a great accomplishment that some newer studies are including these measures, it is regrettable that they were not used when comparing TADs to less aggressive treatments whose aim was to improve cancer-related symptomatology and QoL, such as SC [53].
Given all the above-mentioned limitations, the quantitative results from our SR must be interpreted with caution. Chemotherapy may result in a slight increase in OS of about 3 months and fewer hospital days compared to SC (about 1 week), while targeted/biological therapies may provide little or no difference in OS and a slight increment on PFS, at the expense of more treatment-related serious adverse events. As already said, there is an important uncertainty about the effects of chemotherapy in PFS, QoL and FS, and even more of immunotherapy in OS and FS. Moreover, as there are no RCTs that have assessed the effects of treating advanced PC beyond a second-line therapy, this lack of evidence should be another criterion for establishing treatment limits.
Given the evidence gaps and the low certainty of evidence of our findings, the assessment of the effects of TADs when compared to SC alone for patients with advanced PC may still be considered appropriate. Some clinicians could justify their use of TADs in advanced PC patients based in the superiority of some regimens over others, since chemotherapy is already considered the standard of care [7, 9]. But others can argue that critical and important outcomes for decision making have not been sufficiently studied and that guidelines’ recommendations of TADs are based on limited evidence and a partial evaluation of their effects. Therefore, it would be reasonable and ethical to conduct more and better RCTs considering BSC with no TAD as the control arm in PC patients with an advanced disease. The information obtained from these studies would certainly enhance the future decision-making process.
This SR has several strengths. We conducted an exhaustive search in six databases, as well as citation search to find all relevant studies. We have also conducted selection, data extraction and quality assessment by two reviewers independently, and we present the results with the respective certainty of evidence assessment using the GRADE guidance.
Although our review did not focus on comparing different schemes of TADs with each other, it really challenges the appropriateness of current recommendations of TADs and the way in which they have been formulated. Therefore, clinicians should continuously provide advanced PC patients with all relevant information about prognosis, treatment options including BSC as a reasonable alternative, and other aspects of care, thus advocating for patient’s autonomy [9]. In the case of advanced PC patients, it is crucial to assume that their very poor survival prognosis at short term basis would be only slightly improved with any TAD, if successful, and this must be balanced with the risks associated to the therapies that could worsen their QoL. If they are aware of this scenario, some patients could reasonably prefer to receive only the best possible SC and give priority to maintain a good QoL until the end of their lives.
Conclusions
This review found that the evidence driven from RCTs is very uncertain about whether the benefits provided by TADs are greater than their associated harms in patients with advanced PC. When the first chemotherapy lines have failed, there is no evidence to propose further TADs to patients unless accepting their inclusion in a trial. In contrast, BSC is an appropriate alternative to be offered, especially if their functional status is poor or the disease is very advanced. Future research should assess the impact of TADs on all patient-important outcomes, thus providing relevant information to involve patients in establishing their goals of care.
Availability of data and materials
The protocol of the current study is available in the OSF repository, https://osf.io/7chx6/, (DOI https://doi.org/10.17605/OSF.IO/7CHX6). Search strategies are included in the supplementary information files.
Abbreviations
- BSC:
-
Best supportive care
- CI:
-
Confidence interval
- EoL:
-
End-of-life
- ECOG:
-
Eastern Cooperative Oncology Group
- FS:
-
Functional status
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation
- HR:
-
Hazard ratio
- KPS:
-
Karnofsky Performance Status
- MD:
-
Mean Difference
- OS:
-
Overall Survival
- PC:
-
Pancreatic Cancer
- PFS:
-
Progression-free Survival
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PS:
-
Performance status
- QoL:
-
Quality of Life
- QoD:
-
Quality of Death
- RCT:
-
Randomised Clinical Trial
- RoB:
-
Risf of Bias
- RR:
-
Risk Ratio
- SC:
-
Supportive Care
- SoF:
-
Summary of Findings
- SR:
-
Systematic Review
- TAD:
-
Treatment with Anticancer Drugs
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
SEER*Explorer: An interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute. [cited 2022]; Available from: https://seer.cancer.gov/explorer/.
Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326(9):851–62.
Lelond S, Ward J, Lambert PJ, Kim CA. Symptom burden of patients with Advanced Pancreas Cancer (APC): a provincial cancer institute observational study. Curr Oncol. 2021;28(4):2789–800.
Tang CC, Von Ah D, Fulton JS. The symptom experience of patients with advanced pancreatic cancer: an integrative review. Cancer Nurs. 2018;41(1):33–44.
Hui D, Nooruddin Z, Didwaniya N, Dev R, De La Cruz M, Kim SH, et al. Concepts and definitions for “actively dying,” “end of life,” “terminally ill,” “terminal care,” and “transition of care”: a systematic review. J Pain Symptom Manage. 2014;47(1):77–89.
Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(5):v56–68. (eUpdate published online 20 June 2017. Ann Oncol. 2017;28(4):iv157.
Balaban EP, Mangu PB, Yee NS. Locally advanced unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2017;13(4):265–9.
Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020:Jco2001364.
Fan JQ, Wang MF, Chen HL, Shang D, Das JK, Song J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol Cancer. 2020;19(1):32.
Patel K, Siraj S, Smith C, Nair M, Vishwanatha JK, Basha R. Pancreatic cancer: an emphasis on current perspectives in immunotherapy. Crit Rev Oncog. 2019;24(2):105–18.
Leroux C, Konstantinidou G. Targeted therapies for pancreatic cancer: overview of current treatments and new opportunities for personalized oncology. Cancers (Basel). 2021;13(4):799.
Salazar J, Pérez-Bracchiglione J, Salas-Gama K, Antequera A, Auladell-Rispau A, Dorantes-Romandía R, et al. Efficacy of systemic oncological treatments in patients with advanced pancreatic cancer at high risk of dying in the short or medium-term: overview of systematic reviews. Eur J Cancer. 2021;154:82–91.
Salazar J, Bracchiglione J, Acosta-Dighero R, Meza N, Meade A-G, Quintana MJ, et al. Systemic oncological treatments in patients with advanced pancreatic cancer: a scoping review and evidence map. Support Care Cancer. 2023;31(2):100.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Bracchiglione Pérez J, Salazar J, Santero M, Requeijo C, Rodriguez Grijalva G, Acosta-Dighero R, et al. Efficacy of systemic oncological treatments in patients with advanced, non-intestinal digestive cancer at high risk of dying in the middle and short term: Evidence synthesis. Open Science Framework. 2022. Available from https://doi.org/10.17605/OSF.IO/7CHX6.
Guyatt G. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice, 3E: McGraw-Hill Education / Medical; 2014. Available from: https://books.google.com/books/about/Users_Guides_to_the_Medical_Literature_A.html?hl=&id=jgZYngEACAAJ.
Mo L, Urbauer DL, Bruera E, Hui D. Recommendations for supportive care and best supportive care in NCCN clinical practice guidelines for treatment of cancer: Differences between solid tumor and hematologic malignancy guidelines. Support Care Cancer. 2021;29(12):7385–92.
Sanz Rubiales Á, Sánchez-Gutiérrez ME, Flores Pérez LA, Del Valle Rivero ML. How is best supportive care provided in clinical trials for patients with advanced cancer? A review of registered protocols of clinical trials. Curr Oncol. 2020;27(2):e100–5.
Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. ACM International Conference Proceeding Series. 2014. https://doi.org/10.1145/2601248.2601268.
Haddaway NR, Grainger MJ, Gray CT. Citationchaser: a tool for transparent and efficient forward and backward citation chasing in systematic searching. Research Synthesis Methods. 2022;13(4):533–45.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handbook for Systematic Reviews of Interventions2019. p. 143–76.
Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clinical Epidemiology and Global Health. 2019;7(2):192–8.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Frey C, Twomey P, Keehn R, Elliott D, Higgins G. Randomized study of 5-FU and CCNU in pancreatic cancer: report of the veterans administration surgical adjuvant cancer chemotherapy study group. Cancer. 1981;47(1):27–31.
Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7(6):593–600.
Huguier M, Barrier A, Valinas R, Flahault A, Adloff M, Pezet D, et al. Randomized trial of 5-fluorouracil, leucovorin and cisplatin in advanced pancreatic cancer. Hepatogastroenterology. 2001;48(39):875–8.
Mallinson CN, Rake MO, Cocking JB, Fox CA, Cwynarski MT, Diffey BL, et al. Chemotherapy in pancreatic cancer: results of a controlled, prospective, randomised, multicentre trial. Br Med J. 1980;281(6255):1589–91.
Mallinson CN, Rake MO, Fox CA. Final results of a controlled trial of chemotherapy for inoperable pancreatic cancer. Irish J Medic Science. 1977;146(suppl.1). ABSTRACT.
A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. The Gastrointestinal Tumor Study Group. Ann Surg. 1979;189(2):205–8.
Xinopoulos D, Dimitroulopoulos D, Karanikas I, Fotopoulou A, Oikonomou N, Korkolis D, et al. Gemcitabine as palliative treatment in patients with unresectable pancreatic cancer previously treated with placement of a covered metal stent. A randomized controlled trial J buon. 2008;13(3):341–7.
Xinopoulos, D, Dimitroulopoulos, D., Fotopoulou, A., Korkolis, D., Tsamakidis, K., Kypreos, D., Basioukas, S., Pasavela, S., Loukou, A., Paraskevas, E. Palliation with previously gemcitabine in patients with advanced pancreatic cancer treated with the placement of a covered metal biliary stent. Annals Gastroenetology. 2009;22(1):0–15.
Ciuleanu TE, Pavlovsky AV, Bodoky G, Garin AM, Langmuir VK, Kroll S, et al. A randomised Phase III trial of glufosfamide compared with best supportive care in metastatic pancreatic adenocarcinoma previously treated with gemcitabine. Eur J Cancer. 2009;45(9):1589–96.
Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47(11):1676–81.
Oettle H, Pelzer U, Stieler J, Hilbig A, Roll L, Schwaner I, Adler M, Detken S, Dorken B, Riess H. Oxaliplatin/Folinic acid/5-fluorouracil 24h (OFF) plus Best Supportive Care versus Best Supportive Care alone (BSC) in second-line therapy of gemcitabine-refractory advanced pancreatic cancer (CONKO 003). J Clinic Oncol. 2005;23:4031.
Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994;81(6):882–5.
Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53(1):146–50.
Takada T, Nimura Y, Katoh H, Nagakawa T, Nakayama T, Matsushiro T, et al. Prospective randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C for non-resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology. 1998;45(24):2020–6.
Oortgiesen JM DL, Weidman JR, Soeder T, Cato A, Sutton LY. A prospective, randomized, double-blind, placebo controlled, group sequential trial of polyclonal antibody stimulator (pas) for the treatment of advanced pancreatic cancer. Annals of Oncology. 2010;21(8):viii234. ABSTRACT.
Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, et al. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012;41(3):374–9.
Gilliam AD, Topuzov EG, Garin AM, Pulay I, Broome P, Watson SA, Rowlands BJ, Takhar A, Beckingham IJ. Randomised, double blind, placebo-controlled, multi-centre, group-sequential trial of G17DT for patients with advanced pancreatic cancer unsuitable or unwilling to take chemotherapy. J Clinic Oncol. 2004;22:2511.
Propper D, Davidenko I, Bridgewater J, Kupcinskas L, Fittipaldo A, Hillenbach C, et al. Phase II, randomized, biomarker identification trial (MARK) for erlotinib in patients with advanced pancreatic carcinoma. Ann Oncol. 2014;25(7):1384–90.
Ducreux M, Davidenko I, Bridgewater J, Kupcinskas L, Johannsdottir H, Van Der Horst T, Klughammer B, Propper D. Investigating potential biomarkers for survival with erlotinib in patients with advanced pancreatic cancer - Results of the phase II BO21129 study. European J Cancer. 2011;47:S464-S.
Herman JM, Kitchen H, Degboe A, Aldhouse NVJ, Trigg A, Hodgin M, et al. Exploring the patient experience of locally advanced or metastatic pancreatic cancer to inform patient-reported outcomes assessment. Qual Life Res. 2019;28(11):2929–39.
Palmieri LJ, Dubreuil O, Bachet JB, Trouilloud I, Locher C, Coriat R, et al. Reasons for chemotherapy discontinuation and end-of-life in patients with gastrointestinal cancer: a multicenter prospective AGEO study. Clin Res Hepatol Gastroenterol. 2021;45(1): 101431.
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Xue P, Zhu L, Wan Z, Huang W, Li N, Chen D, et al. A prognostic index model to predict the clinical outcomes for advanced pancreatic cancer patients following palliative chemotherapy. J Cancer Res Clin Oncol. 2015;141(9):1653–60.
Chung V, Sun V, Ruel N, Smith TJ, Ferrell BR. Improving palliative care and quality of life in pancreatic cancer patients. J Palliat Med. 2022;25(5):720–7.
Acknowledgements
The authors would like to thank Juan Carlos Vásquez and Daniel Simancas for their collaboration in the selection process of this review.
Funding
This study has been funded by Instituto de Salud Carlos III through the project "PI18/00034" (Co-funded by European Regional Development Fund "A way to make Europe").
Author information
Authors and Affiliations
Consortia
Contributions
JS, JB, and XB conceived and designed the review. JS, JB, and OSE participated in the study selection, data extraction and analysis. AA, DBP, MGV, SMP, CP, AT, and XB contributed to the interpretation of the findings. JS prepare the draft of the manuscript and JB and XB provided supervisory support. All authors contributed with their vision and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy for PUBMED and CENTRAL.
Additional file 2.
Excluded reports.
Additional file 3.
Characteristics of included studies.
Additional file 4.
Judgements for each risk of bias domain for included studies.
Additional file 5.
Results for chemotherapy and targeted/biological therapy versus no-TAD per outcome.
Additional file 6.
Summary of findings for chemotherapy compared to no-TAD for advanced pancreatic cancer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salazar, J., Bracchiglione, J., Savall-Esteve, O. et al. Treatment with anticancer drugs for advanced pancreatic cancer: a systematic review. BMC Cancer 23, 748 (2023). https://doi.org/10.1186/s12885-023-11207-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11207-4