Abstract
Purpose
To establish a prognostic index model for advanced pancreatic cancer patients receiving palliative chemotherapy based on clinical variables.
Methods
The clinical data of 118 patients with advanced pancreatic cancer who received palliative chemotherapy between January 2006 and August 2013 in our center were retrospectively analyzed. Prognostic factors for overall survival were identified using Cox proportional hazards model. A prognostic index model was established by these pretreatment factors to predict prognosis. Kaplan–Meier estimation and log-rank test were performed to compare the overall survival difference between low-risk and high-risk group of patients.
Results
Median overall survival time for all patients was 8.8 months [95 % confidence interval (CI) 7.0–10.6 months]. Multivariate analysis identified ECOG score = 2 (hazard ratio 2.03; 95 % CI 1.07–3.85; P = 0.030), CA19-9 levels of ≥1000 U/mL (hazard ratio 2.07; 95 % CI 1.09–3.92; P = 0.026), and CRP levels of ≥5 mg/L (hazard ratio 2.05; 95 % CI 1.06–3.96; P = 0.033) as independent poor prognostic factors for overall survival. For the three factors, ECOG score = 2, CA19-9 levels of ≥1000 U/mL, and CRP levels of ≥5 mg/L were allocated 1 point each. There were 84 (71.2 %) patients allocated to low-risk group with total score 0–1 point, and 34 (28.8 %) patients were categorized as high-risk group with total scores 2–3 points. The median overall survival for low-risk group and high-risk group was 9.9 months (95 % CI 6.8–13.0) and 5.3 months (95 % CI 4.1–6.5), respectively (hazard ratio 0.27; 95 % CI 0.14–0.52; P < 0.001). The estimated 1-year survival rates for low-risk group and high-risk group were 40.5 and 5.9 %, respectively (P < 0.05).
Conclusions
A novel prognostic index model based on three clinical parameters was established to predict the prognosis of patients with advanced pancreatic cancer receiving palliative chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a devastating malignant disease represented by almost equal morbidity and mortality annually (Siegel et al. 2013). It is estimated to be the second leading cause of cancer-related death in USA around 2020 (Rahib et al. 2014). Although surgical resection is the only potential curative modality, only 10–20 % of patients were first diagnosed with resectable tumor (Heinemann et al. 2012). Even after curative resection, a large proportion of patients develop recurrence within 1 year (Katz et al. 2009; Barugola et al. 2009). The 5-year survival rate remains dismal (Stathis and Moore 2010).
In clinical practice, most patients present with locally advanced or metastatic disease at the first diagnosis (Heinemann et al. 2012; Stathis and Moore 2010). Palliative chemotherapy with gemcitabine (Burris et al. 1997; Heinemann et al. 2008) or S-1 (Okusaka et al. 2008; Ueno et al. 2013) based regimen for advanced pancreatic cancer (APC) has been established as the standard of care in recent years. However, the prognosis of patients receiving palliative chemotherapy varies depending on a number of clinical characteristics (Papadoniou et al. 2008). It is crucial to identify the subgroup of APC patients who would benefit from palliative chemotherapy (Philip et al. 2009). Previous studies have identified several prognostic factors for survival, such as pretreatment CA19-9 (Saad et al. 2002; Reni et al. 2009), C-reactive protein (CRP) (Haas et al. 2010; Wang et al. 2012), serum albumin levels (Maréchal et al. 2007), neutrophil-to lymphocyte ratio (NLR) (Xue et al. 2014), and performance status (Tas et al. 2013). However, the predictive value of these factors for palliative chemotherapy of APC patients is still controversial. Therefore, the development of an alternative method to predict the prognosis for APC patients is attracting more attention. Glasgow Prognostic Score (GPS) (McMillan 2013), a notable prognostic scoring method, which was developed a decade ago, represented a sensitive measure of the systemic inflammatory response and nutritional status of patient. Glen et al. (2006) verified its prognostic value in patients with inoperable pancreatic cancer. However, the host-related factor of GPS alone can hardly reflect the overall status of patients with pancreatic cancer. Therefore, we sought to develop a comprehensive and feasible prognostic index model to predict the prognosis for APC patients in daily practice.
In this study, we investigated multiple pretreatment variables that are easily accessible in clinical practice to predict the outcomes of APC patients receiving palliative chemotherapy. Furthermore, a prognostic index model derived from the independent prognostic factors was established to effectively identify the high-risk group of APC patients undergoing gemcitabine- or S-1-based palliative chemotherapy. It could be valuable for clinicians in decision making of treatment strategies and assessment of APC patients who may likely benefit from palliative chemotherapy.
Materials and methods
Patients and treatment
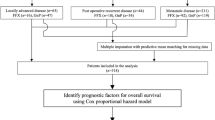
Clinical data of 145 consecutive APC patients, who received gemcitabine- or S-1-based palliative chemotherapy in our cancer center between January 2006 and August 2013, were retrospectively analyzed. Twenty-seven cases were excluded from this study due to deficient pathological or clinical data, and 118 patients who met the following inclusion criteria were included in our study: (1) patients with pathologically confirmed invasive ductal carcinoma of the pancreas, either by surgical resection or needle biopsy; (2) patients presented with locally advanced unresectable or metastasis disease diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI); and (3) patients with available clinical data at the first administration of gemcitabine- and/or S-1-based palliative chemotherapy. All patients enrolled had signed informed consent previously for the purpose of clinical research. This study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiaotong University.
Palliative chemotherapy consisted of gemcitabine- or S-1-based regimens, including gemcitabine monotherapy (n = 68) (Burris et al. 1997), gemcitabine and cisplatin combination therapy (n = 8) (Heinemann et al. 2006), gemcitabine and oxaliplatin combination therapy (n = 12) (Louvet et al. 2005), gemcitabine and nab-paclitexal combination therapy (n = 4) (Von Hoff et al. 2013), gemcitabine and erlotinib combination therapy (n = 4) (Moore et al. 2007), and S-1 monotherapy (n = 22) (Okusaka et al. 2008; Ueno et al. 2013). The specific dosage and schedule of each regimen were adjusted by the physicians based on the individual patient’s general condition.
Prognostic factors
The integrated clinical data included patients’ demographics, the medical treatment records, pathological reports, tumor-node-metastasis stage, imaging scan of body, and pretreatment laboratory data were collected for analysis. Fourteen clinical variables were chosen as potential prognostic factors, among which continuous parameters were divided into two categories according to the previous studies (Papadoniou et al. 2008; Haas et al. 2010; Xue et al. 2014; Tanaka et al. 2008) for the convenience of prognostic analysis as follows: age (<65 or ≥65 years), gender (male or female), Eastern Cooperative Oncology Group (ECOG) score (0–1 or 2), primary tumor location (head or body/tail), prior tumor resection (no or yes), distant metastasis (no or yes), levels of carbohydrate antigen 19-9 (CA19-9 <1000 or ≥1000 U/mL), carcinoembryonic antigen (CEA <5 or ≥5 ng/mL), C-reactive protein (CRP <5 or ≥5 mg/L), hemoglobin (<100 or ≥100 g/L), neutrophil-to-lymphocyte ratio (NLR <5 or ≥5), platelet to lymphocyte ratio (PLR <150 or ≥150), and albumin (<35 or ≥35 g/L).
Statistical analysis
All of the analyses were performed using SPSS statistical software (version 17.0, SPSS Inc, Chicago, IL, USA). The primary end point of the study was overall survival (OS). OS was calculated from the initiation of palliative chemotherapy to the date of death for any reason or the last follow-up visit of patient. OS were estimated by the Kaplan–Meier method, and the difference in OS was compared by log-rank tests. Prognostic variables associated with OS were identified through univariate analysis by Cox regression models. The hazard ratio (HR) and 95 % confidence interval (CI) were calculated using Cox regression models. A two-tailed P value of <0.05 was considered statistically significant. The independent prognostic variables associated with OS were confirmed by multivariate analysis using Cox proportional hazards model. A prognostic index model was established based on independent variables that were significantly associated with OS in the multivariate analysis.
Results
Patient characteristics
A total of 118 consecutive patients with APC treated with first-line palliative chemotherapy between January 2006 and August 2013 were investigated. The median age of these patients was 62 years (range 34–82). Seventy-four patients (62.7 %) had relatively good general conditions with ECOG score 0–1. Forty-six patients (39.0 %) had pancreatic head carcinoma, while seventy-two patients (61.0 %) had carcinoma in the body and tail of pancreas. Twenty-four patients (20.3 %) had received primary pancreatic lesion resection before recurrence. Forty-six patients (39.0 %) had unresectable locally advanced lesion, while seventy-two patients (61.0 %) had distant metastatic disease. Of these patients, sixty-four (54.2 %) had liver metastasis, sixty (50.8 %) had celiac lymph node metastasis, and thirty-four (28.8 %) had ascites or peritoneum metastasis. Twenty-two patients (18.6 %) received S-1 monotherapy; other ninety-six patients (81.4 %) received gemcitabine-containing regimen treatment, among which sixty-eight had received gemcitabine monotherapy and twenty-eight had received gemcitabine-based combination therapy. Patients’ baseline characteristics are shown in Table 1.
Univariate and multivariate analysis of prognostic factors
Univariate analysis of potential prognostic factors associated with OS in this cohort showed that ECOG score of 2, unresectable disease, distant metastasis, CA19-9 levels of ≥1000 U/mL, CRP levels of ≥5 mg/L, and NLR ≥5 were significantly associated with poor OS (P < 0.05). Performing the subsequent multivariate analysis, a total of three factors, including ECOG score = 2, CA19-9 levels of ≥1000 U/mL, and CRP levels of ≥5 mg/L, were identified as independent prognostic factors for poor OS in APC patients following palliative chemotherapy (Table 2).
Overall survival
With median follow-up period of 8.5 months (range 0.5–65.8), 88 patients (74.6 %) were reported dead at time of last follow-up. The median OS time for all the 118 patients was 8.8 months [95 % confidence interval (CI), 7.0–10.6 months] since initiation of palliative chemotherapy (Fig. 1). The 1- and 2-year survival rates were 32.2 and 10.2 %, respectively.
To compare OS of patients with different profiles of prognostic factors, we divided patients in subgroups according to the independent prognostic factors identified in the multivariate analysis, including ECOG score, CA19-9 levels, and CRP levels, and compared the Kaplan–Meier curves for OS by log-rank test (Fig. 2). The median OS was 5.5 months (95 % CI 4.4–6.6) in ECOG score 2 group and 9.9 months (95 % CI 6.9–12.9) in ECOG score 0–1 group [hazard ratio (HR) 0.47; 95 % CI 0.26–0.86; P = 0.015]. For patients with CA19-9 levels of ≥1000 versus <1000 U/mL, median OS was 5.3 months (95 % CI 4.0–6.6) versus 9.9 months (95 % CI 6.7–13.1) (HR 0.36; 95 % CI 0.19–0.66; P = 0.001). The median OS for patients with CRP levels ≥5 mg/L was 5.9 months (95 % CI 4.4–7.4) compared with 10.6 months (95 % CI 6.6–14.6) in patients with CRP levels <5 mg/L (HR 0.49; 95 % CI 0.27–0.89; P = 0.019).
Prognostic index model
The pretreatment ECOG score, CA19-9 levels, and CRP levels identified as independent prognostic factor by multivariate analysis were used to establish the prognostic index model. Table 3 shows the criteria of prognostic index model as follows: ECOG score 2, CA19-9 levels of ≥1000 U/mL, and CRP levels ≥5 mg/L were allocated 1 point each; ECOG score 0–1, CA19-9 levels of <1000 U/mL, and CRP levels <5 mg/L were allocated 0 point each. The total score ranging from 0 to 3 was categorized into two prognostic index risk groups as defined as follows: low-risk group, 0 or 1 point; high-risk group, 2 or 3 point. There were 84 (71.2 %) patients allocated to low-risk group, and 34 (28.8 %) patients were categorized as high-risk group. The median OS for the low-risk group was 9.9 months (95 % CI 6.8–13.0), which was significantly longer than that of 5.3 months (95 % CI 4.1–6.5) in high-risk group (HR 0.27; 95 % CI 0.14–0.52; P < 0.001). The estimated 1-year survival rates for low-risk group and high-risk group were 40.5 and 5.9 %, respectively (P < 0.05) (Fig. 3).
Discussion
The incidence of pancreatic cancer has gradually increased in developing countries in recent years (Ma et al. 2013). Several clinical trials confirmed that gemcitabine- or S-1-containing palliative chemotherapy possess similar efficacy and safety for patients with APC; nevertheless, the prognosis of APC patients remains dismal (Stathis and Moore 2010; Burris et al. 1997; Heinemann et al. 2008; Okusaka et al. 2008; Ueno et al. 2013; Michl and Gress 2013). It is clinically relevant to identify the prognostic factors for APC patients in determination of the treatment strategy.
Previous studies have reported several prognostic factors related to poor outcomes for APC patients, including elevated pretreatment levels of CA19-9 (Saad et al. 2002; Reni et al. 2009), CRP (Haas et al. 2010; Wang et al. 2012; Pine et al. 2009), and LDH (Haas et al. 2010; Tas et al. 2001), increased ratio of neutrophil to lymphocyte (Xue et al. 2014), poor performance status (Tas et al. 2013), and status of initial unresectable disease (Xue et al. 2014). However, the prognostic factors reported were different in various studies. In the current study, we devised a convenient prognostic index model by retrospectively analyzing various clinicopathological factors and pretreatment parameters in APC patients receiving palliative chemotherapy. Three factors, including ECOG score of 2, CA19-9 levels of ≥1000 U/mL, and CRP levels ≥5 mg/L, were identified as independent adverse prognostic factors for OS in APC patients in our cohort. Creating a prognostic index model with these three factors, patients classified as low risk with this model showed significant survival benefit from gemcitabine- or S-1-based chemotherapy, with a median survival duration of 9.9 months and 1-year survival rate of 40.5 %, which was much better than the patients categorized as high risk, with median survival duration of 5.3 months and a 1-year survival rate of 5.9 %. Based on these values, palliative chemotherapy of gemcitabine- or S-1-containing regimen showed no significant survival benefit for the patients in high-risk group. As to the low-risk group of patients, it should be noted that those who have fewer poor prognostic factors may do well even without chemotherapy. Nevertheless, this prognostic index model would help oncologists to avoid excessive medical treatment for APC patients in high-risk group; meanwhile, it is urgent to develop novel therapeutical strategies for these intractable high-risk groups of APC patients.
Previous studies have proposed various prognostic models for APC patients (details in Table 4). Hamada et al. (2014) recently designed a nomogram derived from analysis of their prospectively collected 531 patients with inoperable pancreatic cancer receiving palliative chemotherapy, which contained six potential prognostic factors of age, sex, ECOG PS score, tumor size, regional lymph node metastasis, and distant metastasis status. This nomogram provided improved ability to predict clinical outcome for each patient with APC. Yi et al. (2011) devised a risk-stratified prognostic model derived from 298 APC patients who received gemcitabine-based chemotherapy, which consists of four prognostic factors, including serum CRP levels, albumin levels, metastasis to liver, and ascites dissemination. The survival outcomes differed remarkably according to the prognostic model stratification. However, only 84.9 % patients in their study had definite pathological diagnosis of ductal adenocarcinoma. Maréchal et al. (2007) also reported a prognostic index consists of three variables of KPS, weight loss, and AST for APC patients who received gemcitabine-containing chemotherapy. Using this index model, patients were categorized into three groups with significantly different survival time. By contrast, three variables of ECOG score, CA19-9 levels, and CRP levels identified in our prognostic index model represent the general condition, tumor burden, and systemic inflammatory reaction of patient, respectively, making our result more close to clinical practice. Consistently, previous study has demonstrated that these three prognostic factors are significantly associated with the survival of a cohort of 103 consecutive patients with APC (Ueno et al. 2000). The median OS of this cohort was 3.2 months in contrast to 8.8 months in this study, and about 30 % of the patients had died within 2 months from the beginning of systemic chemotherapy. This may be because patients with locally advanced APC were excluded from this study. Additionally, the chemotherapeutic regimen used in this cohort varied among patients, having only a few patients treated with gemcitabine monotherapy which is not consistent to current clinical practice. Because of the disparity of categorization criteria for the continuous parameters, such as CA19-9 and CRP, between studies (Yi et al. 2011; Maréchal et al. 2007; Ueno et al. 2000), universally accepted cutoff values of these parameters have yet established. This underscores the importance of conducting further study to elucidate this issue. Our data suggests that the cutoff value defined in this study based on previous studies and our clinical practice could be utilized successfully to predict prognosis for APC patients. Obvious limitations should be addressed that the sample size of the current study is relatively small compared with other previous studies. Furthermore, the reliability of the predictive model developed in retrospectively single center should be externally validated using another independent cohort data.
In this study, all the APC patients have received either gemcitabine- or S-1-based chemotherapy. Although the chemotherapy regimens differed among patients in our study, it is unlikely that the treatment regimens would influence the prognosis of patients. Ueno et al. (2013) demonstrated in GEST study that efficacy of gemcitabine, S-1, or gemcitabine/S-1 combination therapy for patients with APC were not statistically different. In addition, previous meta-analysis studies (Heinemann et al. 2008) showed no significant improvement in OS for gemcitabine-based combinations over gemcitabine monotherapy. Furthermore, in our results of univariate analysis for different treatment regimens, the prognosis of patients received gemcitabine- or S-1-containing regimens showed no statistical difference (HR 1.55; 95 % CI 0.74–3.26; P = 0.245).
In conclusion, our study identified three independent adverse prognostic factors, ECOG, CA19-9, and CRP, in patients with APC who had received palliative chemotherapy. A prognostic index model calculated based on these factors was developed to stratify patients with low and high risk of poor prognosis. These readily accessible pretreatment parameters of patients and prognostic index model could assist clinicians to identify high-risk patients and propose individualized therapeutic approach to APC patients in clinical practice.
Abbreviations
- APC:
-
Advanced pancreatic cancer
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PLR:
-
Platelet-to-lymphocytes ratio
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CRP:
-
C-reactive protein
- LDH:
-
Lactate dehydrogenase
- TB:
-
Total bilirubin
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- ALP:
-
Alkaline phosphatase
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- GPS:
-
Glasgow Prognostic Score
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Barugola G, Partelli S, Marcucci S et al (2009) Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol 16:3316–3322
Burris HA III, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Glen P, Jamieson NB, McMillan DC et al (2006) Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology 6(5):450–453
Haas M, Laubender RP, Stieber P et al (2010) Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol 31:351–357
Hamada T, Nakai Y, Yasunaga H et al (2014) Prognostic nomogram for nonresectable pancreatic cancer treated with gemcitabine-based chemotherapy. Br J Cancer 110(8):1943–1949
Heinemann V, Quietzsch D, Gieseler F et al (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24(24):3946–3952
Heinemann V, Boeck S, Hinke A et al (2008) Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8:82
Heinemann V, Haas M, Boeck S (2012) Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev 38:843–853
Katz MH, Wang H, Fleming JB et al (2009) Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 16:836–847
Louvet C, Labianca R, Hammel P et al (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23(15):3509–3516
Ma C, Jiang YX, Liu SZ et al (2013) Trend and prediction on the incidence of pancreatic cancer in China. Zhonghua Liu Xing Bing Xue Za Zhi 34(2):160–163
Maréchal R, Demols A, Gay F et al (2007) Prognostic factors and prognostic index for chemonaïve and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology 73(1-2):41–51
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39(5):534–540
Michl P, Gress TM (2013) Current concepts and novel targets in advanced pancreatic cancer. Gut 62(2):317–326
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Okusaka T, Funakoshi A, Furuse J et al (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Papadoniou N, Kosmas C, Gennatas K et al (2008) Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res 28:543–549
Philip PA, Mooney M, Jaffe D et al (2009) Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 27:5660–5669
Pine JK, Fusai KG, Young R et al (2009) Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol 35(6):605–610
Rahib L, Smith BD, Aizenberg R et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11):2913–2921
Reni M, Cereda S, Balzano G et al (2009) Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 115:2630–2639
Saad ED, Machado MC, Wajsbrot D et al (2002) Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer 32(1):35–41
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7(3):163–172
Tanaka T, Ikeda M, Okusaka T et al (2008) Prognostic factors in japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol 38:755–761
Tas F, Aykan F, Alici S et al (2001) Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 24(6):547–550
Tas F, Sen F, Odabas H et al (2013) Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 18(5):839–846
Ueno H, Okada S, Okusaka T et al (2000) Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology 59(4):296–301
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703
Wang DS, Luo HY, Qiu MZ et al (2012) Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 29(5):3092–3100
Xue P, Kanai M, Mori Y et al (2014a) Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 3(2):406–415
Xue P, Kanai M, Mori Y et al (2014b) Comparative outcomes between initially unresectable and recurrent cases of advanced pancreatic cancer following palliative chemotherapy. Pancreas 43(3):411–416
Yi JH, Lee J, Park SH et al (2011) A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology 80(3–4):175–180
Acknowledgments
This study was supported by the National Natural Science Foundation of China, Grant ID 81171887.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Xue, P., Zhu, L., Wan, Z. et al. A prognostic index model to predict the clinical outcomes for advanced pancreatic cancer patients following palliative chemotherapy. J Cancer Res Clin Oncol 141, 1653–1660 (2015). https://doi.org/10.1007/s00432-015-1953-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1953-y