Abstract
Background
To the best of our knowledge, no previous studies have explored the relationship between visceral obesity and malnutrition. Therefore, this study has aimed to investigate the association between them in patients with rectal cancer.
Methods
Patients with rectal cancer who underwent proctectomy were included. Malnutrition was defined according to the Global Leadership Initiative on Malnutrition (GLIM). Visceral obesity was measured using computed tomography (CT). The patients were classified into four groups according to the presence of malnutrition or visceral obesity. Univariate and multivariate logistic regression analyses were performed to evaluate risk factors for postoperative complications. Univariate and multivariate cox regression analyses were performed to evaluate the risk factors for overall survival (OS) and cancer-specific survival (CSS). Kaplan-Meier survival curves and log-rank tests were performed for the four groups.
Results
This study enrolled 624 patients. 204 (32.7%) patients were included in the well-nourished non-visceral obesity (WN) group, 264 (42.3%) patients were included in the well-nourished visceral obesity (WO) group, 114 (18.3%) patients were included in the malnourished non-visceral obesity (MN) group, and 42 (6.7%) patients were included in the malnourished visceral obesity (MO) group. In the multivariate logistic regression analysis, the Charlson comorbidity index (CCI), MN, and MO were associated with postoperative complications. In the multivariate cox regression analysis, age, American Society of Anesthesiologists (ASA) score, tumor differentiation, tumor node metastasis (TNM), and MO were associated with worsened OS and CSS.
Conclusions
This study demonstrated that the combination of visceral obesity and malnutrition resulted in higher postoperative complication and mortality rates and was a good indicator of poor prognosis in patients with rectal cancer.
Similar content being viewed by others
Introduction
Malnutrition is a major health concern worldwide, as nutritional status is an important prognostic factor in patients with cancer [1]. Patients with cancer-related malnutrition experience increased postoperative complication and mortality rates [2]. Malnutrition has been responsible for 10–20% of deaths in patients with cancer rather than the tumor itself, because of the effect on the progression and therapeutic responses of cancer [3, 4]. Thus, the nutritional status of patients with cancer should be assessed, and nutritional interventions should be provided perioperatively, when necessary.
Pervasive obesogenic lifestyles have led to obesity epidemics worldwide [5]. Obesity is characterized by an increase in adiposity, which adversely affects health and is typically defined using body mass index (BMI) [6]. However, clinicians were unable to differentiate between visceral, subcutaneous, intermuscular, and intramuscular adiposity using BMI because it is a generalized measure and does not consider the composition of each body compartment. Therefore, individuals with obesity were highly heterogeneous [7, 8]. Visceral obesity, the accumulation of visceral adipose tissue (VAT), which is considered a more reliable indicator of obesity than BMI. [9, 10]. Visceral obesity had a negative impact on the outcomes of patients with cancer, including longer operative time, greater intraoperative blood loss, longer hospital stay, higher postoperative complications, and higher mortality rate [11, 12].
According to the World Health Organization (WHO), “a double burden of malnutrition” existed when undernutrition, obesity, and diet-related non-communicable diseases coexist, which is a real and growing global health challenge [13]. Some studies have explored the impact of malnutrition on obese patients’ outcomes, but BMI has remained the only surrogate measure for obesity. [14,15,16]. To the best of our knowledge, no previous studies have explored the relationship between visceral obesity and malnutrition. Therefore, this study has aimed to investigate the association between them in patients with rectal cancer.
Material & Methods
Patients
This study included patients with rectal cancer who underwent proctectomy between February 2013 and March 2019 at the Anorectal Surgery Department of the Second Affiliated Hospital of Wenzhou Medical University. Depending on the distance between the tumor and the rectum, the Dixon or Miles operation was performed. Inclusion criteria: (1) age ≥ 18 years; (2) American Society of Anesthesiologists (ASA) grade ≤ III; and (3) planning to undergo curative proctectomy. Exclusion criteria: (1) an available preoperative abdominal computed tomography (CT) beyond a month; (2) missing data; (3) undergoing palliative or emergency surgery; (4) receiving neoadjuvant treatments; or (5) patients with metastatic disease (stage IV). The data collection protocol for this study was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (LCKY2020–209) and complied with the Declaration of Helsinki. Informed consents were obtained from all participants.
Data collection
Data were collected as follows: (1) general features, including age, gender, BMI, skeletal muscle index (SMI), visceral fat area (VFA), Charlson comorbidity index (CCI) [17], ASA grade, and previous abdominal surgery; (2) clinicopathological features, including tumor size, tumor location, tumor differentiation, tumor stage, node stage, and pathological tumor node metastasis (TNM) stage; and (3) postoperative short-term and long-term outcomes, including postoperative complications (according to the Clavien–Dindo classification grade [18]), postoperative hospital stay and mortality.
Assessment of SMI and VFA
Preoperative abdominal CT images at the level of the third lumbar vertebra were obtained using specialized imaging software (INFINITT Healthcare Co., Ltd.) to calculate muscle mass, which was defined as the area shown in − 29 to + 150 Hounsfield unit (HU). VFA was defined as the area shown in − 150 to − 50 HU. The SMI was calculated as the area of muscle mass divided by the square of the height (m). Low SMI were determined by our previous study, < 40.8 cm2/m2 for males or < 34.9 cm2/m2 for females [19]. Visceral obesity was defined as VFA ≥ 100 cm2 in both males and females [20].
Assessment of malnutrition
Malnutrition was diagnosed using the two-step model, Global Leadership Initiative in Malnutrition (GLIM) [21]. The first step was a Nutritional Risk Screening 2002 (NRS 2002) ≥ 3 to identify the individuals at risk of malnutrition. Second, malnutrition was defined if one of the three phenotypical criteria (non-volitional weight loss, low BMI and reduced muscle mass) was met as the patients in this study had already met the etiological criterion (disease burden) [21]. Non-volitional weight loss was defined as weight loss > 5% within 6 months or > 10% beyond 6 months. A low BMI score was defined when patients aged ≥ 70 years old had a BMI score of < 18.5 kg/m2, or when patients aged < 70 years old had a BMI score of < 20 kg/m2. Reduced muscle mass was defined as low SMI.
Follow-up
Follow-up with patients via telephone was regularly conducted 1 month after surgery, every 3 months for 2 years, and every 6 months thereafter until death, or the end of the study in August 2022, or more than 8 years. Overall survival (OS) was calculated from the date of surgery until death.
Statistical analysis
Regarding continuous variables, the mean and standard deviation (SD) or the median and interquartile range (IQR) were shown. Analysis of variance or Kruskal-Wallis H tests were used to compare continuous variables. Categorical variables were presented as numbers and proportions and Chi-squared or Fisher’s exact tests were used to compare them. Univariate and multivariate logistic regression analyses were performed to evaluate the relationship between the factors and postoperative complications. Univariate and multivariate cox regression analyses were performed to evaluate the risk factors for OS and cancer-specific survival (CSS). Kaplan-Meier survival curves and log-rank tests were performed for the four groups. Multivariate analyses were conducted on factors with P < 0.10 in the univariate analyses. Two-sided P < 0.05 was statistically significant. SPSS 26.0 and R software (version 4.2.1, https://cran.r-project.org) were used.
Results
A total of 624 patients with rectal cancer were enrolled. Based on the diagnostic criteria, 306 (49.0%) and 156 (25.0%) patients were identified as having visceral obesity and malnutrition, respectively. The patients were classified into four groups according to their nutritional and visceral obesity status: 204 (32.7%) in the well-nourished non-visceral obesity (WN) group, 264 (42.3%) in the well-nourished visceral obesity (WO) group, 114 (18.3%) in the malnourished non-visceral obesity (MN) group, and 42 (6.7%) in the malnourished visceral obesity (MO) group.
Table 1 showed the clinical characteristics of the patients with rectal cancer in each group. Among the four groups, there were significant differences in age (P < 0.001), SMI (P < 0.001), VFA (P < 0.001), BMI (P < 0.001), CCI (P = 0.001), and ASA grade (P < 0.001). No significant differences in gender, previous abdominal surgery, tumor size, tumor location, tumor differentiation, tumor stage, node stage, TNM stage, and chemotherapy among the four groups.
Table 2 showed the details of postoperative complications and postoperative hospital stay. The overall postoperative complication rate was 23.2% (n = 145). The postoperative complication rates in the WN, WO, MN, and MO groups were 17.6% (n = 36), 21.2% (n = 56), 28.9% (n = 33), and 47.6% (n = 42), respectively, with a significant difference (P < 0.001). No significant difference in postoperative hospital stay among the four groups (P = 0.194) was noted.
Table 3 showed the univariate and multivariate logistic regression analyses of the factors associated with postoperative complications. The multivariate logistic analysis revealed that CCI (odds ratio [OR]: 2.343; 95% confidence interval [CI]: 1.573–3.491; P < 0.001), MN (OR: 1.952; 95% CI: 1.126–3.383; P = 0.017), and MO (OR: 4.112; 95% CI: 2.007–8.426; P < 0.001) were associated with postoperative complications.
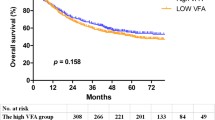
The median follow-up time was 4.95 (3.38–6.72) years. A total of 133 deaths (21.3%) occurred during the follow-up, of which 117 were cancer-specific. Table 4 showed the univariate and multivariate cox regression analyses of the factors associated with OS. In the multivariate cox regression analysis, age (OR: 2.029; 95% CI: 1.358–3.034; P = 0.001), ASA grade (OR: 1.989; 95% CI: 1.350–2.929; P < 0.001), tumor differentiation (OR: 2.075; 95% CI: 1.336–3.223; P = 0.001), TNM stage ([II vs. Tis, I OR: 2.243; 95% CI: 1.222–4.118; P = 0.009] [ III vs. Tis, I, OR: 2.243; 95% CI: 2.310–7.003; P < 0.001]), and MO (OR: 2.615; 95% CI: 1.404–4.871; P = 0.002) were associated with worsened OS. Figure 1. showed the Kaplan-Meier curves for OS according to nutritional and visceral obesity status in patients with rectal cancer.
Table 5 showed the univariate and multivariate cox regression analyses of the factors associated with CSS. In the multivariate cox regression analysis, age (OR: 1.913; 95% CI: 1.253–2.921; P = 0.003), ASA grade (OR: 1.792; 95% CI: 1.176–2.731; P = 0.007), tumor differentiation (OR: 1.938; 95% CI: 1.215–3.090; P = 0.005), TNM stage ([II vs. Tis, I OR: 9.920; 95% CI: 3.030–32.480; P < 0.001] [ III vs. Tis, I, OR: 20.158; 95% CI: 6.356–63.924; P < 0.001]), and MO (OR: 2.627; 95% CI: 1.363–5.063; P = 0.004) were associated with worsened CSS. Figure 2. showed the Kaplan-Meier curves for CSS according to nutritional and visceral obesity status in patients with rectal cancer.
Discussion
To the best of our knowledge, this is the first study to investigate the impact of visceral obesity and GLIM-defined malnutrition on short- and long-term outcomes in patients with rectal cancer. This study demonstrated that patients with both visceral obesity and GLIM-defined malnutrition were more likely to experience postoperative complications, poorer OS and CSS.
GLIM, a universal malnutrition model, was used in this study, in which the prevalence of GLIM-defined malnutrition was 25.0%. GLIM consisted of three phenotypical and two etiological criteria. In our study, cancer diagnosis was considered as the etiological criterion. GLIM-defined malnutrition was diagnosed when at least one of the phenotypic criteria was met. Non-volitional weight loss as a phenotypical criterion, was the most traditional indicator of malnutrition and was present in almost all malnutrition screening tools [21]. Skeletal muscle mass had attracted much attention in recent years. Many articles demonstrated the correlation between muscle mass and survival [19, 22]. When alternative phenotypical criteria were met, malnutrition might also be diagnosed despite the high BMI values when other phenotypical criteria were met. Thus, the GLIM criteria offer a major conceptual advancement in the diagnosis of malnutrition, even in patients with high BMI and adiposity. Different combinations of phenotypical and etiological criteria allowed for a wide range of GLIM applications [21]. Previous articles have pointed out that GLIM-defined malnutrition was a predictor of OS [23, 24]. Therefore, we used GLIM to evaluate the nutritional status.
While BMI was a convenient way to measure body weight, it failed to distinguish body composition, body fat distribution, fluid accumulation, and absolute weight gain among other factors [8, 25]. Therefore, visceral obesity was used in this study and the prevalence of visceral obesity was 50.8%. Many trials had demonstrated that visceral obesity was associated with longer operative time, greater blood loss during surgery, longer postoperative hospital stay, and higher postoperative complication rates after elective colorectal surgery [10, 11]. Furthermore, visceral obesity showed no influence on OS; however, it was significantly associated with disease-free survival in patients with resectable colorectal cancer [12]. However, it had not been verified in this article, which may be caused by our separation of the MO group from total visceral obesity group.
It was no surprise that TNM stage and tumor differentiation correlated with survival. In this study, CCI was a significant predictor of postoperative complication, but showed no influence on OS. Furthermore, ASA index was a significant predictor of OS; however, it showed no influence on postoperative complications. It showed that preoperative comorbidities were related to postoperative complications, while preoperative comorbidities that limited activity were related to OS. A low BMI score was associated with postoperative complications in the univariate analysis, but not in the multivariate analysis because it was one of the indicators for the diagnosis of malnutrition and was excluded as a confounding factor in the multivariate analysis. It had been reported that the association between BMI and colorectal cancer survival is U- or J-shaped [26]. In our study, high BMI was a protective factor in the univariate cox analysis and was excluded in the multivariate cox analysis. In this study, MN group was a protective factor for OS in the univariate cox analysis and was excluded in the multivariate cox analysis. It may be caused by the fact that we cut out the MO group from the malnutrition group.
With the growth of urbanization and industrialization in Asia, foods with low nutritional value were easily available and affordable, and sedentary lifestyles were promoted, contributing to the double burden of obesity and malnutrition [13]. Our study showed that visceral obesity and malnutrition were well evaluated using CT and GLIM. How to intervene in it was also a challenge. Previous studies have indicated that preventing malnutrition should be a part of the obesity medical care plan, which included a lifestyle change, making the right food choices and avoiding unhealthy foods [27]. Moreover, fortified foods such as specific vitamin supplements should be included to prevent shortages. It was not widely accepted that multi-model pre-habilitation should include exercise therapy, nutritional supplementation, and hematologic optimization preoperatively [28]. Further research was needed to explain the factors shaping MO in patients, as well as appropriate treatment strategies.
This study had some limitations that should be considered. Firstly, despite the attempts to minimize the confounding factors, the retrospective nature of our analysis posed a risk of selection bias. Secondly, we are supposed to keep in mind whether our cut-points are appropriate to define low SMI and visceral obesity. Several studies have established definitions for low SMI and visceral obesity according to different criteria [11, 29, 30]. Owing to the lack of a uniform age-specific threshold for CT measured low SMI and visceral obesity, we applied a unified cutoff value for SMI based on our previous study [19] and cutoff values for visceral obesity based on the Japanese research [20]. There still remained the need for definitive criteria of CT measured low SMI and visceral obesity for Asian. Finally, this was a single-center study among Chinese patients with rectal cancer, which may not be applicable to other ethnic populations and regions. In the future, a multicenter prospective study in different populations is required to validate our findings.
Conclusion
In conclusion, this study demonstrated that the combination of visceral obesity and malnutrition resulted in higher postoperative complication and mortality rates and was a good indicator of poor prognosis in patients with rectal cancer. According to our findings, visceral obesity and malnutrition had a double burden. Therefore, health lifestyle programs and policies are required to reduce visceral obesity and promote nutritional health.
Data availability
The datasets generated during and analyzed during the current study are not publicly available, because they contain information that could compromise the privacy of research participants, but they are available from the corresponding author on reasonable request.
References
Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr. 2018;72:1255–9.
Lee DU, Fan GH, Hastie DJ, Addonizio EA, Suh J, Prakasam VN, et al. The clinical impact of malnutrition on the postoperative outcomes of patients undergoing colorectal resection surgery for colon or rectal cancer: propensity score matched analysis of 2011–2017 US hospitals. Surg Oncol. 2021;38:101587.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021;40:2898–913.
Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37:1787–93.
Samocha-Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, et al. Metabolically healthy and unhealthy obese–the 2013 stock Conference report. Obes Rev. 2014;15:697–708.
Watanabe J, Tatsumi K, Ota M, Suwa Y, Suzuki S, Watanabe A, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. Int J Colorectal Dis. 2014;29:343–51.
Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity Paradox: the Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26:1008–15.
Aquina CT, Rickles AS, Probst CP, Kelly KN, Deeb AP, Monson JR, et al. Visceral obesity, not elevated BMI, is strongly associated with incisional hernia after colorectal surgery. Dis Colon Rectum. 2015;58:220–7.
Park BK, Park JW, Ryoo SB, Jeong SY, Park KJ, Park JG. Effect of visceral obesity on Surgical Outcomes of patients undergoing laparoscopic colorectal surgery. World J Surg. 2015;39:2343–53.
Chen WZ, Chen XD, Ma LL, Zhang FM, Lin J, Zhuang CL, et al. Impact of visceral obesity and Sarcopenia on short-term outcomes after colorectal Cancer surgery. Dig Dis Sci. 2018;63:1620–30.
Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 2008;15:1918–22.
Chien SC, Chandramouli C, Lo CI, Lin CF, Sung KT, Huang WH, et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in asian adults: a cohort study. PLoS Med. 2021;18:e1003661.
Huang DD, Wu GF, Luo X, Song HN, Wang WB, Liu NX, et al. Value of muscle quality, strength and gait speed in supporting the predictive power of GLIM-defined malnutrition for postoperative outcomes in overweight patients with gastric cancer. Clin Nutr. 2021;40:4201–8.
van Vliet IMY, Gomes-Neto AW, de Jong MFC, Bakker SJL, Jager-Wittenaar H, Navis GJ. Malnutrition screening on hospital admission: impact of overweight and obesity on comparative performance of MUST and PG-SGA SF. Eur J Clin Nutr. 2021;75:1398–406.
Van de Louw A, Zhu X, Frankenfield D. Obesity and malnutrition in critically ill patients with acute myeloid leukemia: prevalence and impact on mortality. Nutrition 2020;79–80:110956.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term Survival after Radical Gastrectomy for gastric Cancer: analysis from a large-scale cohort. Med (Baltim). 2016;95:e3164.
Examination Committee of Criteria for. Obesity disease’ in J and Japan Society for the study of O. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–92.
Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207–17.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Zhang Q, Zhang KP, Zhang X, Tang M, Song CH, Cong MH, et al. Scored-GLIM as an effective tool to assess nutrition status and predict survival in patients with cancer. Clin Nutr. 2021;40:4225–33.
Song HN, Wang WB, Luo X, Huang DD, Ruan XJ, Xing CG, et al. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn J Clin Oncol. 2022;52:466–74.
Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391–406.
Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body Mass Index and Mortality in patients with Colorectal Cancer using Causal Diagrams. JAMA Oncol. 2016;2:1137–45.
Kobylinska M, Antosik K, Decyk A, Kurowska K. Malnutrition in obesity: is it possible? Obes Facts. 2022;15:19–25.
Bolshinsky V, Li MH, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal Prehabilitation Programs as a bundle of care in gastrointestinal Cancer surgery: a systematic review. Dis Colon Rectum. 2018;61:124–38.
Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2019;269:924–31.
Feng H, Wang X, Zhao T, Mao L, Hui Y, Fan X, et al. Myopenic obesity determined by visceral fat area strongly predicts long-term mortality in cirrhosis. Clin Nutr. 2021;40:1983–9.
Acknowledgements
We would like to thank the Editage (https://app.editage.com/) for its excellent polishing work.
Funding
This study was funded by the National Natural Science Foundation of China (grant numbers 82274530).
Author information
Authors and Affiliations
Contributions
Chong-Jun Zhou wrote the manuscript; Xi-Yi Chen and Chen-Guo Zheng contributed to the design of the research; Yi Lin and Jie-Yu Liu contributed to the interpretation of the data; Chong-Jun Zhou and Zhong-Lin Wang contributed to the acquisition and analysis of the data. Chong-Jun Zhou, Xi-Yi Chen and Chen-Guo Zheng drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The data collection protocol for this study was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (LCKY2020–209) and complied with the Declaration of Helsinki. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, CJ., Lin, Y., Liu, JY. et al. Malnutrition and visceral obesity predicted adverse short-term and long-term outcomes in patients undergoing proctectomy for rectal cancer. BMC Cancer 23, 576 (2023). https://doi.org/10.1186/s12885-023-11083-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11083-y