Abstract
Purpose
An assessment is being conducted to determine the safety and effectiveness of using Cone-beam computed tomography (CBCT)-guided transcatheter arterial chemoembolization (TACE) and microwave ablation (MWA) sequentially to treat small hepatocellular carcinomas (HCCs) located in the hepatic dome.
Materials and methods
Fifty-three patients with small HCCs in the hepatic dome who underwent TACE combined with simultaneous CBCT-guided MWA were studied. Inclusion criteria were a single HCCs ≤ 5.0 cm or a maximum of three. The safety and interventional-related complications were monitored, and local tumor progression (LTP), overall survival (OS), and prognostic factors for LTP/OS were evaluated.
Results
The procedures were successfully accomplished in all patients. According to Common Terminology Criteria for Adverse Events (CTCAE), adverse reactions and complications are mainly Grade 1 or 2 (mild symptoms, no or local/noninvasive intervention indicated). Liver and kidney function and alpha-fetoprotein (AFP) levels remained within a reasonable range after 4 weeks of treatment (both p < 0.001). The mean LTP was 44.406 months (95% CI: 39.429, 49.383) and the mean OS rate was 55.157 months (95% CI: 52.559, 57.754). The combination treatment achieved 1-, 3-, and 5-year LTP rates of 92.5%, 69.6%, and 34.5%, respectively; and 1-, 3-, and 5-year OS rates of 100.0%, 88.4%, and 70.2%, respectively. Results from both univariate and multivariate Cox regression analyses showed that the tumor diameter (< 3 cm) and the distance to the hepatic dome (≥ 5 mm, < 10 mm) had a significant impact on the patient’s LTP and OS, and were related to better survival.
Conclusion
CBCT-guided TACE combined with simultaneous MWA was a safe and successful treatment of HCCs located under the hepatic dome.
Similar content being viewed by others
Introduction
Hepatocellular Carcinoma (HCC) is a leading cause of cancer-related deaths worldwide, with a higher prevalence among elderly patients as life expectancy increases [1, 2]. The management of older HCC patients with other comorbidities will increasingly become a global issue. Data from the aging Chinese population showed that over a quarter of HCC patients and HCC-related deaths were over 70 years old [3]. Although surgical resection is still the first-line treatment for patients with small HCC, elderly patients are often deemed a high-risk group for this due to the presence of additional underlying diseases [4, 5]. Those elderly patients who undergo surgical resection tend to experience longer hospital stays, higher complication rates, and poorer overall survival (OS) than younger patients [6, 7]. Therefore, transcatheter arterial chemoembolization (TACE) combined with microwave ablation (MWA) may be a more suitable alternative for elderly patients with small HCC.
Ultrasound (US) and computed tomography (CT) have traditionally been the main image-guided modes used to perform percutaneous ablation in the treatment of HCC [8, 9]. However, due to the interference of the gas at the bottom of the lung and acoustic shadowing from the ribs, the HCC in the hepatic dome has been a blind spot for ultrasound-guided ablation [10]. Additionally, unenhanced US and CT often yield poor visualization of smaller HCC with cirrhosis in the hepatic dome. Cone-beam computed tomography (CBCT) offers multi-plane functionality, rapid image reconstruction, and superior tissue resolution, making it possible to integrate TACE and ablation processes in a single interventional procedure. After TACE, tumors can be more easily identified by CBCT due to the deposition of iodized oil. CBCT thus allows for TACE and MWA to be completed in one interventional procedure, greatly improving efficiency and reducing the duration of the interval [11, 12]. In this study, we completed TACE sequential MWA treatments on 53 small HCC patients abutting the hepatic dome.
Materials and methods
Patients
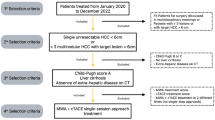
Patients with HCC included in this study were consistent with the diagnostic criteria of the American Association for the Study of Liver Diseases or European Association for the Study of the Liver criteria [5]. Given the retrospective nature of this project, our Institutional Review Board approved the study and waived the patient’s informed consent requirement. In this retrospective study, we included 53 patients (65.6 ± 8.9 years; range 47–79 years) who received TACE sequential MWA guided by CBCT in the treatment of small HCC under the hepatic dome. The patient characteristics are shown in (Table 1). The inclusion and exclusion criteria are listed in (Table 2).
Procedure
TACE treatment
Two experienced interventional radiologists (with more than 10 years of experience each) performed all TACE procedures. First, a 5-Fr catheter was used for hepatic artery angiography to identify the tumor and its feeder(s). Subsequently, a 2.0 F microcatheter (Progreat, Terumo Corporation, Tokyo, Japan) was used for superselective catheterization of the feeding artery. Pirarubicin (THP; 60–80 mg; Shenzhen Meirui Pharmaceutical Co., Ltd. China) and iodized oil (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) were administered at an average dose of 10–20 mg (average 14.3 mg) and 4–6 ml (average 5.1 ml), respectively. Finally, microspheres (100–300 μm; Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) were used for complete embolization of the artery supplying the tumor after lipiodol was evenly deposited in the tumor (Fig. 1).
Patients with HCC with a diameter of 3 mm adjacent to the diaphragm; A. The tumor first completes the transcatheter arterial chemoembolization under the guidance of CBCT;B. HCC is marked by iodized oil; C. preoperative puncture route planning under the guidance of CBCT; D-F. The patient completes the MWA process immediately after TACE treatment
MWA treatment
All patients receiving TACE and ablation procedures were instructed to perform breathing exercises prior to treatment. Specifically, under CBCT-guided local anesthesia (Syngo-DynaCT; Siemens AG, Germany), a microwave antenna (ECO-100AI10, ECO Microwave System Co, Nanjing, China) was percutaneously inserted for tumor ablation. The patients were asked to hold their breath for 10 s, and the data collection was performed at 200° rotation and 0.37 µGy/frame X-ray dose to complete the volumetric reconstruction. iGuide VNS (Siemens puncture navigation software) was used to plan the puncture path, to align the skin entry point and the target tumor site for real-time perspective presentation, and to puncture according to the path; the power and duration of ablation were determined by the physician based on the quality of the surrounding hepatic tissue, lesion depth, and demarcation line length; usually, the tumor was ablated at 40.6 ± 0.9.7wt for 7.4 ± 2.5 min. Then, the pre- and post-ablation CT scans were superimposed to evaluate the ablation zone and any direct complications.
Definitions and evaluation of data
The study’s primary outcome measures were overall survival (OS), Local Tumor Progression (LTP), and radiological response. OS was defined as the period between initial treatment and death from any cause. LTP was considered present if nodular enhancement was detected in the ablation area on follow-up imaging. The radiological response was evaluated using the modified response evaluation criteria in solid tumors (mRECIST; 2020 edition [13]) 4 weeks after MWA.
Follow-up
One month post-MWA, laboratory tests such as alpha-fetoprotein (AFP) and liver function tests, as well as imaging studies including enhanced CT or enhanced MR were performed. Patients were followed up at 3-month intervals to monitor for any signs of recurrence or residuals. Using the 2020 edition of mRECIST, treatment progress was evaluated. In the event that complete response (CR) was not attained, additional treatments were carried out until CR was achieved according to the physician’s discretion.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as proportions (%), and continuous variables presented as mean ± standard deviation (SD). Survival analysis was based on Kaplan–Meier curves. Univariate and multivariate Cox proportional hazards regression models were used to analyze prognostic factors for LTP and OS. A P-value of < 0.05 was considered statistically significant.
Results
Patient characteristics
The mean age of the patients was 65.6 ± 8.9 years (range, 47–79 years). Of the 53 patients, 17 (32.1%) were 65years old or younger, 23 (43.3%) were male, and 34 (64.1%) of the small HCC patients were associated with hepatitis B. In all of the patients, the mean target tumor size was 3.6 ± 0.9 (range, 2.6–4.9) cm, and 41 (77.4%) had tumors < 3 cm in diameter. Furthermore, 15 patients had tumors within 5 mm of the hepatic dome, and the remaining 38 patients were beyond 5 mm. Additionally, 35 (66.0%) patients were considered to be Child–Pugh A, while 18 (34.0%) were considered to be Child–Pugh B. The mean energy, ablation duration per tumor and the mean safety margin were 40.6 ± 0.9.7 kJ and 7.4 ± 2.5 min, respectively.
Safety
All patients underwent liver/kidney laboratory tests and alpha-fetoprotein (AFP) determination over the course of the first and fourth weeks post-procedure. The mean blood urea nitrogen (BUN) stayed within its normal range for the duration of 4 weeks after treatment. However, their mean total bilirubin (TBIL; P = 0.001) level saw a slight increase within the first week after the procedure, but was brought back to its normal levels by the fourth week. The mean albumin (ALB) was also largely back in its normal range after the fourth week. Additionally, combined treatment yielded a rapid decline in AFP levels (P<0.001), and was kept within an acceptable range after 4 weeks (Fig. 2).
Retrospective analysis of 53 patients with TACE combined with simultaneous CBCT-guided MWA in HCC with before (M0) and after 1 week(1 W) and 4 weeks (4 W) of laboratory test results. The black dotted line indicates the normal range of laboratory inspection indicators. (A) Mean TBIL increased significantly at 1 W and returned to normal at 4 W; (B) Mean ALB increased slightly after 4 W of treatment, and most patients were in the normal range; (C) Mean BUN still remained normal at 1 and 4 W; (D) Mean AFP level decreased rapidly after treatment, and almost returned to normal level after 4 W(P<0.001); ALB, albumin; BUN, blood urea nitrogen; PT, prothrombin time; TBIL, total bilirubin; AFP ,Alpha-fetoprotein; TACE, transarterial chemoembolization; CBCT, cone-beam computed tomography; HCC, hepatocellular carcinoma; MWA, microwave ablation
Interventional-related complications
Most adverse events and complications were CTCAE grade 1 or 2 (mild symptoms, no or local/noninvasive intervention indicated), or interventional radiology society Grade A or B (no or nominal treatment, no consequences). Exceptions included six patients (11.3%) who had localized atelectasis of the lung parenchyma caused by localized thermal injury, three patients (5.7%) with perihepatic effusion requiring thoracic drainage, and one patient (1.9%) with pneumothorax who needed closed thoracic drainage and returned to stable within 3 days after treatment. None of the patients experienced life-threatening complications during or after treatment (Table 3).
LTP and OS
The survival analysis of CBCT-guided TACE sequential MWA for the treatment of small HCCs under the hepatic dome revealed a mean LTP of 44.406 months (95% CI: 39.429, 49.383) and mean OS of 55.157 months (95% CI: 52.559, 57.754) in the combination therapy. The 1-, 3- and 5-year LTP rates of the combination treatment were 92.5%, 69.6% and 34.5%, respectively (Fig. 3A); the 1-, 3- and 5-year OS rates were 100.0%, 88.4% and 70.2%, respectively (Fig. 3B). Univariate Cox proportional hazard regression indicated that Child-Pugh (A vs. B), liver cirrhosis (YES vs. NO) and the number of lesions (single vs. 2–3 lesions) were not associated with longer LTP and OS (both P > 0.05). Additionally, both univariate and multivariate Cox regression revealed that the tumor diameter (< 3 cm) and the distance to hepatic dome (⩾10 mm, <5 mm) did have a significant impact on the patient’s LTP and OS and were related to better survival (Table 4).
Mean Local tumor progression (LTP) was 44.406 months (95% CI: 39.429, 49.383) and mean overall survival (OS) rates was 55.157 months (95% CI: 52.559, 57.754) in the combination therapy; A; The 1-, 3-, and 5-year LTP rates of combination group were 92.5%, 69.6% and 34.5%, respectively; B. The 1-, 3- and 5-year OS rates were 100.0%, 88.4% and 70.2%, respectively; LTP, Local tumor progression; OS, overall survival;
Tumor diameter and the distance to hepatic dome
The mean LTP for procedures with tumor diameter < 3 cm was 50.622 months (95% CI: 46.183, 55.060), compared to 23.367 months (95% CI: 15.116, 31.617) for those with tumor diameter ≥ 3 cm, <5 cm (p = 0.000, log-rank test). The 1-, 3- and 5-year LTP-free survival rates for patients with tumor diameter < 3 cm were 95.1%, 85.3% and 42.2%, respectively, and those with tumor diameter ≥ 3 cm, <5 cm were 83.3%, 8.3% and 8.3%, respectively (Fig. 4A). The mean OS was 58.342 months (95% CI: 56.732, 59.952) for those with tumor diameter < 3 cm, and 44.650 months (95% CI: 37.492, 51.808) for those with tumor diameter ≥ 3 cm, <5 cm (p = 0.000, log-rank test). The 1-, 3- and 5-year OS rates for patients with tumor diameter < 3 cm were 100.0%, 97.5% and 81.3%, respectively, and those for tumor diameter ≥ 3 cm, <5 cm were 100.0%, 58.3% and 33.3%, respectively (Fig. 4B). Regarding procedures with HCC distance to hepatic dome < 5 mm, the mean LTP was 19.360 months (95% CI: 13.719, 57.263); for those with distance ≥ 10 mm, <5 mm, it was 54.350 months (95% CI: 51.437, 57.263) (p = 0.000, log-rank test). The 1-, 3- and 5-year LTP-free survival rates for patients with HCC distance to hepatic dome < 1 mm were 73.3%, 6.7% and 0.0%, respectively; for those with distance ≥ 10 mm, <5 mm they were 100.0%, 91.9% and 48.3%, respectively (Fig. 5A). The mean OS for procedures with HCC distance to hepatic dome < 5 mm was 44.962 months (95% CI: 38.906, 51.019), compared to 59.339 months (95% CI: 58.314, 60.365) for those with distance ≥ 10 mm, <5 mm (p = 0.000, log-rank test). The 1-, 3- and 5-year OS rates for patients with HCC distance to hepatic dome < 5 mm were 100.0%, 60.0% and 17.8%, respectively, and those for distance ≥ 10 mm, <5 mm were 100.0%, 100.0% and 91.3%, respectively (Fig. 5B).
A. Comparison of LTP between tumor diameter<3 cm and tumor diameter ≥ 3 cm,<5 cm after TACE sequential MWA treatment. The mean LTP was 50.622 months (95% CI: 46.183, 55.060) for procedures with tumor diameter<3 cm versus 23.367 months (95% CI: 15.116, 31.617) for procedures with tumor diameter ≥ 3 cm,<5 cm (p = 0.000, log-rank test). The 1-, 3-, and 5-year LTP-free survival rates in patients with tumor diameter<3 cm were 95.1%, 85.3% and 42.2%, respectively, and the 1-, 3- and 5-year LTP-free survival rates in patients with tumor diameter ≥ 3 cm,<5 cm were 83.3%, 8.3% and 8.3%, respectively; B. Comparison of OS between tumor diameter<3 cm and tumor diameter ≥ 3 cm,<5 cm after TACE sequential MWA treatment. The mean OS was 58.342 months (95% CI: 56.732, 59.952) for procedures with tumor diameter<3 cm versus 44.650 months (95% CI: 37.492, 51.808) for procedures with tumor diameter ≥ 3 cm,<5 cm (p = 0.000, log-rank test). The 1-, 3-, and 5-year OS rates in patients with tumor diameter<3 cm were 100.0%, 97.5% and 81.3%, respectively, and the 1-, 3- and 5-year OS rates in patients with tumor diameter ≥ 3 cm,<5 cm were 100.0%, 58.3% and 33.3%, respectively
A. Comparison of LTP between HCC distance to hepatic dome<5 mm and distance ≥ 5 mm,<10 mm after TACE sequential MWA. The mean LTP was 19.360 months (95% CI: 13.719, 57.263) for procedures with HCC distance to hepatic dome <5 mm versus 54.350 months (95% CI: 51.437, 57.263) for procedures with distance ≥ 5 mm,<10 mm (p = 0.000, log-rank test). The 1-, 3-, and 5-year LTP-free survival rates in patients with HCC distance to hepatic dome<5 mm were 73.3%, 6.7% and 0.0%, respectively, and the 1-, 3- and 5-year LTP-free survival rates in patients with distance ≥ 5 mm,<10 mm were 100.0%,91.9% and 48.3%, respectively; B. Comparison of OS between HCC distance to hepatic dome<5 mm and distance ≥ 5 mm,<10 mm after TACE sequential MWA. The mean OS was 44.962 months (95% CI: 38.906, 51.019) for procedures with HCC distance to hepatic dome <5 mm versus 59.339 months (95% CI: 58.314, 60.365) for procedures with distance ≥ 5 mm,<10 mm (p = 0.000, log-rank test). The 1-, 3-, and 5-year OS rates in patients with HCC distance to hepatic dome<5 mm were 100.0%, 60.0% and 17.8%, respectively, and the 1-, 3- and 5-year OS rates in patients with distance ≥ 5 mm,<10 mm were 100.0%, 100.0% and 91.3%, respectively
Discussion
With the increased life expectancy in many countries, the conventional management model for HCC is not appropriate for elderly patients [14]. While surgical resection is considered a reasonable first-line treatment for small HCC, the long-term benefits of radical resection for elderly patients remain unclear due to the impacts of compromised liver function or regenerative capacity, portal hypertension, tumor location, and comorbidities [15,16,17,18]. For small HCC, TACE combine thermal ablation are usually not recommended as a single ablation is equally effective [19]. However, for tumor located close to the diaphragm, it is difficult to determine the puncture path under conventional ultrasound and CT equipment [20]. The 3-year LTP for small HCC near the diaphragm treated with ablation alone is as high as 62%, whereas TACE combined with thermal ablation for small HCC near the diaphragm has shown promising therapeutic effects, with a 5-year LTP rate of only 3% [21,22,23,24]. Therefore, most centers adopt a combination of TACE and thermal ablation as the generally accepted resection alternative for small HCC patients near the diaphragm.
Radiofrequency ablation (RFA) and MWA are commonly employed thermal ablation techniques for hepatic malignancies. In comparison to RFA, MWA has similar benefits such as larger volume of necrosis, shorter procedure time, and quicker attainment of higher temperatures, and is less affected by heat-sink effects from adjacent vasculature [25, 26]. A propensity score analysis of MWA and RFA for the treatment of perivascular HCC demonstrated similar disease control rates in both groups (94% vs. 91%, p = 0.492). Moreover, MWA had better control of tumor progression for periportal HCC or single-nodule perivascular HCC patients compared to RFA [27]. In a meta-analysis of MWA and RFA for HCC showed no difference in terms of complete response (risk ratio (RR) 1.01, 95% CI 0.99–1.02). The local recurrence rate was similar between MWA and RFA, but MWA had significantly lower distant recurrence rate (RR 0.60, 0.39–0.92) [28]. Moreover, the study of TACE combined with either RFA or MWA for the treatment of HCC indicated that TACE + MWA (TM) group had better overall survival (hazard ratio [HR]: 1.55; 95% confidence interval [CI]: 1.09–2.21, p = 0.01) and higher rate of complete response (RR: 0.87; 95% CI: 0.79–0.96, p = 0.003) than TACE + RFA (TR) group. The advantage of TM was greater for those with tumor diameter less than 3 cm [29].
Despite its widespread acceptance in clinical centers, conventional computed tomography (cCT)-guided MWA is limited in its ability to accurately delineate the precise location of tumors and the boundaries of ablation lesions [31]. Multiple contrast agent administrations can further increase the burden on the kidneys. As an alternative, CBCT-guided TACE sequential MWA is a reliable treatment option [32]. The first TACE procedure facilitates the deposition of iodine-containing oil inside the tumor and the utilization of CBCT-mounted flat detector technology for improved spatial resolution to acquire richer CT information and real-time fluorescence imaging for the guidewire to realign the puncture angle, direction and depth in accordance with the precise location of the lesion. This approach allows for the completion of two treatments in a single procedure without transferring the patient to the CT room, thereby reducing the interval between the two treatments and minimizing the patient’s risk. In this retrospective research, satisfactory results were obtained from CBCT-guided TACE sequential MWA treatment of small HCC in the hepatic dome. The mean LTP was 44.406 months (95% CI: 39.429, 49.383) and the mean OS was 55.157 months (95% CI: 52.559, 57.754). The LTP rate was 92.5%, 69.6%, and 34.5% at 1, 3, and 5 years, respectively, while the OS rate was 100.0%, 88.4%, and 70.2% at 1, 3, and 5 years, respectively.
Although CBCT imaging can provide high-quality spatial resolution, poor density resolution is a major problem, which can make it difficult to accurately visualize the extent of tumor ablation during treatment. To address these issues, we primarily utilize the following methods to evaluate the degree of tumor ablation: (1) Using CBCT perfusion imaging after MWA, complete ablation was indicated when there was no abnormal staining around the lesion. (2) Adjusting the window width range to 120–350 HU and the window level range to 25–45 HU for visual observation after ablation. (3) Immediately pre- and post-ablation CT scans were superimposed to evaluate the ablation zone and any direct complications.
However, our study presents several limitations that should be taken into consideration. Firstly, the study is retrospective and the small sample size increases the possibility of bias. Secondly, CBCT imaging has its own challenges, such as the density of iodinated oil causing artifacts, as well as breath holding and immobility being necessary for successful image reconstruction. Moreover, only tumors with well-defined borders were selected as target lesions, disregarding those surrounded by streak artifacts from catheters or located in truncated segments of the liver, which may affect the accuracy of the results. Nevertheless, we have ensured the effectiveness of the study conclusions by strict inclusion and exclusion criteria, rational statistical methods, and high-quality follow-up data. Thus, to provide better treatment decisions for small hepatocellular carcinoma located in the hepatic dome.
Conclusion
In summary, CBCT-guided TACE sequential MWA treatment of small HCCs under the hepatic dome has demonstrated to be of clinical value. Furthermore, CBCT can be used to guide accurate puncture, which would assist in the decision-making process for interventional procedures and improve the safety of the treatment by minimizing associated risks.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Villanueva A, Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–62.
Toh MR, Wong E, Wong SH, Ng A, Loo LH, Chow PK, Ngeow J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164(5):766–82.
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray C, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. LANCET 2019, 394(10204): 1145–1158.
Kim JM, Cho BI, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, Paik SW, Park CK. Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. AM J SURG. 2015;209(2):391–7.
Cammarota A, D’Alessio A, Pressiani T, Rimassa L, Personeni N. Systemic treatment for older patients with Unresectable Hepatocellular Carcinoma. Drugs Aging. 2021;38(7):579–91.
Galun D, Bogdanovic A, Zivanovic M, Zuvela M. Short- and long-term outcomes after Hepatectomy in Elderly patients with Hepatocellular Carcinoma: an analysis of 229 cases from a developing country. J Hepatocell Carcinoma. 2021;8:155–65.
Kishida N, Hibi T, Itano O, Okabayashi K, Shinoda M, Kitago M, Abe Y, Yagi H, Kitagawa Y. Validation of hepatectomy for elderly patients with hepatocellular carcinoma. ANN SURG ONCOL. 2015;22(9):3094–101.
Lencioni R, de Baere T, Martin RC, Nutting CW, Narayanan G. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of Novel Thermal and Non-Thermal Technologies - A Western Perspective. Liver Cancer. 2015;4(4):208–14.
Meng Y, Jiang B, Yan K, Wang S, Zhang Z, Chen L, Wu W, Yang W. Evaluation of the safety and efficacy of ultrasound-guided percutaneous radiofrequency ablation for hepatocellular carcinoma and liver metastases adjacent to the gallbladder. Int J Hyperthermia. 2023;40(1):2182748.
Takai TR, Okano A, Yamakawa G, Mizukoshi K, Obayashi H, Ohana M. Impact of an ultrasound-guided radiofrequency ablation training program on the outcomes in patients with hepatocellular carcinoma. Diagn Interv Imaging. 2019;100(12):771–80.
Li Z, Jiao D, Han X, Si G, Li Y, Liu J, Xu Y, Zheng B, Zhang X. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided microwave ablation in the treatment of small hepatocellular carcinoma. Cancer Imaging. 2020;20(1):13.
Li Z, Hao D, Jiao D, Zhang W, Han X. Transcatheter arterial chemoembolization combined with simultaneous cone-beam computed tomography-guided microwave ablation in the treatment of small Hepatocellular Carcinoma: clinical Experiences from 50 procedures. ACAD RADIOL. 2021;28(Suppl 1):64–S70.
Winters AC, Viramontes M, Buch A, Najarian L, Yum J, Yang L, Saab S. Older patients with Hepatocellular Carcinoma are less knowledgeable about Survivorship Issues: outcomes from a Survey-based study. J CLIN GASTROENTEROL. 2021;55(1):88–92.
Rho SY, Lee HW, Kim DY, Kim KS. Current status of therapeutic choice and feasibility for patients with Hepatocellular Carcinoma aged >/= 70 years: a Nationwide Cancer Registry Analysis. J Hepatocell Carcinoma. 2021;8:321–32.
Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small Hepatocellular Carcinoma: systematic review of Randomized controlled trials with Meta-analysis and Trial Sequential Analysis. Radiology. 2018;287(2):461–72.
Shapey IM, Malik HZ, de Liguori CN. Data driven decision-making for older patients with hepatocellular carcinoma. Eur J Surg Oncol. 2021;47(3 Pt A):576–82.
Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? RADIOLOGY 2009, 252(3): 905–13.
Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, Kwon WK. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol. 2009;82(983):908–15.
Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. KOREAN J RADIOL. 2009;10(1):34–42.
Head HW, Dodd GR, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243(3):877–84.
Yamakado K, Nakatsuka A, Takaki H, Sakurai H, Isaji S, Yamamoto N, Shiraki K, Takeda K. Subphrenic versus nonsubphrenic hepatocellular carcinoma: combined therapy with chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2010;194(2):530–5.
Ding H, Su M, Zhu C, Wang L, Zheng Q, Wan Y. CT-guided versus laparoscopic radiofrequency ablation in recurrent small hepatocellular carcinoma against the diaphragmatic dome. Sci Rep. 2017;7:44583.
Barrow B, Martin IR. Microwave ablation for hepatic malignancies: a systematic review of the technology and differences in devices. SURG ENDOSC. 2023;37(2):817–34.
Zou YW, Ren ZG, Sun Y, Liu ZG, Hu XB, Wang HY, Yu ZJ. The latest research progress on minimally invasive treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2023;22(1):54–63.
Feng Y, Wang L, Lv H, Shi T, Xu C, Zheng H, Qi J, Zhao X, Li J, Gao Y, Qin C, Zhu Q. Microwave ablation versus radiofrequency ablation for perivascular hepatocellular carcinoma: a propensity score analysis. HPB (Oxford). 2021;23(4):512–9.
Facciorusso A, Abd EAM, Tartaglia N, Ramai D, Mohan BP, Cotsoglou C, Pusceddu S, Giacomelli L, Ambrosi A, Sacco R. Microwave ablation Versus Radiofrequency ablation for treatment of Hepatocellular Carcinoma: a Meta-analysis of Randomized controlled trials. Cancers (Basel) 2020, 12(12).
Zhao J, Wu J, He M, Cao M, Lei J, Luo H, Yi F, Ding J, Wei Y, Zhang W. Comparison of transcatheter arterial chemoembolization combined with radiofrequency ablation or microwave ablation for the treatment of unresectable hepatocellular carcinoma: a systemic review and meta-analysis. Int J Hyperthermia. 2020;37(1):624–33.
Duan L, Zhang BH, Liao JX, Zheng XH. Iodized oil CT-guided radiofrequency ablation for hepatocellular carcinoma within Milan criteria. HEPATOL INT. 2022;16(1):207–8.
Liu Y, Wu K, Xu K, Tian C, Jiao D, Han X. Cone beam computed tomography-guided microwave ablation for hepatocellular carcinoma under the hepatic dome: a retrospective case-control study. Quant Imaging Med Surg. 2022;12(10):4837–51.
Acknowledgements
We would like to thank the patients and all employees of the First Affiliated Hospital of Zhengzhou University Department of Interventional Radiology. We would also like to thank Ms. Chen Xinyue from CT collaboration NE Asia, Siemens Healthcare, Beijing, China, who provided extensive technical support as well as language editing for this article.Thank you, Mr. Zhi Yongxiang, for your assistance.
Funding
This work was supported by the Science and Technology Project of Henan Province [grant number: 172102310388], the Key Scientific Research Projects of Higher Education Institutions in Henan Province [grant number: 20A320024] and Provincial and Ministerial Youth Projects, Henan Medical Science and Technology Public Relations Program 2019 [grant number: SB201902014].
Author information
Authors and Affiliations
Contributions
Zhaonan Li have made substantial contributions to the conception and design of the work,and the acquisition, analysis of data, as well as manuscript writing. Kaihao Xu have made contributions to the design of the work. Xueliang Zhou have made contributions to the acquisition, analysis of data. Dechao Jiao have made contributions to analysis, interpretation of data,and manuscript writing. Xinwei Han have made contributions to the data collection and image analysis. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. Xinwei Han is corresponding author,and responsible for ensuring that all listed authors have approved the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The medical ethics committee of our college (The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province) approved the retrospective study and gave up the written informed consent. During follow-up, we informed patients about the study and they agreed to use their data. We confirm that all methods were performed in accordance with the relevant guidelines and Declaration of Helsinki.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Xu, K., Zhou, X. et al. TACE sequential MWA guided by cone-beam computed tomography in the treatment of small hepatocellular carcinoma under the hepatic dome. BMC Cancer 23, 600 (2023). https://doi.org/10.1186/s12885-023-11066-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11066-z