Abstract
Purpose
Microwave ablation (MWA) and conventional transarterial chemoembolization (cTACE) are locoregional treatments commonly performed in very early, early and intermediate stages of hepatocellular carcinoma (HCC). Despite combined locoregional approaches have shown encouraging results in obtaining complete tumor necrosis, their application in a single session is poorly described.
Our aim was to evaluate the safety and efficacy of single-session MWA and cTACE treatment in 5-cm HCCs and its influence on liver function.
Materials and methods
All 5-cm HCCs treated by MWA and cTACE performed in a single-session in our Interventional Radiology unit between January 2020 and December 2022 were retrospectively recorded and analyzed. Patients with poor or missing pre- and post-treatment imaging were excluded. Technical success, clinical success, and complications rate were examined as primary endpoints. Pre- and post-treatment liver function laboratory parameters were also evaluated.
Results
A total of 15 lesions (mean lesion diameter, 5.0 ± 1.4 cm) in 15 patients (11 men; mean age, 67.1 ± 8.9 years) were retrospectively evaluated. Technical and clinical success were 100% and 73%, respectively. Four (27%) cases of partial response and no cases of progressive or stable disease were recorded. AST and ALT values have found to be significantly higher in post-treatment laboratory tests. No other significant differences between pre- and post-treatment laboratory values were registered. AST and ALT pre- and post-treatment higher differences (ΔAST and ΔALT) were significantly associated with a lower clinical success rate.
Conclusion
MWA and cTACE single-session approach is safe and effective for 5-cm HCCs, without significant liver function impairment. A post-treatment increase in AST and ALT values may be a predictor for clinical failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and a leading cause of cancer-related death [1, 2]. Although cirrhosis is a well-known principal risk factor for HCC, several minor co-factors with synergic effect exist [3]. At-risk patients are closely monitored with abdominal ultrasound, alpha-fetoprotein (AFP) biomarker and liver function laboratory tests, whereas CT and MRI are not performed routinely [4,5,6,7,8]. Over the past decade, HCC incidence and mortality have remained stable due to the active surveillance programs, the multidisciplinary approach and the improvements of locoregional and systemic treatments [4, 9,10,11].
Locoregional minimally invasive procedures, such as microwave ablation (MWA) and transarterial chemoembolization (TACE), are, respectively, indicated for very early/early stage HCC and unresectable intermediate-stage HCC, according to Barcelona Clinic Liver Cancer staging [12, 13]. In addition, multimodal and combined locoregional approaches have been developed with the aim of reaching the best match for every clinical scenario [14,15,16,17,18,19,20]. The combination of TACE with ablative therapies has been proven to increase treatment efficacy, mostly preventing incomplete peripheral tumor necrosis and especially in largest tumors sized 3–7 cm, without adjuntive risks [18,19,20].
The rationale of the combined application of these two strategies stems from the variable responses of large HCCs to TACE, due to the development of hypoxia and consequent angiogenesis, often hesitating in tumor progression or recurrence. This phenomenon could be mitigated by MWA, usually not performed in large, intermediate-stage HCCs but efficiently applicable in viable tumors after TACE [21].
Despite the known advantages of combined (early or late) sequential MWA and TACE, its application in single session has been poorly described in literature due to the assumption that a shorter time interval between the MWA and TACE deteriorates patient liver function [21, 22]. Our study aims to evaluate safety, technical and clinical success, and the effect on liver function of single-step MWA and conventional TACE (cTACE) combined procedures.
Materials and methods
Study design and patient population
The study was approved by the Internal Review Board (IRB) and was conducted in conformity to the ethics guidelines of the 1990 Declaration of Helsinki and its amendments. All patients provided an informed written consent to the procedures.
This single-center retrospective study analyzed all the consecutive patients referred to our Interventional Radiology unit for unresectable HCCs with main diameter ≤ 6-cm from January 2020 to December 2022. All the unresectable HCCs ≤ 5-cm treated by MWA followed by cTACE in a single-session approach, with available pre- and post-treatment Contrast Enhanced Computed Tomography (CECT) performed in our center, were included.
Files and images were extracted from RIS (Radiology Information) and PACS (Picture Archiving and Communication Systems—GE Medical System, Milwaukee) of the hospital. The searched key words were “hepatic chemoembolization” and “hepatic ablation”. Demographic, clinical and laboratory data were obtained from digital medical records.
Treatment decision of all the included cases were based on a multidisciplinary consensus obtained during dedicated tumor board meetings attended by a dedicated oncologic radiologist, an interventional radiologist, an oncologist, an hepatologist and a liver transplant surgeon. All patients were deemed unfit for surgery due to lesion characteristics, patient’s comorbidities, or refusal.
Inclusion criteria were Child–Pugh score A, liver cirrhosis, single unresectable ≤ 6-cm HCC or < 3 multinodular HCC and a target lesion ≤ 6-cm, absence of extrahepatic disease on CT imaging.
Exclusion criteria were age < 18 years, known allergy to iodinated contrast medium, impaired platelet count, and coagulation parameters (INR > 1.5; PLT < 45,000/μL) (Fig. 1).
Clinical and laboratory characteristics
Patient general characteristics (age and sex), principal comorbidities, previous surgical and locoregional therapies, HCC nodules number, size (main diameter) and volume were assessed. Aspartate Transferase (AST), Alanine Aminotransferase (ALT), Gamma-Glutamyl Transferase (GGT), Alkaline Phosphatase (ALP), INR (International Normalized Ratio), aPPT (activated partial thromboplastin time), platelet (PLT) count (× 103/mmc), albumin (ALB); total, direct and indirect bilirubin values (Bil Tot, Bil Dir and Bil Ind, respectively), and Alpha-fetoprotein (AFP) levels measured within 24 h before and 24 h after a single-session locoregional procedure were evaluated. The difference between pre- and post-procedural values of each parameter was calculated and defined as Δ (delta). In addition, the variation of these values between pre- and post-treatment, reported as ΔAST, ΔALT, ΔGGT, ΔALP, ΔPLT, ΔALB, ΔBilDir, ΔBilInd, ΔBilTot and ΔAFP were evaluated and included in the statistical analysis. Laboratory values were also registered 7 days after the procedure.
Diagnostic imaging
All cases had previously undergone thorax and abdomen CECT pretreatment study. One and three-month CECT follow-up imaging was available.
CT standard protocol consisted of basal unenhanced phase followed by intravenous injection of contrast medium (1.5 ml/kg, Iopamiro®370, Bracco, Milan, Italy) and dedicated triple-phase liver protocol (arterial phase, portal venous phase and delayed phase acquisition). All CT scans were realized with 64-Multi Detector CT (Lightspeed VCT, GE Healthcare, USA) or with 256-slice Dual Scan CT (SOMATOM Force, Siemens Healthineers, Forchheim, Germany). Furthermore, all the patients had previously being submitted to a liver Ultrasound (US; RS80a, Samsung) to evaluate the feasibility of US guidance as an aid to MW-probe positioning in addition to CBCT guidance.
Cone beam CT imaging
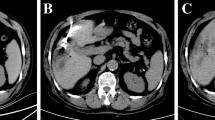
CBCT imaging was obtained during end-expiration apnea and using two different angiographic units both equipped with a digital monoplane C-arm Cone beam CT (Artis zee, Siemens Healthcare GmbH, Erlangen, Germany) and (Azurion, Philips Medical Systems, Amsterdam, Netherlands).
The department’s standard protocol, summarized in Fig. 2, consisted of three acquisitions including:
-
1.
Pretreatment dual-phase contrast enhanced CBCT (ceCBCT), obtained by intra-arterial contrast medium injection through the diagnostic catheter with the aim of confirming the size and location of the target lesion, identifying any additional nodule undetected at preliminary CT, mapping the feeding arteries, and excluding potential arteriovenous shunts;
-
2.
Post-MWA-ceCBCT, obtained by intra-arterial contrast medium injection through the diagnostic catheter to evaluate the ablated area, to visualize the residual tumor where cTACE needs to be performed;
-
3.
Post-cTACE-CBCT obtained without contrast medium injection to evaluate lipiodol retention and its distribution compared to the ablation area.
Single-Session combined procedure
All the combined treatments were performed in two fully equipped angio-suites, in a single-session approach, with patient monitoring and anaesthesiologic assistance. MWA and subsequent cTACE procedures were performed by an interventional radiologist with at least 5 years of experience.
A right common femoral artery access was performed under local anaesthesia (10ml of Mepivacaine 2% solution), and a 4Fr introducer sheath was positioned. The main hepatic artery was catheterized using 4Fr catheters such as Cobra-2 and Simmons-1 (Cordis, Santa Clara, CA, USA), and a preliminary selective arteriography was performed. Pretreatment imaging was completed by a contrast enhanced CBCT protocol, as previously described.
Following conscious sedation, percutaneous US-guided MWA (Solero, Angiodynamics, Latham, NY, USA) was performed after placing the MW-probe tip in the optimal central position, according to the predetermined MWA scheme.
Once MWA was completed and the MW-probe was withdrawn, a post-MWA CBCT was performed and the patient underwent cTACE following catheterization of the tumor-feeding arteries using 2.4–2.7 Fr microcatheters (Progreat, Terumo Medical, Tokyo, Japan), as selectively as possible. An emulsion of 2–20 mL of Ethiodized oil (Lipiodol Ultra-Fluid; Laboratoire Andre Guerbet, Aulnay-sous-Bois, France) and 75 mg of doxorubicin hydrochloride was then administered with a dosage determined according to tumor size and patient’s liver function, until flow stasis was achieved. Consequently, Gelatin sponge was administered in “slurry” preparations (Spongostan®, Johnson & Johnson Medical NV, NJ, USA). Finally, post-cTACE-CBCT scan was performed, and the catheter was removed. Haemostasis of the puncture site was obtained by manual compression. The average time between the end of MWA and the beginning of drug injection was approximately 5 min. The main steps of the single-session approach are summarized in Fig. 3. No antibiotic prophylaxis was realized before and after the single-session combined treatment.
technical steps of combined single-session MWA + cTACE procedure. Preliminary liver US evaluation (a); interventional sterile table and 4Fr introducer sheath preparation (b–c); femoral access with positioning of 4Fr introducer sheath (d); MW-probe opening and testing (e–f); second ultrasound evaluation before MW-probe positioning (g); ultrasound-guided ablation (h); panoramic view of the two accesses, one percutaneous with the antenna-probe onsite, and one on the right groin for cTACE (i); ultrasound monitoring during ablation showing typical acoustic shadowing artifacts (j)
Definitions and outcomes
Technical success was defined as a MWA followed by cTACE successfully performed in a single-session approach. Inadequate US window preventing safe percutaneous access for MWA, failure of arterial branch catheterization, and inefficient catheterization resulting in vessel damage were considered as cases of technical failure.
Clinical success was defined as the absence of residual HCC at 1- and 3-month CECT follow-up, defined as complete response (CR) according to mRECIST criteria [23]. Cases of stable disease (SD) or partial response (PR) were deemed clinical failures. Complications were evaluated based on the CIRSE classification system [24, 25].
The assessment of single-session MWA + cTACE impact on liver function was also evaluated as a secondary endpoint. This analysis was conducted in terms of post-procedural variation of the liver laboratory panel parameters listed above.
Statistical analysis
Data were analyzed using GraphPad Prism version 9.1.1 (GraphPad Software, Boston, MA) statistical software. Counts and percentage were used to report nominal variables whereas means and standard deviations (95% confidence interval) were used for continuous variables.
The Shapiro Wilk Test was performed to determine the categorical variables as appropriate.
Student’s t test was used for continuous variables with normal distribution (Gaussian continuous variables) whereas for continuous variables with abnormal distribution (non-Gaussian continuous variables) and for ordinal variables, Mann–Whitney U test was used.
A p value < 0.05 was considered statistically significant. For each statistical comparison, confidence intervals (CI) and p values were reported.
Results
A total of 15 HCCs in 15 patients (11 male; 71.4%) were treated. The mean age was 67.1 ± 8.9 years (range:51–85 years). Clinical comorbidities were present in 12/15 patients (86%).
The mean lesion diameter was 5 ± 1.4 cm. Four (27%) target lesions were localized in left liver lobe, and 11 (73%) in the right lobe. Demographics, clinical findings, and lesion characteristics are summarized in Table 1.
Mean laboratory values of AST, ALT, GGT, ALP, BilDir, BilInd, BilTot, ALB, PLT and AFP as well as their variations between pre- and post-procedural values (Δ), and their values registered 7 days after procedures are analytically reported in Table 2.
Mean procedural duration was 108 ± 20 min.
Technical success was obtained in all cases (15/15; 100%). Clinical success was obtained in 11 cases (73%) while in 4 cases (27%), partial response was reported. Just 1 partial response case had a rapid progression of systemic disease and comorbidities up to death; for this reason, no re-treatment was realized, whereas the other 3 partial response cases were re-treated with cTACE alone to chemoembolize the residual HCC. No major complications were noted. In one case (4.8%), the development of segmental arterial-portal fistula was detected at one-month follow-up.
The results of univariate analysis are reported in Table 3.
Statistically significant results in terms of ALT (p = 0.00062) and AST (p = 0.0093) between pre- and post-procedural values were obtained, with significantly higher ALT and AST values in post-procedural measurements (Fig. 4).
Correlation of clinical outcome with difference between pre-procedural and post-procedural AST values (ΔAST, p = 0.023, central value expressed by the median of the measurements) (a) and with difference between pre-procedural and post-procedural ALT values (ΔALT, p = 0.0003) (b). Modification of AST values between pre- and post-treatment (p = 0.00062) (c) and of ALT values between pre- and post-treatment (p = 0.0093) (d)
A significant difference was also observed in terms of ΔALT (p = 0.023) and ΔAST (p = 0.043) values between clinical success and clinical failure cases, with a significantly higher number of clinical failure cases registered in presence of higher ΔALT and ΔAST values (Fig. 3).
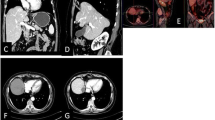
A representative case of HCC lesion treated with MWA followed by cTACE in a single session, is presented in Fig. 5.
Seventy-four-years-old patient with presence of HCC lesion in the VII hepatic segment as shown in triphasic contrast enhanced CT pretreatment, arterial phase (a), venous phase (b), delayed phase (c). After initial angiographic study (d), single-session approach with MWA (e) followed by cTACE was performed. CBCT with intra-arterial contrast medium injection was performed before treatment (f) and after MWA (g), whereas CBCT without medium of contrast was performed after cTACE (h). Follow-up imaging with contrast enhanced CT shows no residual HCC and peri-lesional lipiodol distribution with central necrotic area, basal acquisition (i), arterial phase (j), venous phase (k), delayed phase (l)
Discussion
The combination of different types of locoregional treatments has progressively gain importance in HCC management due to its potential clinical implications. Firstly, combined treatments have demonstrated to improve local tumor control, particularly for larger tumors and for those located between early and intermediate BCLC stages [20, 26]. Additionally, combined strategies may contribute to improve patient overall survival and reduce recurrence rates [27, 28]. Although surgery and transplant remain the gold standard of HCC treatment, combined approaches are today considered an excellent option for bridging and down-staging to surgery or transplant [29].
Until today, the target of combined locoregional therapies was focused on single unresectable HCC lesions with main diameter ≥ 3 cm and classified as BCLC-A, due to the high recurrence rates after ablation as stand-alone treatment [20, 28, 30,31,32]. Nevertheless, several meta-analyses have obtained promising results of combined approach both in early stage and in intermediate-stage HCCs compared to single TACE or ablation [33,34,35,36,37,38].
The rationale of the combined approach arises from the possibility of overcoming the main limitations of each individual treatment through a synergistic action. The heat sink effect of ablation can be mitigated by the decreased arterial flow following TACE, thereby increasing the ablation area.
The retention of lipiodol within the nodule treated by TACE can make less challenging the evaluation of ablative margins, particularly those not clearly visible during US guidance and those at high risk of damaging the adjacent structures. TACE can also ensure the treatment of satellite nodules otherwise undetectable with ablation alone. Conversely, ablation may reduce several negative effects correlated with arterial recanalization and angiogenesis often occurring after repeated (often suboptimal) TACE procedures and commonly resulting in residual tumor and intrahepatic recurrences as well as progressive impairment of liver function [38,39,40]. Especially in larger tumor volumes, ablation can also reduce the expression of vascular growth factors induced by hypoxia, thus improving long-term outcomes [33, 36,37,38].
Despite the known theoretical advantages, precise indications regarding combined therapies are still missing in current guidelines due to several unresolved issues.
Firstly, which techniques should be preferred in combined approaches for both TACE and ablation remains controversial. Recent meta-analyses, despite the greater reported experience with radiofrequency ablation (RFA), suggest that MWA has a better tumor response and long-term survival than any other ablation technique [34, 41]. In this study, cTACE was preferred to DEB-TACE according to literature, due to many advantages such as a stronger heat conduction, the peri-portal embolization, and a better visibility of treated areas and satellite lesions [30, 42].
Treatment sequence remains another matter of debate. Despite most authors prefer TACE followed by ablation, in our series, MWA before cTACE was adopted due to known benefits such as the stimulation of necrosis on peripheral tumor areas thus exposed to higher drug concentrations, the increased vascular permeability in the surrounding ablation area due to the exposure to sublethal heating, and the higher drug concentration on a relatively smaller volume of residual viable tissue [43, 44]. In contrast to the previously hypothesized loss in efficacy of drugs injected by TACE if exposed to high temperatures, recent studies demonstrated that the sublethal hyperthermia of the periablation area enhances the anticancer activity of doxorubicin [45].
Lastly, there is a lack of relevant literature regarding the timing of combination therapy. It is known that time interval should be carefully decided in order to ensure the balance between successful tumor eradication and preservation of liver function. Most studies recommend a variable time interval from 2 to 5 weeks between ablation and TACE to allow liver function recovery, discouraging single-session approaches [46,47,48]. Others believe that a time interval of 0–2 days is sufficient [20].
In accordance with combined early sequential RFA + TACE results, our single-session approach demonstrated encouraging technical and clinical success rates, respectively, of 100% and 73%, without major complications [46]. Furthermore, no significant modifications between pre- and post-treatment values in most of laboratory liver function parameters were observed, except for a transitory increase in AST and ALT values. All the liver injury indices have subsequently return to normal values within one week after combined procedures, suggesting a transitory impairment rather than an acute liver injury.
An increase in AST and ALT values had been previously reported by Yuan et al., despite the fact that the more conservative sequential approach was preferred in this series [48]. Even in cases of solely TACE treatment, higher variations in AST and ALT were observed [49]. This could be explained by non-target chemoembolization and consequent higher hepatic toxicity due to a greater drug spreading within the healthy liver parenchyma [49].
Other pre-procedural laboratory values have been extensively analyzed to identify possible predictive factors of treatment failure, most of them with limited availability [27, 50, 51]. In our preliminary series, higher AST and ALT variations were significantly related with a poor clinical success, suggesting these parameters could be monitored in the first days after single-session combined treatments to predict negative outcomes.
This study has several and important limitations including the retrospective and single-center design, the small sample size, and the limited follow-up. Moreover, missing data regarding comorbidities, as well as previous locoregional and systemic treatments might have biased the local progression outcomes.
To the best of our knowledge, the present series is the first investigating the outcomes of combined treatments performed in single-session and employing exclusively MWA as ablative technology and conventional TACE as intra-arterial strategy. Furthermore, the results demonstrate that minimizing the time interval between the two different treatment modalities resulted in only a mild liver function impairment despite the maximum synergistic effect.
In conclusion, the single-session approach showed encouraging results in terms of safety, technical and clinical success with the advantage of reducing number of hospitalizations, length of stay and patient discomfort. Larger populations, multicentric and prospective controlled studies comparing single-session and sequential combined therapies are required to validate these preliminary results.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2
Vitale A, Svegliati-Baroni G, Ortolani A, Cucco M, Dalla Riva GV, Giannini EG, Piscaglia F, Rapaccini G, Di Marco M, Caturelli E, Zoli M, Sacco R, Cabibbo G, Marra F, Mega A, Morisco F, Gasbarrini A, Foschi FG, Missale G, Masotto A, Nardone G, Raimondo G, Azzaroli F, Vidili G, Oliveri F, Pelizzaro F, Ramirez Morales R, Cillo U, Trevisani F, Miele L, Marchesini G, Farinati F (2023) Italian Liver Cancer (ITA.LI.CA) group Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: the ITA.LI.CA database. Gut 72(1):141–152. https://doi.org/10.1136/gutjnl-2021-324915
Toh MR, Wong EYT, Wong SH, Ng AWT, Loo L-H, Chow PK-H, Ngeow J (2023) Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology 164(5):766–782. https://doi.org/10.1053/j.gastro.2023.01.033
Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH (2023) AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 78(6):1922–1965. https://doi.org/10.1097/HEP.0000000000000466
Gong W, Wu J, Wei H, Jiang Z, Wan M, Wu C, Xue W, Ma R, Zhou X, Zhou H (2023) Combining serum AFP and CEUS LI-RADS for better diagnostic performance in Chinese high-risk patients. Radiol Med 128(4):393–401. https://doi.org/10.1007/s11547-023-01614-9
Cellina M, Cè M, Rossini N, Cacioppa LM, Ascenti V, Carrafiello G, Floridi C (2023) Computed tomography urography: state of the art and beyond. Tomography 9(3):909–930. https://doi.org/10.3390/tomography9030075
Ruan SM, Huang H, Cheng MQ, Lin MX, Hu HT, Huang Y, Li MD, Lu MD, Wang W (2023) Shear-wave elastography combined with contrast-enhanced ultrasound algorithm for noninvasive characterization of focal liver lesions. Radiol Med 128(1):6–15. https://doi.org/10.1007/s11547-022-01575-5
Cellina M, Cacioppa LM, Cè M, Chiarpenello V, Costa M, Vincenzo Z, Pais D, Bausano MV, Rossini N, Bruno A, Floridi C (2023) Artificial intelligence in lung cancer screening: the future is now. Cancers (Basel) 15(17):4344. https://doi.org/10.3390/cancers15174344
Cacioppa LM, Floridi C, Cocozza MA, Bruno A, Modestino F, Martella C, Rosati M, Paccapelo A, Mosconi C, Candelari R (2023) The prominent role of percutaneous transarterial embolization in the treatment of anterior abdominal wall hematomas: the results of three high volume tertiary referral centers. Radiol Med 128(9):1125–1137. https://doi.org/10.1007/s11547-023-01678-7
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Yamada A, Kamagata K, Hirata K, Ito R, Nakaura T, Ueda D, Fujita S, Fushimi Y, Fujima N, Matsui Y, Tatsugami F, Nozaki T, Fujioka T, Yanagawa M, Tsuboyama T, Kawamura M, Naganawa S (2023) Clinical applications of artificial intelligence in liver imaging. Radiol Med 128(6):655–667. https://doi.org/10.1007/s11547-023-01638-1
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76(3):681–693. https://doi.org/10.1016/j.jhep.2021.11.018
Mosconi C, O’Rourke J, Kloeckner R, Sturm L, Golfieri R, Celsa C, Fateen W, Odisio BC, Garanzini EM, Peck-Radosavljevic M, Borghi A, Ma YT, Stoehr F, Bettinger D, Giuffrida P, Aithal GP, Lin YM, Spreafico C, Giampalma E, Johnson P, Cucchetti A (2023) Textbook outcome after trans-arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol 46(4):449–459. https://doi.org/10.1007/s00270-023-03375-4
Chen QW, Ying HF, Gao S, Shen YH, Meng ZQ, Chen H, Chen Z, Teng WJ (2016) Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 40(3):309–314. https://doi.org/10.1016/j.clinre.2015.07.008
Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY (2013) Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 31(4):426–432. https://doi.org/10.1200/JCO.2012.42.9936
Renzulli M, Brandi N, Argalia G, Brocchi S, Farolfi A, Fanti S, Golfieri R (2022) Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol Med 127(2):129–144. https://doi.org/10.1007/s11547-022-01449-w
Lee HJ, Kim JW, Hur YH, Shin SS, Heo SH, Cho SB, Kang YJ, Lim HS, Seon HJ, Jeong YY (2017) Combined therapy of transcatheter arterial chemoembolization and radiofrequency ablation versus surgical resection for single 2–3 cm hepatocellular carcinoma: a propensity-score matching analysis. J Vasc Interv Radiol 28(9):1240-1247.e3. https://doi.org/10.1016/j.jvir.2017.05.015
Dan Y, Meng W, Li W, Chen Z, Lyu Y, Yu T (2022) Transarterial chemoembolization combined with radiofrequency ablation versus hepatectomy for hepatocellular carcinoma: a meta-analysis. Front Surg 9:1–13. https://doi.org/10.3389/fsurg.2022.948355
Ni JY, Liu SS, Xu LF, Sun HL, Chen YT (2013) Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 19(24):3872–3882. https://doi.org/10.3748/wjg.v19.i24.3872
Yan L, Ren Y, Qian K, Kan X, Zhang H, Chen L, Liang B, Zheng C (2021) Sequential transarterial chemoembolization and early radiofrequency ablation improves clinical outcomes for early-intermediate hepatocellular carcinoma in a 10-year single-center comparative study. BMC Gastroenterol 21(1):1–10. https://doi.org/10.1186/s12876-021-01765-x
Jiang C, Cheng G, Liao M, Huang J (2021) Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol 19(1):1–13. https://doi.org/10.1186/s12957-021-02188-4
Lee SK, Yang H, Kwon JH, Shim DJ, Kim D, Nam SW, Yoo SH, Bae SH, Lee A, Lee YJ, Jeon C, Jang JW, Sung PS, Chun HJ, Kim SH, Choi JI, Oh JS, Yang YJ (2023) Chemoembolization combined radiofrequency ablation versus chemoembolization alone for treatment of beyond the Milan criteria viable hepatocellular carcinoma (CERFA): study protocol for a randomized controlled trial. Trials 24(1):1–9. https://doi.org/10.1186/s13063-023-07266-4
Llovet JM, Lencioni R (2020) mRECIST for HCC: performance and novel refinements. J Hepatol 72(2):288–306. https://doi.org/10.1016/j.jhep.2019.09.026
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL (2017) Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol 40(8):1141–1146. https://doi.org/10.1007/s00270-017-1703-4
Vacirca A, Faggioli G, Pini R, Gallitto E, Mascoli C, Cacioppa LM, Gargiulo M, Stella A (2019) The outcome of technical intraoperative complications occurring in standard aortic endovascular repair. Ann Vasc Surg 56:153–162. https://doi.org/10.1016/j.avsg.2018.08.092
Wang X, Hu Y, Ren M, Lu X, Lu G, He S (2016) Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol 17(1):93–102. https://doi.org/10.3348/kjr.2016.17.1.93
Long J, Wang H, Zhao P, Sheng SP, Shi Q-S, Long M, Zheng JS (2020) Transarterial chemoembolization combined with radiofrequency ablation for solitary large hepatocellular carcinoma ranging from 5 to 7 cm: an 8-year prospective study. Abdom Radiol (NY) 45(9):2736–2747. https://doi.org/10.1007/s00261-020-02612-5
Ren Y, Cao Y, Ma H, Kan X, Zhou C, Liu J, Shi Q, Feng G, Xiong B, Zheng C (2019) Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer 19(1):1–10. https://doi.org/10.1186/s12885-019-6237-5
Yi PS, Huang M, Zhang M, Xu L, Xu MQ (2018) Comparison of transarterial chemoembolization combined with radiofrequency ablation therapy versus surgical resection for early hepatocellular carcinoma. Am Surg 84(2):282–288
Lee HJ, Kim JW, Hur YH, Cho SB, Lee BC, Lee BK, Hwang EC, Cho YS, Seon HJ (2019) Conventional chemoembolization plus radiofrequency ablation versus surgical resection for single, medium-sized hepatocellular carcinoma: propensity-score matching analysis. J Vasc Interv Radiol 30(3):284-292.e1. https://doi.org/10.1016/j.jvir.2018.09.030
Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Kuang M (2018) Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol 28(8):3522–3531. https://doi.org/10.1007/s00330-017-5166-4
Iezzi R, Pompili M, Posa A, Carchesio F, Siciliano M, Annicchiarico BE, Agnes S, Giuliante F, Garcovich M, Cerrito L, Ponziani FR, Basso M, Cassano A, Rapaccini GL, De Gaetano AM, Gasbarrini A, Manfredi R (2019) HepatoCATT study group for the multidisciplinary management of HCC. Interventional oncology treatments for unresectable early stage HCC in patients with a high risk for intraprocedural bleeding: is a single-step combined therapy safe and feasible? Eur J Radiol 114:32–37. https://doi.org/10.1016/j.ejrad.2019.02.030
Yang Y, Yu H, Qi L, Liu C, Feng Y, Qi J, Li J, Zhu Q (2022) Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: a systematic review and meta-analysis. Int J Hyperthermia 39(1):455–465. https://doi.org/10.1080/02656736.2022.2048095
Keshavarz P, Raman SS (2022) Comparison of combined transarterial chemoembolization and ablations in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY) 47(3):1009–1023. https://doi.org/10.1007/s00261-021-03368-2
Liu C, Li T, He JT, Shao H (2020) TACE combined with microwave ablation therapy versus TACE alone for treatment of early—and intermediate-stage hepatocellular carcinomas larger than 5 cm: a meta-analysis. Diagn Interv Radiol 26(6):575–583. https://doi.org/10.5152/dir.2020.19615
Zhao J, Wu J, He M, Cao M, Lei J, Luo H, Yi F, Ding J, Wei Y, Zhang W (2020) Comparison of transcatheter arterial chemoembolization combined with radiofrequency ablation or microwave ablation for the treatment of unresectable hepatocellular carcinoma: a systemic review and meta-analysis. Int J Hyperthermia 37(1):624–633. https://doi.org/10.1080/02656736.2020.1774667
Wang L, Ke Q, Lin N, Huang Q, Zeng Y, Liu J (2019) The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 36(1):1288–1296. https://doi.org/10.1080/02656736.2019.1692148
Yang DJ, Luo KL, Liu H, Cai B, Tao GQ, Su XF, Hou XJ, Ye F, Li XY, Tian ZQ (2017) Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma. Oncotarget 8(2):2960–2970. https://doi.org/10.18632/oncotarget.13813
Hatzidakis A, Müller L, Krokidis M, Kloeckner R (2022) Local and regional therapies for hepatocellular carcinoma and future combinations. Cancers (Basel) 14(10):1–25. https://doi.org/10.3390/cancers14102469
Mosconi C, Cacioppa LM, Cappelli A, Gramenzi AG, Vara G, Modestino F, Renzulli M, Golfieri R (2023) Update of the Bologna Experience in Radioembolization of Intrahepatic cholangiocarcinoma. Technol Cancer Res Treat. https://doi.org/10.1177/15330338231155690
Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O (2018) Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol 68(4):783–797. https://doi.org/10.1016/j.jhep.2017.10.004
Tan J, Mathy RM, Chang DH, Tang T, Zhang ZS, Xiao YD (2022) Combined transarterial iodized oil injection and computed tomography-guided thermal ablation for hepatocellular carcinoma: utility of the iodized oil retention pattern. Abdom Radiol (NY) 47(1):431–442. https://doi.org/10.1007/s00261-021-03305-3
Iezzi R, Posa A, Tanzilli A, Carchesio F, Pompili M, Manfredi R (2020) Balloon-occluded MWA (b-MWA) followed by balloon-occluded TACE (b-TACE): technical note on a new combined single-step therapy for single large HCC. Cardiovasc Intervent Radiol 43(11):1702–1707. https://doi.org/10.1007/s00270-020-02583-6
Iezzi R, Cesario V, Siciliani L, Campanale M, De Gaetano AM, Siciliano M, Agnes S, Giuliante F, Grieco A, Pompili M, Rapaccini GL, Gasbarrini A, Bonomo L (2013) HepatoCATT Group for the Multidisciplinary Management of HCC. Single-step multimodal locoregional treatment for unresectable hepatocellular carcinoma: balloon-occluded percutaneous radiofrequency thermal ablation (BO-RFA) plus transcatheter arterial chemoembolization (TACE). Radiol Med 118(4):555–569. https://doi.org/10.1007/s11547-012-0914-7
Wang Q, Zhang H, Ren QQ, Ye TH, Liu YM, Zheng CS, Zhou GF, Xia XW (2021) Sublethal hyperthermia enhances anticancer activity of doxorubicin in chronically hypoxic HepG2 cells through ROS-dependent mechanism. Biosci Rep 41(6):1–12. https://doi.org/10.1042/BSR20210442
Yu Y, Fu J, Xia P, Chu C (2022) A systematic review and meta-analysis on the efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of primary liver cancer. Transl Cancer Res 11(5):1297–1308. https://doi.org/10.21037/tcr-22-816
Xu Z, Xie H, Zhou L, Chen X, Zheng S (2019) The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst) 2019:1–7. https://doi.org/10.1155/2019/8619096
Yuan P, Zhang Z, Kuai J (2019) Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON 24(1):163–170
Miksad RA, Ogasawara S, Xia F, Fellous M, Piscaglia F (2019) Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: the LiverT study. BMC Cancer 19(1):1–8. https://doi.org/10.1186/s12885-019-5989-2
Yamada R, Bassaco B, Bracewell S, Volin S, Collins H, Hannegan C, Guimarares M (2020) Combined conventional transarterial chemoembolization with Mitomycin and percutaneous ablation for unresectable hepatocellular carcinoma. J Gastrointest Oncol 11(2):298–303. https://doi.org/10.21037/jgo.2019.01.07
Minici R, Siciliano MA, Ammendola M, Santoro RC, Barbieri V, Ranieri G, Laganà D (2022) Prognostic role of neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-c reactive protein ratio (LCR) in patients with hepatocellular carcinoma (HCC) undergoing chemoembolizations (TACE) of the liver: the unexplored corner linking tumor microenvironment, biomarkers and interventional radiology. Cancers (Basel) 15(1):257. https://doi.org/10.3390/cancers15010257
Acknowledgements
Not applicable.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Chiara Floridi, Laura Maria Cacioppa, Nicolò Rossini, Marco Macchini and Alessandra Bruno. The first draft of the manuscript was written by Chiara Floridi, Laura Maria Cacioppa, Nicolò Rossini, and Roberto Candelari. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial interests to disclose.
The authors Chiara Floridi, Laura Maria Cacioppa and Andrea Agostini are members of the Editorial Board and Deputy Editor panel of the journal La Radiologia Medica.
Ethics approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
This study obtained the approval of the Internal Review Board (IRB) of University Politecnica Delle Marche.
Informed consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors. The authors affirm that human research participants provided informed consent for publication of the images in Figs. 2 and 4.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Floridi, C., Cacioppa, L.M., Rossini, N. et al. Microwave ablation followed by cTACE in 5-cm HCC lesions: does a single-session approach affect liver function?. Radiol med (2024). https://doi.org/10.1007/s11547-024-01842-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11547-024-01842-7