Abstract
Background
Preoperative assessment of lymphovascular invasion(LVI) of rectal cancer has very important clinical significance. However, accurate preoperative imaging evaluation of LVI is highly challenging because the resolution of MRI is still limited. Relatively few studies have focused on prediction of LVI of rectal cancer with the tool of radiomics, especially in patients with negative statue of MRI-based extramural vascular invasion (mrEMVI).The purpose of this study was to explore the preoperative predictive value of biparametric MRI-based radiomics features for LVI of rectal cancer in patients with the negative statue of mrEMVI.
Methods
The data of 146 cases of rectal adenocarcinoma confirmed by postoperative pathology were retrospectively collected. In the cases, 38 had positive status of LVI. All patients were examined by MRI before the operation. The biparametric MRI protocols included T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI). We used whole-volume three-dimensional method and two feature selection methods, minimum redundancy maximum relevance (mRMR) and least absolute shrinkage and selection operator (LASSO), to extract and select the features. Logistics regression was used to construct models. The area under the receiver operating characteristic curve (AUC) and DeLong’s test were used to evaluate the diagnostic performance of the radiomics based on T2WI and DWI and the combined models.
Results
Radiomics models based on T2WI and DWI had good predictive performance for LVI of rectal cancer in both the training cohort and the validation cohort. The AUCs of the T2WI model were 0.87 and 0.87, and the AUCs of the DWI model were 0.94 and 0.92. The combined model was better than the T2WI model, with AUCs of 0.97 and 0.95. The predictive performance of the DWI model was comparable to that of the combined model.
Conclusions
The radiomics model based on biparametric MRI, especially DWI, had good predictive value for LVI of rectal cancer. This model has the potential to facilitate the clinical recognition of LVI in rectal cancer preoperatively.
Similar content being viewed by others
Background
Colorectal cancer is a common malignant tumor of the digestive tract. The global morbidity and mortality rates associated with colorectal cancer have been increasing in recent years. This cancer is the second most common cause of cancer-related death, and rectal cancer accounts for one-third of the incidence of colorectal cancer [1, 2]. At present, surgical resection combined with neoadjuvant chemoradiotherapy is the main treatment for rectal cancer [2,3,4]. Previous studies have shown that invasion of tumor cells into the microcirculation around the rectum increases the risk of spread and metastasis of rectal cancer. Vascular invasion of rectal cancer is an independent risk factor for postoperative tumor recurrence and low tumor-free survival [5, 6]. A recent study showed that lymphovascular invasion(LVI), rather than the traditionally believed depth of tumor invasion, is even a key risk factor for early rectal cancer metastasis [7]. Therefore, preoperative assessment of LVI of rectal cancer, including identification of small lymphatic and vascular invasion, has very important clinical significance for predicting postoperative recurrence, metastasis, and tumor-free survival of rectal cancer.
High-resolution magnetic resonance imaging (MRI) is the main imaging method for rectal cancer, and it plays a very important role in assessment of the tumor in the preoperative and postoperative periods. Biparametric MRI, including T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI), has demonstrated reliable results in the detection and staging of rectal cancer [8]. High-resolution MRI shows good performance in the demonstration of larger vessels. In the detection of extramural vascular invasion(EMVI),MRI has moderate sensitivity and high specificity. However, MRI cannot definitively depict vessels with a diameter of < 3 mm, including micro-arteriovenous and lymphatic vessels [9, 10]. Therefore, accurate preoperative imaging evaluation of LVI of rectal cancer is highly challenging.
Radiomics relies on algorithm models to extract quantitative imaging features that cannot be captured by visual inspection. The selected radiomics features can be used to establish predictive model related to disease diagnosis and treatment combined with clinical and pathological data. At present, radiomics as an emergent imaging analysis is widely used in the imaging studies of tumor diagnosis, post-treatment, and prognosis evaluation [11,12,13]. In the study of rectal cancer, research has shown that radiomics has good predictive value for nodal metastasis and extramural venous invasion as well as evaluation of neoadjuvant radiotherapy and chemotherapy in patients with rectal cancer [14]. However, relatively few studies have focused on preoperative prediction of LVI of rectal cancer especially in patients with the negative statue of MRI-based extramural vascular invasion(mrEMVI).
The present study was performed to explore the preoperative predictive value and role of biparametric MRI-based radiomic features for LVI of rectal cancer in patients with the negative statue of mrEMVI.
Materials and methods
Patients
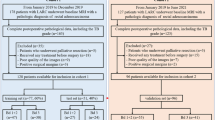
Cases of rectal adenocarcinoma confirmed by surgery and postoperative pathology were retrospectively collected from 2019 to 2021. The inclusion criteria were rectal adenocarcinoma confirmed by surgery and postoperative pathology, examination by noncontrast-enhanced MRI before the operation, and confirmation of the status of LVI by postoperative pathology. The exclusion criteria were patients with treatment before the operation; patients with the positive statue of mrEMVI; incomplete imaging or clinical and pathological data; poor image quality of MRI before the operation, affecting the imaging diagnosis; and a > 1-month interval between the MRI examination and operation. Finally, 146 cases were included. A study flow chart is shown in Fig. 1.
Image acquisition
All patients underwent preoperative MRI examinations with a Siemens Skyra 3.0 T system (Siemens Healthineers, Erlangen, Germany), and intestinal cleaning and preparation were performed before the examination. The biparametric MRI protocols included T2WI and DWI. Images from each sequence were obtained in the oblique coronal, oblique axial, and sagittal planes. The oblique axial plane was determined by the perpendicular axis of the tumor, and the oblique coronal plane was parallel to the tumor axis or anal canal depending on the location of the tumor. The details of the scanning sequences are shown in Table 1.
Reference standard for lymphovascular invasion
The reference standard for LVI of rectal cancer was the presence of tumor cells in lymphatic vessels and/or blood vessels in the resected specimen by postoperative pathology. The surface of tumor cells is covered with endothelial cells or thrombosis is found in blood vessels.
Image interpretation and segmentation
The patients were randomly divided into a training group (n = 102) and a validation group (n = 44) at a proportion of 7:3. Previous research has established that the signal intensity of rectal adenocarcinoma on T2WI is higher than that of the muscularis propria but lower than that of the submucosa. On high b-value DWI, the foci of the lesions show significantly higher signal intensity than the normal rectal wall, and the corresponding apparent diffusion coefficient map shows significantly lower signal intensity [15]. In the present study, a physician who had specialized in abdominal imaging for 8 years (reader1) used ITK-SNAP software (version 3.8.0, http://www.itksnap.org/pmwiki/pmwiki.php?n=Main.Publications) to evaluate T2WI and DWI of all planes and delineate the regions of interest on oblique axial images independently. The region of the tumor was identified with reference to the relevant imaging diagnostic criteria [16,17,18]. The whole-volume three-dimensional delineation method was used in this process as shown in Fig. 2. After one week, 30 cases were randomly selected and delineated by reader 1 and another physician with 12 years of diagnostic experience (reader 2), respectively, after training. The delineation interval between T2WI and DWI of the same patient was more than one day.
Example of segmentation. Images from a 73-year-old man with rectal adenocarcinoma and pathologically proven lymphovascular invasion. a, b Delineation of the tumor on T2-weighted images. d, e Delineation of the tumor on diffusion-weighted images. c, f Three-dimensional rendering of segmentation of tumor
Radiomics feature extraction and model construction
The radiomics features were extracted using PyRadiomics software version 3.0.1 (https://pyradiomics.readthedocs.io/en/latest/index.html) [19], which met the criteria of the Image Biomarker Standardization Initiative (https://theibsi.github.io). The first-order features, shape-based features, and texture features based on Gray-level co-occurrence matrix(GLCM), Gray-level run-length matrix (GLRLM), Gray-level size zone matrix(GLSZM) and Gray-level dependence matrix(GLDM) were extracted, except for the original image.The texture features are also described as the second-order features that describe the intensity level of the spatial distribution of voxels.We also transformed the image using the Laplacian of Gaussian (sigma: 2, 3, 4, 5) and wavelet transformations. In total, 1218 radiomics features were obtained for each image. The default MRI extraction settings provided by PyRadiomics were adopted to extract the features. The basic settings were as follows: resample pixel spacing: 1 × 1 × 1, bin width: 5, normalize: true, and normalize scale: 100. Further details are provided in the Supplemental materials 1 and 2.
The radiomics features of 30 cases delineated by 2 physicians were calculated, and the intra-observer and inter-observer reproducibility of the radiomics features were evaluated. The radiomics features with poor reproducibility should he ruled out.We then used two feature selection methods, minimum redundancy maximum relevance (mRMR) and least absolute shrinkage and selection operator (LASSO), to select the features. First, mRMR was performed to eliminate the redundant and irrelevant features, and 30 features were retained. LASSO was then conducted to choose the optimized subset of features to construct the final model. LASSO involved choosing the regular parameter λ and determining the number of features. After the number of features had been determined, the most predictive subset of features was chosen and the corresponding coefficients were evaluated.
The radiomics score (Radscore) was calculated by summing the selected features weighted by their coefficients using the following formula: \(\mathrm y^\wedge=\mathrm b1\mathrm X1+\mathrm b2\mathrm X2+\cdots+\mathrm{biXi}+\mathrm b\), where y^ is the Radscore, b is the intercept, bi is the coefficient of the feature i, and Xi is the value of the feature i. In this way, the Radscore was calculated by summing the selected features weighted by their coefficients.
Statistical analysis
All statistical analyses were performed with R software version 4.0.3 (https://cran.r-project.org). Continuous variables are expressed as mean and standard deviation, and categorical variables are expressed as frequency and percentage. The t test or rank sum test was used to analyze differences between groups of continuous variables, the chi-square test or Fisher’s test was used for unordered categorical variables, and the rank sum test was used for ordered categorical variables. The intraclass correlation coefficients (ICCs) was used to assess the intra-observer and inter-observer reproducibility of extraction. The ICCs < 0.7 was considered to have poor reproducibility. LASSO regression was used to select radiomics feature parameters, and logistics regression was used to construct model equations. The receiver operating characteristic (ROC) Curves and the area under the receiver operating characteristics curve (AUC) and DeLong’s test were used to evaluate the predictive performance among different models. A P-value of < 0.05 was considered statistically significant.
Results
In total, 146 cases of rectal cancer were included in this study (102 cases in the training group, 44 cases in the validation group). Of these 146 cases, 38 were positive for lymphovascular invasion. There were no significant differences in the clinical or pathological results between the groups. The details are shown in Table 2.
The ICCs of the intra-observer and inter-observer reproducibility were satisfactory. Finally,11 and 14 radiomics features were retained from the T2WI and DWI sequences, respectively. 14 characteristic parameters were retained from the combined model. The details are shown in Fig. 3. The Radscore equations were included in Supplemental materials 1 and 2.
The receiver operating characteristics curve showed that the radiomics model based on T2WI and DWI had good predictive performance for lymphovascular invasion of rectal cancer in both the training cohort and the validation cohort. The AUCs of the T2WI model were 0.87 and 0.87 and the AUCs of the DWI model were 0.94 and 0.92. DeLong’s test showed that the DWI model had better performance than the T2WI model (P < 0.05) and that the combined model had better performance than the T2WI model, with AUCs of 0.97 and 0.95 in the training cohort and validation cohort, respectively (P < 0.05). The DWI model had predictive performance comparable to that of the combined model (P > 0.05) (Table 3, Fig. 4). There was an example as shown in Fig. 5. The calibration curves of the combined model demonstrated the best agreement with the ideal curve ( Fig. 6). The decision curves analysis showed that the combined model had the highest standardized net benefit across the range of 0.1–1.0 of the high risk threshold( Fig. 7).
Discussion
High-resolution MRI is currently the main imaging modality for preoperative and postoperative evaluation of patients with rectal cancer, but it cannot be used to obtain a clear diagnosis of small lymphovascular invasion. The present study was performed to explore the preoperative predictive value of biparametric MRI based radiomics features for LVI of rectal cancer, and the results showed that the radiomics models based on T2WI and DWI had good performance. A recent study of rectal cancer showed that a multiparameter radiomics model had good performance in predicting extramural venous invasion of rectal cancer [20]. In the present study, pathologically confirmed cases of LVI were included. Therefore, the evaluation of microcirculation invasion of rectal cancer was more comprehensively in our study. We selected biparametric images, including those obtained from T2WI and DWI sequences, to delineate lesions in this study for several reasons. On the one hand, the biparametric images are easier to obtain clinically than enhanced images with lower economic cost and a shorter inspection time, which is helpful for multicenter research. Without intravenous injection of gadolinium contrast agent, there is no potential risk of residual contrast agent in the body [21, 22]. On the other hand, previous studies have shown that enhanced T1WI has a limited role in staging of rectal cancer and cannot effectively distinguish the signal intensity of the lesions from that of the rectal wall [23, 24]. T2WI and DWI are the most important sequences for the imaging staging of rectal cancer and are effective in demonstrating the lesions [18, 25]. In addition, the whole-tumor three-dimensional method of delineation was used in this study for two reasons. First, because of the limited resolution of MRI, we could not identify the small-diameter arteriovenous and lymphatic vessels on the images directly. It was difficult to accurately delineate. Second, the three-dimensional method can more accurately and fully characterize the voxel characteristics than the two-dimensional method, as shown in previous studies [26, 27]. In recent years, there have been several studies similarly exploring the role of MRI based radiomics for LVI in patients with rectal cancer [28, 29].But differently, we focused on those patients with negative statue of mrEMVI. As the previous evidence showed, MRI has high specificity but moderate sensitivity in the detection of EMVI. There is no need for patients with positive statue of mrEMVI on the basis of routine MRI evaluation to offer extra imaging method for prediction of lymphovasular invasion.
In this study, the results showed that the AUCs of the radiomics models based on T2WI and DWI in the training cohort were 0.87 and 0.87, and those in the validation cohort were 0.94 and 0.92. The AUCs of the combined model were 0.97 and 0.95 in the training cohort and validation cohort, respectively.The results were better than previous study mentioned above.The probable reasons were as follows. First, the patients in our cohort were all evaluated as mrEMVI(-) preoperately. Consequently, the ratio of early stage patients was higher in our study compared with the previous studies. The cystic and necrotic components in early stage rectal cancer are less than advanced stage.Second, we delineated on biparametric MR images rather than multiparametric or multimodal images,which made the process easier and avoid the possibility of making mistakes in delineation on enhamced images.Third, we ruled out the cases of rectal mucinous adenocarcinoma. Rectal mucinous adenocarcinoma is a rare subtype of rectal adenocarcinoma characterized by lakes of stromal mucin containing scant malignant epithelial cells [30, 31]. Thus, it is difficult to identify the solid component on images. The extent of the tumor can be overestimated. As a result, the delineation of the lesions in our study was easier and more accurate.
Moreover, the discrimination of each model was compared. The results showed that the combined model was better than the T2WI model. Interestingly, the AUC of the DWI model was higher than that of the T2WI model and comparable to that of the combined model. As we know, DWI can detect the motion of water molecules in the extracellular and intracellular spaces in vivo, which reflects the cell membrane integrity and cell density [32]. Previous studies have shown that high b-value DWI can improve the accuracy of imaging staging of rectal cancer and has a role in distinguishing tumor foci and fibrous reactions after neoadjuvant chemoradiotherapy [33,34,35]. In rectal adenocarcinoma, although the component of necrosis and cysts can increase the diffusion, the solid component consisting of the tumor cells always causes diffusion to become restricted. Consequently, the region of tumor cells demonstrates much higher signal intensity than the rectal wall on DWI, which is helpful in detecting the tumor regions and facilitating more accurate delineation. Additionally, the regions of interest on DWI may better characterize tumors and include more biological information than T2WI because DWI has the capability to reflect the tumor microenvironment. Furthermore, the calibration and clinical applicability of the models were taken into consideration. The results showed the combined model demonstrated the best degree of calibration and clinical applicability. The results showed that the combined model can effectively predict LVI of rectal cancer in patients with the mrEMVI (-). DWI was helpful in discriminating the status of LVI in this study.
This study had some limitations. First, the sample size was not large enough. Large-sample multicenter studies are still needed. Second, patients who underwent preoperative neoadjuvant radiotherapy and chemotherapy were excluded. There will be selection bias in this cohort of patients.Third, because of the limited sample size, cases of vascular and lymphatic invasion were not separately divided into groups. Fourth, this was a retrospective study. Finally, the patients’ clinicopathological data was not included in the predictive model.
In conclusion, the radiomics model based on bipapametric MRI showed good preoperative predictive value for lymphovascular invasion of rectal cancer with the mrEMVI (-). Radiomics from bipapametric MRI has the potential to serve as a noninvasive and effective biomarker to predict the status of lymphovascular invasion in rectal cancer preoperatively.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the receiver operating characteristics curve
- CI:
-
Confidence interval
- DWI:
-
Diffusion-weighted imaging
- FOV:
-
Field of view
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size zone matrix
- GLDM:
-
Gray-level dependence matrix
- ICCs:
-
Intraclass correlation coefficients
- LASSO:
-
Least absolute shrinkage and selection operator
- LVI:
-
Lymphovascular invasion
- MRI:
-
Magnetic resonance imaging
- mRMR:
-
Minimum redundancy maximum relevance
- Radscore:
-
Radiomics score
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation T2WI:T2-weighted imaging
- TR:
-
Repetition time
- TE:
-
Echo time
- mrEMVI:
-
MRI-based extramural vascular invasion
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Franke AJ. Skelton WPt, George TJ, Iqbal A: A Comprehensive Review of Randomized Clinical Trials Shaping the Landscape of Rectal Cancer Therapy. Clin Colorectal Cancer. 2021;20(1):1–19.
Alawawdeh A, Krishnan T, Roy A, Karapetis C, Joshi R, Singhal N, Price T. Curative therapy for rectal cancer. Expert Rev Anticancer Ther. 2021;21(2):193–203.
Wilkinson N. Management of Rectal Cancer. Surg Clin North Am. 2020;100(3):615–28.
Zhang XY, Wang S, Li XT, Wang YP, Shi YJ, Wang L, Wu AW, Sun YS. MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology. 2018;289(3):677–85.
Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, Baek JY, Kim SY, Kim DY, Oh JH. Magnetic Resonance Imaging-Detected Extramural Venous Invasion in Rectal Cancer before and after Preoperative Chemoradiotherapy: Diagnostic Performance and Prognostic Significance. Eur Radiol. 2018;28(2):496–505.
Rönnow CF, Arthursson V, Toth E, Krarup PM, Syk I, Thorlacius H. Lymphovascular Infiltration, Not Depth of Invasion, is the Critical Risk Factor of Metastases in Early Colorectal Cancer: Retrospective Population-based Cohort Study on Prospectively Collected Data, Including Validation. Ann Surg. 2022;275(1):e148–54.
Horvat N, Petkovska I, Gollub MJ. MR Imaging of Rectal Cancer. Radiol Clin North Am. 2018;56(5):751–74.
Chandramohan A, Mittal R, Dsouza R, Yezzaji H, Eapen A, Simon B, John R, Singh A, Ram TS, Jesudason MR, et al. Prognostic significance of MR identified EMVI, tumour deposits, mesorectal nodes and pelvic side wall disease in locally advanced rectal cancer. Colorectal Dis. 2022;24(4):428-438.
Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(3):355–64.
Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19(2):132-146.
Choi YS, Ahn SS, Chang JH, Kang SG, Kim EH, Kim SH, Jain R, Lee SK. Machine learning and radiomic phenotyping of lower grade gliomas: improving survival prediction. Eur Radiol. 2020;30(7):3834–42.
Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–57.
Hou M, Sun JH. Emerging applications of radiomics in rectal cancer: State of the art and future perspectives. World J Gastroenterol. 2021;27(25):3802–14.
Akasu T, Iinuma G, Fujita T, Muramatsu Y, Tateishi U, Miyakawa K, Murakami T, Moriyama N. Thin-section MRI with a phased-array coil for preoperative evaluation of pelvic anatomy and tumor extent in patients with rectal cancer. AJR Am J Roentgenol. 2005;184(2):531–8.
Horvat N. Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ: MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39(2):367–87.
Moreno CC, Sullivan PS, Mittal PK. Rectal MRI for Cancer Staging and Surveillance. Gastroenterol Clin North Am. 2018;47(3):537–52.
Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts H. Computational Radiomics System to Decode the Radiographic Phenotype. Can Res. 2017;77(21):e104–7.
Shu Z, Mao D, Song Q, Xu Y, Pang P, Zhang Y. Multiparameter MRI-based radiomics for preoperative prediction of extramural venous invasion in rectal cancer. Eur Radiol. 2022;32(2):1002-1013.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275(3):772–82.
Kanda T, Matsuda M, Oba H, Toyoda K, Furui S. Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;277(3):924–5.
Gürses B, Böge M, Altınmakas E, Balık E. Multiparametric MRI in rectal cancer. Diagn Interv Radiol. 2019;25(3):175–82.
Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG. Rectal cancer: MR imaging in local staging–is gadolinium-based contrast material helpful? Radiology. 2005;234(1):179–88.
Curvo-Semedo L. Rectal Cancer: Staging. Magn Reson Imaging Clin N Am. 2020;28(1):105–15.
Huang J, Chen Y, Zhang Y, Xie J, Liang Y, Yuan W, Zhou T, Gao R, Wen R, Xia Y, et al. Comparison of clinical-computed tomography model with 2D and 3D radiomics models to predict occult peritoneal metastases in advanced gastric cancer. Abdom Radiol (NY). 2022;47(1):66–75.
Wan Q, Zhou J, Xia X, Hu J, Wang P, Peng Y, Zhang T, Sun J, Song Y, Yang G, et al. Diagnostic Performance of 2D and 3D T2WI-Based Radiomics Features With Machine Learning Algorithms to Distinguish Solid Solitary Pulmonary Lesion. Front Oncol. 2021;11:683587.
Zhang Y, He K, Guo Y, Liu X, Yang Q, Zhang C, Xie Y, Mu S, Guo Y, Fu Y, et al. A Novel Multimodal Radiomics Model for Preoperative Prediction of Lymphovascular Invasion in Rectal Cancer. Front Oncol. 2020;10:457.
Zhang K, Ren Y, Xu S, Lu W, Xie S, Qu J, Wang X, Shen B, Pang P, Cai X, et al. A clinical-radiomics model incorporating T2-weighted and diffusion-weighted magnetic resonance images predicts the existence of lymphovascular invasion / perineural invasion in patients with colorectal cancer. Med Phys. 2021;48(9):4872–82.
Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg. 2021;13(12):1567–83.
Horvat N, Hope TA, Pickhardt PJ, Petkovska I. Mucinous rectal cancer: concepts and imaging challenges. Abdom Radiol (NY). 2019;44(11):3569–80.
Schurink NW, Lambregts DMJ, Beets-Tan RGH. Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. Br J Radiol. 2019;92(1096):20180655.
Kalisz KR, Enzerra MD, Paspulati RM. MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy. Radiographics. 2019;39(2):538–56.
Lambregts D, Rao S, Sassen S, Martens M, Heijnen L, Buijsen J, Sosef M, Beets G, Vliegen R, Beets-Tan R. MRI and Diffusion-weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg. 2015;262(6):1034–9.
Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, Filip C, Haustermans K. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2012;82(2):863–70.
Acknowledgements
Not applicable.
Funding
This work was mainly supported by the program for Gusu Medical talent of Suzhou city(grant number GSWS2020009), the Translational Research Grant of NCRCH(grant number2020WSB06), National Natural Science Foundation of China (grant number 81671743), the clinical key diseases diagnosis and therapy special project of Health and Family Planning Commission of Suzhou (LCZX201801), the program for Advanced Talents within Six Industries of Jiangsu province (WSW-057), and the High-level Health Personnel “six-one” Project of Jiangsu province in China (LGY2016035).
Author information
Authors and Affiliations
Contributions
JM.N. ( Jianming Ni)and YG.L.(Yonggang Li) designed the design of the whole study. PF.T.(Pengfei Tong) and DQ.S. (Danqi Sun) performed image interpretation and segmentation. PF.T.(Pengfei Tong),DQ.S. (Danqi Sun) and GQ.C.(Guangqiang Chen) did the statistical analysis. PF.T. was a major contributor in writing the manuscript.Clinical-Pathological data were collected by PF.T.(Pengfei Tong),DQ.S. (Danqi Sun) and JM.N. ( Jianming Ni). JM.N. ( Jianming Ni)and YG.L. (Yonggang Li)contributed equally to this work and should be considered as co-corresponding authors. PF.T.(Pengfei Tong) and DQ.S. (Danqi Sun) contributed equeally to this paper and should be considered as co-first authors. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by Ethics Committee of the Affiliated Wuxi NO.2 People's Hospital of Nanjing Medical University,First Affiliated Hospital of Soochow University and the Second Affiliated Hospital of Soochow University.All methods were carried out in accordance with relevant guidelines and regulations.Informed consent was obtained from all participants or, if participants are under 18, from a parent and/or legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tong, P., Sun, D., Chen, G. et al. Biparametric magnetic resonance imaging-based radiomics features for prediction of lymphovascular invasion in rectal cancer. BMC Cancer 23, 61 (2023). https://doi.org/10.1186/s12885-023-10534-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10534-w