Abstract
Background
Lymphovascular invasion (LVI) is a factor correlated with a poor prognosis in oesophageal squamous cell carcinoma (ESCC). Lymphatic invasion (LI) and vascular invasion (VI) should be reported separately because they may indicate a difference in prognosis. The prognostic role of LI and VI in ESCC patients remains controversial. A meta-analysis was conducted to resolve this question.
Methods
We searched the PubMed, EMBASE, Web of Science, Scopus and Cochrane Library databases for studies on the association between LI and VI and the prognosis of patients with ESCC. The PICOs (Participant, Intervention, Comparison, Outcome) strategy were selected for the systematic review and meta-analysis. The effect size (ES) was the hazard ratio (HR) or relative ratio (RR) with 95% confidence intervals (CI) for overall survival (OS) and recurrence-free survival (RFS).
Results
A total of 27 studies with 5740 patients were included. We calculated the pooled results from univariate and multivariate analysis using the Cox proportional hazards method. The heterogeneity was acceptable in OS and RFS. According to the pooled results of multivariate analysis, both LI and VI were correlated with a worse OS. VI was a negative indicator for RFS, while the p value of VI was greater than 0.05. The prognostic role was weakened in subgroup analysis with studies using haematoxylin–eosin staining method.
Conclusions
Both LI and VI were indicators of a worse OS outcome. LI was a more significant indicator in predicting a worse RFS. More larger sample studies with immunohistochemical staining and good designs are required to detect the prognostic value of separate LI and VI in ESCC.
Similar content being viewed by others
Introduction
Oesophageal cancer (EC) morbidity is increasing worldwide and it is the seventh most common malignant cancer in the world [1]. The mortality from EC is the sixth leading cause of cancer-related death worldwide, with a low 5-year survival rate of EC patients ranging from 15%-34% [1, 2]. Oesophageal squamous cell carcinoma (ESCC) is one of the main types of EC. The most important prognostic factors are tumour characteristics (such as tumour size, tumour location, depth of invasion, differentiation) and whether there are any affected regional lymph nodes and/or metastatic sites (nonregional lymph nodes and organs outside the oesophagus) according to the 8th AJCC Cancer Staging Manual [3]. Our previous studies suggested that lymphovascular invasion, which was correlated with the ability of the cancer to metastasize, was associated with a poor prognosis in EC patients [4,5,6].
Lymphovascular invasion (LVI) refers to the presence of malignant cells within lymph vessels and/or vascular vessels. The distinction between lymphatic invasion and vascular invasion could be made by the presence of erythrocytes in the endothelial line and a thick vessel wall [7]. Lymphatic invasion and vascular invasion should be reported separately because they may indicate a difference in prognosis on the basis of the 8th AJCC Cancer Staging Manual [3]. However, studies about the prognostic role of LI and VI were controversial. Sarbia et al. reported that LI and VI were both poor prognostic indicator [8]. Waraich et al. reported that VI is not a prognostic indicator of recurrence [9]. Additionally, the prognosis of thoracic ESCC patients with both LI and VI was worse than that of patients with LI or VI alone [10]. However, there is no study with a large sample concentrating on the prognostic value of lymphatic invasion and vascular invasion in ESCC separately. Therefore, we conducted this meta-analysis and systematic review to evaluate the relationship between separate lymphatic invasion, vascular invasion and prognosis in ESCC patients.
Materials and methods
This meta-analysis and systematic review were carried out in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines [11].
Search strategy
PubMed, EMBASE, Web of Science, Scopus and Cochrane Library databases were searched for relevant studies published through Nov. 4, 2022. The combination of search bar was: ((((lymphovascular invasion OR lymph vessel invasion OR angiolymphatic invasion OR lymphatic invasion OR lymphangiogenesis OR venous invasion OR vascular invasion OR blood vessel invasion)) AND (esophageal cancer OR esophageal carcinoma)) AND (survival OR prognosis)). Only studies published in English were reviewed.
Selection criteria and data extraction
The PICOs (Participant, Intervention, Comparison, Outcome) strategy were adopted for the selection of studies. The preliminary screen was carried out by title and abstract on the basis of PICOs. Exclusion criteria included: (1) duplicate reports, letters, conference papers, and reviews, (2) studies that did not contain prognostic information, (3) oesophagogastric junction cancer (EJC), and (4) sample size less than 100 patients. Inclusion criteria: (1) the histology type of cancer was ESCC, (2) included articles published in English, (3) high quality of included studies and (4) studies included in this meta-analysis must provide sufficient survival data about LI and VI, and (5) patients must undergo operation. The prognostic indicator was hazard ratio (HR) or relative ratio (RR). The prognostic outcome was overall survival (OS) or relapse-free survival (RFS). The prognostic outcome was analysed with the Cox proportional hazards method. If studies were published on the same group of patients, the newest or the most informative article was selected. The K agreement between the reviewers is 0.76. The K agreement was based on measurement consistency assessment in the Cochrane handbook for systematic reviews of interventions [12].
Quality assessment of included studies
To ensure the quality of the included studies, the criteria derived from Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) were used to assess the included studies [13]. The selected criteria derived from REMARK guidelines were listed in Table 1.
Risk of bias appraisal
The risk of bias of included studies was assessed by the Cochrane Risk of Bias Assessment Tool [14]. This tool contains nine domains: representativeness of the exposed cohort; selection of the non-exposed cohort; ascertainment of exposure; outcome was not present at start of study; comparability of cohorts on the basis of the design or analysis (main and other confounding factors were not statistically different); assessment of outcome; enough follow-up; adequacy of follow up of cohorts. Each domain was scored as “high risk of bias”, “low risk of bias”, or “unclear risk of bias”. The plot of risk of bias was generated by Review Manger 5.3.
Data extraction
Potential articles were independently reviewed by 2 investigators (Wang A. and Tan Y.) against the above criteria. Disagreements were discussed and resolved by a third author (Wang S.). Data from the included studies were independently extracted by two authors (Wang A. and Tan Y.) using a standardized form. A third investigator (Wang S.) checked the collected data for accuracy. The following information was extracted: surname of the first author, years included, country, sample size of included studies, patients’ characteristics, stage information, staining method, number of LI and VI, survival statistics, statistical method and compliance to REMARK criteria.
Statistical analysis
Statistical analysis was performed using Stata/SE version 12.0 for Windows (Stata Corporation, College Station, TX, USA). A worse prognosis of ESCC was indicated by pooled HR value > 1. Cochran’s Q test (Chi-squared test; Chi2) and the I2 metric were performed to test the heterogeneity of the pooled results. An I2 value less than 25% indicated no heterogeneity; an I2 value between 25 and 50% suggested moderate heterogeneity; an I2 value between 50 and 75% suggested medium heterogeneity; and an I2 greater than 75% was considered extreme heterogeneity. We adopted a fixed-effects model (the Mantel–Haenszel method) when I2 < 50% with p > 0.05 in this meta-analysis. If not, we used a random-effects model. Subgroup analysis was used to explore heterogeneity when necessary. Begg’s test was used to assess publication bias. Two-tailed tests were adopted to calculate the p value, and p ≤ 0.05 was considered statistically significant.
Results
Characteristics of the studies
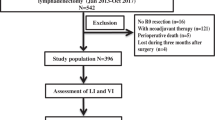
We retrieved 3363 articles after removing the duplicates. A total of 3130 articles were excluded after screening the titles and abstracts. We identified 233 potential articles for full-text review. Finally, 27 articles were eligible for this meta-analysis after 206 articles were excluded. The detailed information of the study inclusion process is listed in the flow chart (Fig. 1).
The remaining 27 articles, which included information on 5740 patients (range: 101–863), were included in the meta-analysis. All of the extracted information was listed in Table 2. A total of five studies adopted RR as the prognostic indicator [8, 15,16,17,18]. The prognostic indicator of the remaining studies was HR.
The risk of bias was assessed by the Cochrane Risk of Bias Assessment Tool. And the results were displayed in Fig. 2. The quality of included studies was elevated by REMARK guidelines. Thirteen studies met all the checklists. Checklist no.2 or no.3 could not be fulfilled in the rest studies.
The prognostic outcomes were OS and RFS. Both the multivariate and univariate Cox proportional hazards methods were used by all of the included studies. All pooled survival outcomes were calculated by multivariate and univariate Cox proportional hazards methods. The results are listed in Tables 3 and 4.
Overall survival
Eighteen studies containing 3989 ESCC patients provided multivariate HR. The pooled multivariate HRs with 95% CIs for LI and VI were 1.33 (1.17–1.50, p < 0.0001) and 1.29 (1.16–1.45, p < 0.0001), respectively. The I2 for LI and VI was 4.1% (Chi2 = 17.72, p = 0.407) and 24.5% (Chi2 = 22.5, p = 0.166), respectively. Thirteen studies concerning 2465 patients provided univariate HR. The pooled univariate HRs with 95% CIs for LI and VI were 2.08 (1.81–2.38, p < 0.0001) and 1.80 (1.60–2.02, p < 0.0001), respectively. The I2 for LI and VI was 46.5% (Chi2 = 22.43, p = 0.033) and 18.4% (Chi2 = 14.70, p = 0.258), respectively. The pooled results are shown in Fig. 3.
Recurrence-free survival
Eight studies containing 1666 ESCC patients provided multivariate HR. The pooled multivariate HRs with 95% CIs for LI and VI were 1.71 (1.24–2.36, p = 0.001) and 1.28 (1.07–1.52, p = 0.07), respectively. The I2 for LI and VI was 50.5% (Chi2 = 14.15, p = 0.049) and 0% (Chi2 = 4.82, p = 0.682), respectively. Seven studies concerning 1270 patients provided univariate HR. The pooled univariate HRs with 95% CIs for LI and VI were 2.62 (1.81–3.78, p < 0.0001) and 1.91 (1.53–2.38, p < 0.0001), respectively. The I2 for LI and VI was 56.4% (Chi2 = 13.75, p = 0.033) and 0% (Chi2 = 3.09, p = 0.798), respectively. The pooled results are listed in Fig. 4.
Publication bias
Begg’s test was used to test the publication bias of the included studies. In pooled multivariate OS, the p values of publication bias for LI and VI were 0.017 and 0.596, respectively. In pooled univariate OS, the p values of publication bias for LI and VI were 0.127 and 0.583, respectively. In pooled multivariate RFS, the p values of publication bias for LI and VI were 0.063 and 1, respectively. In pooled univariate RFS, the p values of publication bias for LI and VI were 0.035 and 0.368, respectively.
Subgroup analysis of studies with haematoxylin–eosin (H&E) staining method
LI and VI were still poor prognostic parameter to OS in pooled multivariate and pooled univariate results. LI and VI were only poor indicator in RFS in pooled univariate analysis, not in pooled multivariate analysis. Detailed information was displayed in Supplementary Fig. 1, Supplementary Fig. 2, Supplementary Table 1 and Supplementary Table 2.
Discussion
Our meta-analysis is the first study to explore the separate prognostic value of LI and VI in ESCC. A total of 27 articles with 5740 patients were enrolled. According to the pooled results of univariate analysis, distinguishing between the prognostic value of LI and VI for OS or RFS is difficult. VI and LI were both associated with adverse OS and RFS. The pooled multivariate results were more convincing than the pooled univariate results. Therefore, we focused on the pooled results from the multivariate analysis. The level of heterogeneity was acceptable for the pooled multivariate results. Both LI and VI were prognostic indicators of a poor OS. For RFS, the p value of VI was greater than 0.05. Therefore, we considered LI as a better predictor of a worse RFS than VI.
Many studies have focused on the relationship between LVI and prognosis in EC patients. The prognostic value of LVI in oesophageal cancer patients, N0 oesophageal cancer patients and superficial oesophageal cancer has been proven by our previous meta-analysis and many studies [4,5,6, 41,42,43,44,45,46,47,48]. Given the possible difference between LI and VI in prognosis, LI should be reported separately from VI according to the 8th AJCC Cancer Staging Manual [3]. An increasing number of studies have emphasized the prognostic value of LI and VI in EC patients. Zhang and his colleagues reported that the prognosis of patients with simultaneous LI and VI was worse than that of patients with LI or VI alone for thoracic ESCC. The 5-year overall survival of LI or VI alone, and simultaneous LI and VI was 54.5% and 33.9% respectively [10]. Tachezy et al. reported that LI was an independent prognostic indicator for both ESCC and adenocarcinoma patients, while VI was a significant factor for adenocarcinoma only [49]. The prognostic role of VI in EC patients is still controversial. Waraich et al. reported that VI was not a risk factor for oesophageal cancer recurrence [9]. Jia’s research suggested that VI was a favourable prognostic parameter in ESCC [50] Moreover, the prognostic value of LI and VI in N0 oesophageal carcinoma patients was not consistent among studies [7, 9, 49, 51, 52]. Due to the uncertain prognostic value of VI and LI in EC patients, a meta-analysis was essential to clarify the problem.
In the current meta-analysis, LI was an indicator of a poor OS and RFS; however, VI was a poor indicator only for OS. How to interpret such results? It could well be due to a type-2 error that vascular invasion did not show a statistical significance with RFS in the current study. Additionally, LI has a more significant role in prognosis in the early stages, such as stage I and stage II oesophageal cancer, than VI. However, the prognostic role of VI is more significant than LI in stage III oesophageal cancer [43]. The pathological information of LI and VI was acquired from surgical specimen. The majority of patients in the enrolled studies were in early stage (stage I and stage II). This may be the reason why LI was more significant than VI in our meta-analysis.
The distinction of blood vessels from lymphatic vessels is made by the presence of erythrocytes in the endothelial line and thick vessel walls. However, the distinction between LI and VI is not obvious by haematoxylin–eosin (H&E) staining [7]. The positive rate of LI, VI or LVI staining by immunohistochemistry (IHC) is higher than that of the H&E staining method in the same group of patients [7, 53, 54]. The IHC method of staining the vascular endothelium (CD34) and the lymphatic endothelium (podoplanin) could increase the possibility of distinguishing lymphatic and vascular invasion [53, 55]. Due to limited number of studies with IHC staining method, we conducted a subgroup analysis of studies with H&E staining method. Pooled results of RFS indicated that the prognostic role of LI and VI was weakened. LI and VI were only poor indicator in RFS in pooled univariate analysis, not in pooled multivariate analysis. This may be explained by the fact that HE staining method is not as specific or sensitive as IHC staining method. However, only three of included papers used IHC staining method and many of the included studies did not provide the staining method. We advocate that IHC staining method should be used when LI and VI need to be separated in future studies.
There were also some limitations of this meta-analysis. First, the studies included were restricted to papers published in English. This may lead to some potential bias. Actually, the p value was less than 0.05 for the publication bias of multivariate LI. Second, tumour stage and staining methods that could lead to different positive rates of LI and VI were not evaluated by the same method among the included studies. Studies investigating the prognostic value of LI and VI should apply the IHC method. Third, I2 was all < 50% which indicated moderate heterogeneity except the I2 of RFS in pooled results of univariate analysis. The heterogeneity among studies should be noticed although they were acceptable. The heterogeneity could influence the credibility of the results. It must be resolved appropriately when the heterogeneity is medium and extreme. Last but not least, the present study only focused on ESCC, therefore the results should be interpreted with caution in western countries.
Conclusions
We hold the opinion that LI and VI are indicators of poor OS in ESCC patients. LI predicts a worse RFS in ESCC patients. Compared to VI, LI is a more significant indicator of a worse RFS. More large-sample studies with immunohistochemical staining and good designs are required to detect the prognostic value of separate LI and VI in ESCC.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Shah MA. Future directions in improving outcomes for patients with gastric and esophageal cancer. Hematol Oncol Clin North Am. 2017;31(3):545–52.
Amin MBES, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, et al. AJCC Cancer Staging Manual. 8th ed. Chicago: Springer; 2016.
Yang J, Lu Z, Li L, Li Y, Tan Y, Zhang D, et al. Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer. 2020;20(1):176.
Wang A, Tan Y, Zhang Y, Xu D, Fang Y, Chen X, et al. The prognostic role of angiolymphatic invasion in N0 esophageal carcinoma: a meta-analysis and systematic review. J Thorac Dis. 2019;11(8):3276–83.
Wang A, Tan Y, Geng X, Chen X, Wang S. Lymphovascular Invasion as a Poor Prognostic Indicator in Thoracic Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Dis Esophagus. 2018;32:1–8.
Imamura Y, Watanabe M, Nagai Y, Baba Y, Hirashima K, Karashima R, et al. Lymphatic vessel invasion detected by the D2–40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinoma. J Surg Oncol. 2012;105(3):277–83.
Sarbia M, Porschen R, Borchard F, Horstmann O, Willers R, Gabbert HE. Incidence and prognostic significance of vascular and neural invasion in squamous cell carcinomas of the esophagus. Int J Cancer. 1995;61(3):333–6.
Waraich N, Rashid F, Jan A, Semararo D, Deb R, Leeder PC, et al. Vascular invasion is not a risk factor in oesophageal cancer recurrence. Int J Surg. 2011;9(3):237–40.
Zhang H, Chen X, Wang S, Fan J, Lu L. Poorer prognosis associated with simultaneous lymphatic and vascular invasion in patients with squamous carcinoma of the thoracic oesophagus. Eur J Cardiothorac Surg. 2017;52(2):378–84.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. 2009;6(7):e1000097.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:142.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–72.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Nakashima Y, Yoshinaga K, Kitao H, Ando K, Kimura Y, Saeki H, et al. Podoplanin is expressed at the invasive front of esophageal squamous cell carcinomas and is involved in collective cell invasion. Cancer Sci. 2013;104(12):1718–25.
Ren HZ, Wang JS, Wang P, Pan GQ, Wen JF, Fu H, et al. Increased expression of prohibitin and its relationship with poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2010;16(4):515–22.
Osugi H, Takemura M, Takada N, Hirohashi K, Kinoshita H, Higashino M. Prognostic factors after oesophagectomy and extended lymphadenectomy for squamous oesophageal cancer. Br J Surg. 2002;89(7):909–13.
Ma Y, Yao X, Li Z, Chen J, Li W, Wang H, et al. The role of vascular invasion and lymphatic invasion in predicting recurrent thoracic oesophageal squamous cell carcinoma. World J Surg Oncol. 2022;20(1):12.
Toriumi T, Yagi K, Ri M, Yajima S, Okumura Y, Aikou S, et al. Lymphatic invasion is a prognostic factor of pathological N0 esophageal squamous cell carcinoma. Dis Esophagus. 2022;35(7):doab087.
Kitamura Y, Oshikiri T, Takiguchi G, Urakawa N, Hasegawa H, Yamamoto M, et al. Impact of lymph node ratio on survival outcome in esophageal squamous cell carcinoma after minimally invasive esophagectomy. Ann Surg Oncol. 2021;28(8):4519–28.
Baba H, Kanda M, Sato Y, Sawaki K, Shimizu D, Koike M, et al. Expression and malignant potential of B4galnt4 in esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27(9):3247–56.
Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K, et al. Prognostic impact of lymphovascular invasion in lymph node-negative superficial esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(11):doz001.
Arigami T, Uchikado Y, Omoto I, Sasaki K, Kita Y, Owaki T, et al. Primary tumor score based on tumor depth and length predicts prognosis in esophageal squamous cell carcinoma. Anticancer Res. 2018;38(9):5447–52.
Nakamura K, Yoshida N, Baba Y, Kosumi K, Uchihara T, Kiyozumi Y, et al. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol. 2017;22(3):469–75.
Okada N, Fujii S, Fujita T, Kanamori J, Kojima T, Hayashi R, et al. The prognostic significance of the positive circumferential resection margin in pathologic T3 squamous cell carcinoma of the esophagus with or without neoadjuvant chemotherapy. Surgery. 2016;159(2):441–50.
Han L, Jia Y, Song Q, Wang N, Wang J, Bai B, et al. Prognostic significance of preoperative absolute peripheral monocyte count in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(7):740–6.
Takata A, Takiguchi S, Okada K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Expression of insulin-like growth factor-ii mrna-binding protein-3 as a marker for predicting clinical outcome in patients with esophageal squamous cell carcinoma. Oncol Lett. 2014;8(5):2027–31.
Takeno S, Takahashi Y, Hashimoto T, Maki K, Shibata R, Yamana I, et al. Is the prognostic impact of tumor location in patients with surgically resected esophageal squamous cell carcinoma affected by surgical approach? Eur Surg Res. 2013;51(1–2):91–8.
Ichikawa H, Miyata G, Miyazaki S, Onodera K, Kamei T, Hoshida T, et al. Esophagectomy using a thoracoscopic approach with an open laparotomic or hand-assisted laparoscopic abdominal stage for esophageal cancer: analysis of survival and prognostic factors in 315 patients. Ann Surg. 2013;257(5):873–85.
Kim HS, Won KY, Kim GY, Kim SC, Park YK, Kim YW. Reduced expression of Raf-1 kinase inhibitory protein predicts regional lymph node metastasis and shorter survival in esophageal squamous cell carcinoma. Pathol Res Pract. 2012;208(5):292–9.
Suzuki S, Miyazaki T, Tanaka N, Sakai M, Sano A, Inose T, et al. Prognostic significance of Cd151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness. Ann Surg Oncol. 2011;18(3):888–93.
Tateno T, Ueno S, Hiwatashi K, Matsumoto M, Okumura H, Setoyama T, et al. Expression of receptor for advanced glycation end products (Rage) is related to prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(2):440–6.
Sano A, Kato H, Sakurai S, Sakai M, Tanaka N, Inose T, et al. Cd24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(2):506–14.
Komatsu S, Imoto I, Tsuda H, Kozaki KI, Muramatsu T, Shimada Y, et al. Overexpression of Smyd2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis. 2009;30(7):1139–46.
Tsujitani S, Saito H, Oka S, Sakamoto T, Kanaji S, Tatebe S, et al. Prognostic significance of Rcas1 expression in relation to the infiltration of dendritic cells and lymphocytes in patients with esophageal carcinoma. Dig Dis Sci. 2007;52(2):549–54.
Natsugoe S, Uchikado Y, Okumura H, Matsumoto M, Setoyama T, Tamotsu K, et al. Snail plays a key role in E-Cadherin-Preserved esophageal squamous cell carcinoma. Oncol Rep. 2007;17(3):517–23.
Dhar DK, Hattori S, Tonomoto Y, Shimoda T, Kato H, Tachibana M, et al. Appraisal of a revised lymph node classification system for esophageal squamous cell cancer. Ann Thorac Surg. 2007;83(4):1265–72.
Takahashi Y, Noguchi T, Takeno S, Kimura Y, Okubo M, Kawahara K. Reduced expression of P63 has prognostic implications for patients with esophageal squamous cell carcinoma. Oncol Rep. 2006;15(2):323–8.
Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, et al. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94(4):929–33.
Tachibana M, Tabara H, Kotoh T, Kinugasa S, Dhar DK, Hishikawa Y, et al. Prognostic significance of perioperative blood transfusions in resectable thoracic esophageal cancer. Am J Gastroenterol. 1999;94(3):757–65.
Nakade H, Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Miyao S, et al. Overexpression of cullin4a correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Int J Clin Oncol. 2020;25(3):446–55.
Gu YM, Yang YS, Hu WP, Wang WP, Yuan Y, Chen LQ. Prognostic value of lymphovascular invasion in patients with esophageal squamous cell carcinoma. Ann Transl Med. 2019;7(12):256.
Wang S, Chen X, Fan J, Lu L. Prognostic significance of lymphovascular invasion for thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23(12):4101–9.
Schoppmann SF, Jesch B, Zacherl J, Riegler MF, Friedrich J, Birner P. Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. 2013;153(4):526–34.
Huang Q, Luo K, Chen C, Wang G, Jin J, Kong M, et al. Identification and validation of lymphovascular invasion as a prognostic and staging factor in node-negative esophageal squamous cell carcinoma. J Thorac Oncol. 2016;11(4):583–92.
Vazquez-Sequeiros E, Wang L, Burgart L, Harmsen W, Zinsmeister A, Allen M, et al. Occult lymph node metastases as a predictor of tumor relapse in patients with node-negative esophageal carcinoma. Gastroenterology. 2002;122(7):1815–21.
Yamashina T, Ishihara R, Nagai K, Matsuura N, Matsui F, Ito T, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108(4):544–51.
Xue LY, Qin XM, Liu Y, Liang J, Lin H, Xue XM, et al. Clinicopathological parameters predicting recurrence of Pt1n0 esophageal squamous cell carcinoma. World J Gastroenterol. 2018;24(45):5154–66.
Tachezy M, Tiebel AK, Gebauer F, Kutup A, Tharun L, Pantel K, et al. Prognostic impact of perineural, blood and lymph vessel invasion for esophageal cancer. Histol Histopathol. 2014;29(11):1467–75.
Jia W, Wang W, Ji CS, Niu JY, Lv YJ, Zhou HC, et al. Coexpression of periostin and egfr in patients with esophageal squamous cell carcinoma and their prognostic significance. Onco Targets Ther. 2016;9:5133–42.
Jeon JH, Lee JM, Moon DH, Yang HC, Kim MS, Lee GK, et al. Prognostic significance of venous invasion and maximum standardized uptake value of (18)F-Fdg Pet/Ct in surgically resected T1n0 esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43(2):471–7.
Zhu CM, Ling YH, Xi SY, Luo RZ, Chen JW, Yun JP, et al. Prognostic significance of the Pn classification supplemented by vascular invasion for esophageal squamous cell carcinoma. PLoS One. 2014;9(4):e96129.
Perry C, Soomro I, Kaye P, Hardy E, Parsons SL, Ragunath K, et al. Analysis of lymphatic and blood vessel invasion biomarkers in T1 esophagogastric adenocarcinomas for improved patient prognostication. Dis Esophagus. 2015;28(3):262–8.
Mitobe J, Ikegami M, Urashima M, Takahashi H, Goda K, Tajiri H. Clinicopathological investigation of lymph node metastasis predictors in superficial esophageal squamous cell carcinoma with a focus on evaluation of lympho-vascular invasion. Scand J Gastroenterol. 2013;48(10):1173–82.
Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, et al. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol. 2011;24(6):774–85.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: S Wang; (II) Administrative support: X Chen; (III) Search of literature: A Wang, Y Tan; (IV) Collection and assembly of data: A Wang, Y Tan, S Wang; (V) Data analysis and interpretation: A Wang, S Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Forrest plot showing the pooled HR for OS, (A) LI from multivariate analysis, (B) VI from multivariate analysis, (C) LI from univariate analysis, (D) VI from univariate analysis.
Additional file 2: Supplementary Figure 2.
Forrest plot showing the pooled HR for RFS, (A) LI from multivariate analysis, (B) VI from multivariate analysis, (C) LI from univariate analysis, (D) VI from univariate analysis.
Additional file 3: Supplementary Table 1.
Pooled results of multivariate analysis. Supplementary Table 2. Pooled results of univariate analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, A., Tan, Y., Wang, S. et al. The prognostic value of separate lymphatic invasion and vascular invasion in oesophageal squamous cell carcinoma: a meta-analysis and systematic review. BMC Cancer 22, 1329 (2022). https://doi.org/10.1186/s12885-022-10441-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10441-6