Abstract
Background
Most patients with cancer and their caregivers desire honest, clear prognostic communication, yet oncologists often disclose prognosis inconsistently. Prognostic communication becomes even more challenging when disease progression is unclear or equivocal. Presently, oncologist approaches for discussing uncertain disease findings are poorly understood.
Methods
In this prospective, longitudinal study, we audio-recorded serial disease reevaluation conversations between children with high-risk cancer, their families, and their primary oncologists over 24 months and conducted content analysis at recorded timepoints when oncologists categorized disease progression as equivocal.
Results
Of the 265 medical discussions recorded across the illness course for 33 patient-parent dyads, a total of 40 recorded discussions took place at equivocal timepoints, comprising > 500 min of medical dialogue. Prognosis talk encompassed < 3% of dialogue and was absent in nearly half of equivocal discussions (17/40, 42.5%). Curability statements were identified in only two conversations. Inductive content analysis of dialogue revealed four distinct patterns for communicating equivocal disease status: (1) up-front reassurance, (2) softening the message, (3) describing possible disease progression without interpretation, (4) expressing uncertainty without discussing the bigger picture.
Conclusion
Oncologists rarely discuss prognosis with children with high-risk cancer and their families at timepoints when disease progression is not definitive. Formal guidance is needed to better support oncologists in navigating uncertainty while sharing honest, person- and family-centered information about prognosis.
Similar content being viewed by others
Background
Most patients with cancer and their families want to receive honest communication about prognosis from their medical team, including truthful disclosure about poor prognosis. [1,2,3] Sharing prognostic information, however, is rarely straightforward, and evidence suggests that oncologists struggle to discuss prognosis directly, often veiling prognostic information in vague language or avoiding prognostic disclosure altogether. [4,5,6] Individual preferences and cultural differences also influence the ways that patients, families, or clinicians wish for prognostic information to be shared, [7,8,9,10,11,12] adding further complexity to already challenging terrain.
Navigating communication about prognosis becomes even more difficult in the setting of uncertainty. Medical professionals often struggle to discuss prognosis directly when the outcome is not definite. [13,14,15] In analyses of physician-patient encounters, medical oncologists rarely discussed prognostic uncertainty. [15,16,17] When simply reviewing hypothetical patient vignettes, most oncologists felt comfortable telling the patient about an incurable disease, yet fewer were willing to disclose uncertainty regarding life expectancy. [18].
Over the past decade, however, dexterity in navigating prognostic uncertainty has become increasingly integral to provision of cancer care. In spite, or perhaps because, of increasingly sophisticated diagnostics and therapeutics, uncertainty with anticipating outcomes for patients with high-risk cancer is common. [19] In pediatric oncology, in particular, predicting outcomes for children with rare cancers treated with novel therapeutics is challenging, [20, 21] and communication approaches for navigating this uncertain space remain poorly understood.
The U-CHAT (Understanding Communication in Healthcare to Achieve Trust) trial was designed to identify and describe strategies used by pediatric oncologists to communicate prognostic information with patients and families across advancing illness. In this analysis, we focused on disease reevaluation conversations between pediatric oncologists, patients with high-risk cancer, and their parents, which oncologists categorized as “equivocal,” meaning data were ambiguous and difficult for the oncologist to characterize as either “good news” or “bad news.” Through this targeted analysis, we aimed to (1) quantify the frequency of prognostic communication in the setting of equivocal disease status for children with high-risk cancer and (2) identify thematic patterns in oncologist approaches for navigating prognostic information when disease progression is ambiguous.
Methods
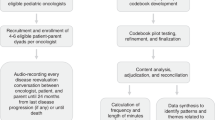
An interdisciplinary team of pediatric oncology and hospice and palliative medicine experts collaborated with the St. Jude Children’s Hospice Bereaved Parent Steering Council to develop the U-CHAT trial. The protocol was approved by the St. Jude Children’s Hospital Institutional Review Board (U-CHAT [Pro00006473]; approval date: July 12, 2016. Data were collected between 2016 and 2020. We present study methods and findings following the Consolidated Criteria for Reporting Qualitative Research (COREQ) reporting guideline and checklist (Supplemental Table 1). [22] Data included in this analysis included recorded disease reevaluation conversations between oncologists and patients’ parents, as well as surveys and recorded interviews of oncologists following those conversations.
Participant Enrollment and Data Collection.
Detailed eligibility criteria, enrollment, and informed consent processes were published previously [6, 23,24,25] and are summarized in Table 1. Briefly, patients with high-risk solid tumor cancers and their families were identified by the research team and approached if their primary oncologist estimated survival as ≤ 50% and expected the patient to have ≥ 2 future disease reevaluation timepoints. Following a standardized informed consent process, patient-parent dyads were enrolled on study and followed longitudinally for 24 months from last disease progression or until death, whichever occurred first. All medical discussions where the oncologist planned to disclose findings from disease reevaluation studies (e.g., laboratory tests, imaging, pathology, etc.) were audio-recorded serially. Conversations were recorded in the clinic or hospital setting, as well as rarely via telephone if patients/families were unable to come to the hospital to discuss disease reevaluation findings. Following each discussion, the recorded conversation was categorized by the primary oncologist as “good news” (i.e., no evidence of disease progression), “bad news” (i.e., clear evidence of disease progression), or “equivocal news” (i.e., ambiguous, unclear findings; unable to definitively describe as good or bad news).
In addition to collecting recorded dialogue, following any “bad news” disease reevaluation discussions, oncologists and parents participated in surveys and audio-recorded semi-structured interviews conducted by research team members trained in qualitative interviewing (CW, EK), using prompts read-aloud to participants that had been pilot tested previously. Interview duration averaged 20 min (range 5 min to > 2 h, dictated by participant preference). Both surveys and interviews included a validated question previously tested in this population: “How likely do you think it is that your child [or patient] will be cured of cancer?” [26,27,28,29] Data about patient demographics and illness course were abstracted from the electronic medical record using a standardized tool, and interviewers wrote memos following interviews.
Codebook Development, Coding, Adjudication, and Analysis.
A team of pediatric oncology and palliative medicine clinicians and researchers (Supplemental Table 2) reviewed the literature and found limited frameworks to conceptualize prognostic communication in pediatric cancer. Building upon adult oncology communication standards, [30, 31] the American Society of Clinical Oncology’s communication consensus guidelines, [32] and the Prognostic and Treatment choices scale, [33] the team developed an a priori codebook to explore prognostic communication between oncologists, children with high-risk cancer, and their families across evolving illness. The codebook categorized prognostic communication into six language domains: prognostic uncertainty, assessing prognostic understanding, disease changing for the worse, best- and worst-case scenarios, survival time, and curability. Codes and definitions are presented in Supplemental Table 3.
To ensure consistency in code application, qualitative analysts (CW, MS, SV, EK) independently pilot-tested the codebook across a series of medical dialogue recordings until consensus was reached. The research team (CW, MS, SV, JB, EK) met to reconcile variances and achieve consensus, modifying the codebook as needed to improve dependability, confirmability, and credibility of independent codes. [34] The codebook was finalized following deep review of sufficient raw data to reach saturation, with no new concepts emerging from transcripts. [35].
Content analysis was conducted per COREQ guidelines, [22] using MAXQDA to organize data (VERBI Software, 2020). [36] Coding was performed by four analysts with training in and experience with content analysis (AP, CW, MS, SV), with each recording coded by at least two independent coders. The research team held weekly meetings for review of coding variances and third-party adjudication to reach consensus. Consistency in code segmentation also was reviewed to ensure a standardized approach.
To maximize opportunities for examination of prognostic communication in the context of uncertainty, this analysis focused on recorded disease reevaluation discussions categorized by oncologists as “equivocal.” Across equivocal discussions, code frequency, temporal duration, and distribution were analyzed and reported as descriptive statistics (AP, CW, EK). Iterative review and serial memo writing of coded dialogue [37] (AP, EK) informed the development of inductive themes describing the communication approaches used by oncologists to navigate discussion about unclear disease status.
Results
A total of 265 medical discussions were recorded across the illness course for 33 patient-parent dyads, comprising more than 4,000 min of recorded dialogue. Data on patient-parent dyads who declined enrollment in U-CHAT were previously published; [6, 25] briefly, 17% of approached dyads (n = 7 dyads) did not enroll due to hesitation or refusal by the patient (n = 3), parent (n = 3), or both (n = 1). Refusal rates did not appear to disproportionately exclude dyads based on race or ethnicity, [6, 25] although small numbers precluded formal scrutiny.
More than half of participating dyads experienced one or more equivocal disease reevaluation timepoints during the study period (17/33, 51.5%); of these, about half (9/17) had more than one equivocal discussion (mean 3.6 equivocal discussions per dyad, range 2–9). Approximately 15% of recorded conversations (40/265) and 12.5% of total dialogue time (510/4,050 min) took place at timepoints with equivocal disease reevaluation findings, comprising > 500 min of medical dialogue and making 40 the denominator for this analysis. All participating oncologists (n = 6) presented equivocal findings to patients and families in at least one disease reevaluation discussion.
Of the dyads involved in equivocal discussions, most were white (15/17, 88.2%), and gender was roughly equivalently divided. Adolescents and young adults (aged ≥ 12 years) comprised more than half of patient participants (9/17, 52.9%). Full participant demographic variables are presented in Table 2. No participants formally dropped out of the study, although one dyad transferred care to another institution prior to death. Most equivocal discussions (34/40, 85%) were followed by disease progression within the 24-month study duration. Among the 17 dyads who experienced at least one equivocal discussion, 13 patients had disease progression, and at the time of publication of this paper, all 13 had died.
Frequency of prognostic communication in equivocal disease reevaluation discussions.
Frequencies and time duration of dialogue coded as prognostic communication (prognostic uncertainty, assessing prognostic understanding, disease changing for the worse, best- and worst-case scenarios, survival time, curability) are presented in Table 3, with representative quotes for each code presented in Table 4. Prognostic communication codes were applied 80 times across 40 equivocal discussions (median 1 code per recorded conversation, range 0–13), totaling < 14 min of prognosis discussion over 510 min of total dialogue time, or 2.9% of total minutes of recorded conversation. Given that this analysis targeted equivocal discussions, it was unsurprising that the most dominant code identified was “prognostic uncertainty” (Table 5). Although oncologists labeled conversations as “equivocal news” rather than “bad news,” the code for “disease changing for the worse” was the code that constituted the most recorded dialogue time across all recordings. Most dialogue coded as “disease changing for the worse” described specific disease reevaluation findings consistent with minimal disease progression within a “big picture” setting that was described as unclear or equivocal.
Prognostic communication dialogue was present in just over half of recorded equivocal discussions (23/40, 57.5%), and when codes were analyzed individually, each code was found in < 50% of recordings: “prognostic uncertainty” 47.5% (19/40), “disease changing for the worse” 42.5% (17/40), and “assessing prognostic understanding” 5% (2/40). Fewer than 10% of recorded equivocal conversations included dialogue addressing whether the cancer could be cured: “best- and worst-case scenarios” was identified in 10% of conversations (4/40), “curability” in 5% (2/40), and no discussions included “survival time” codes. Across all equivocal discussions, the “curability” code was applied a total of twice and the “assessing prognostic understanding” applied a total of four times. When the latter code was applied, the depth and focus with which prognostic understanding was explored was limited (Table 4), representing a cursory assessment of patients’ and families’ awareness of prognosis.
Oncologist communication patterns in settings of uncertainty.
Inductive content analysis of prognostic communication dialogue revealed four thematic patterns for how oncologists shared prognostic information when disease reevaluation findings were worrisome yet lacked evidence of frank disease progression (Table 5).
Up-front reassurance: Although oncologists categorized these discussions as “equivocal” to the research team, when talking with patients and families, they often led with reassurance about the uncertain findings. For example, oncologists frequently opened conversations with a positive statement to offer relief for the waiting family:
“So, everything looks stable on scans, okay. I don’t have the bone marrow test back, but his [labs] are normal. So, I think everything’s where we were a month ago in terms of scans.”
One oncologist opened the conversation with “good” news despite privately classifying the findings as “equivocal”: “So, I know you just want to hear about scans, so we are going to start talking about that first. Everything is stable, and there is nothing new. So, that’s good.” That oncologist went on to relativize the positive framing as good but not “the best”:
I wish that I could say – I mean, the best thing would be if I came in and said everything is gone. So, I don’t want to pretend like that wouldn’t be the best news – that would be the best news.
Softening the message: While conveying equivocal findings, oncologists softened the message of possible disease progression by using minimizing modifiers to downgrade worry. For example, one oncologist said:
The CT of the chest shows a very, very small little nodule which is about 2 mm on the left lung. That maybe just a little blood vessel within the lungs…so what we need to do is just follow that.
Oncologists also used emphatic language (“they definitely don’t” and “so tiny”) to minimize the weight of uncertain data:
These little things, I’m not even sure what they are. I’ll show you the pictures – they definitely don’t light up at all, but they are so tiny, and the radiologist doesn’t even know what to say about them either.
Describing possible disease progression without interpretation: Many oncologists described disease reevaluation findings (e.g., laboratory tests, imaging, pathology, etc.) in detail but did not interpret how the findings may impact prognosis and curability. For example, oncologists pointed out new lesions (“So there is one little spot in your clavicle, which is a fancy word for your collar bone, that is bright…”) or increases in lesion size (“The one over here is a little bit more elongated than it was before but not by a huge extent”) often without connecting these findings to the bigger picture or explaining what the lesions could mean for the patient’s future life.
Expressing uncertainty without discussing the bigger picture: Oncologists offered statements of uncertainty without expressing concerns about the possibility of disease progression or anchoring the moment of uncertainty in the context of a prior high-risk diagnosis. In this approach, language like “I just don’t know” or “I just can’t know” were often used. At times, oncologists expressed their hesitation frankly: “I certainly don’t feel 100% confident, like, I don’t want to say this is [disease] because I don’t know that.” Similarly, another oncologist used the phrase “not entirely sure” repeatedly in interpreting findings:
It looks maybe a collection of fluid…We aren’t entirely sure what that is or why it’s there, but it doesn’t really look like tumor either, so we are not entirely sure what to make of that other than we know that you’re doing well.
Co-occurrence of patterns: The “describing possible disease progression without interpretation” pattern frequently occurred concurrently with “softening the message” or “expressing uncertainty without discussing the bigger picture” patterns. Specifically, when oncologists focused on describing findings in detail, they used modifiers to minimize concern or emphasized inability to confirm bad news:
That’s the one we have been following, and when we look at that one…the difference is a couple of millimeters. Um, so it’s not - I can’t say that it has decreased in size, but it has not gotten bigger to a degree that I could say that this is clearly, you know, something that is blowing up and progressing.
While oncologists rarely voiced concerns about disease progression during recorded equivocal discussions, data from surveys and interviews showed that oncologists generally believed that their patients’ disease would progress and likely be incurable for most patients. Specifically, all 6 participating oncologists completed surveys and interviews following disease progression for all 13 patients who progressed while on study; for each of these patients, the oncologist estimated odds of cure to be very low or zero. In response the question: “How likely do you think it is that your patient/child will be cured of cancer?,” oncologists offered a range of similar responses: “Nearly impossible, but we can hope;” “I do not think she will be cured unfortunately;” “I would still say less than 10%, but we would always love to be proven wrong;” and “I do not think she’ll be cured…less than 5%.” One oncologist explored the complexity of interpreting disease reevaluation data and the challenge of sampling error when responding to this question:
Zero, nothing. We barely got her to transplant…She never cleared her marrow, and the last marrow, by a miracle, it came back negative. I think it was just sampling error. I think there was always disease there.
Another oncologist alluded to the inevitability of disease spread even without visible evidence on imaging:
I think it’s unlikely he’ll have long-term cure. I think he might have a period of disease- free, as best we can tell in terms of pictures. Obviously, you know, if he has disease in his lungs, he probably has micro-mets that we can’t see…
Discussion
Pediatric oncologists often face prognostic uncertainty, particularly when interpreting indefinite or equivocal findings. In this qualitative study, equivocal conversations occurred relatively often: more than half of patient-parent dyads experienced one or more equivocal conversations, and all oncologists participated in discussions about equivocal findings. The prevalence of this experience suggests the need for oncologists to receive training and be prepared to navigate communication about disease status in the setting of uncertainty.
Notably, all patients in this study were considered “high-risk,” with their primary oncologist estimating survival at ≤ 50% to qualify patients for enrollment. Despite patients’ high-risk status, little discussion of prognosis occurred during equivocal timepoints where disease progression was suspected but not definitive. These findings corroborate prior exploratory work suggesting that opportunities exist for oncologists to consider “seed planting” communication approaches, including anticipatory discussion to explore a patient’s or family’s hopes, worries, and goals with the intention of laying groundwork for future conversations about prognosis. [6].
In lieu of seed planting, we found that oncologists are more likely to reassure, soften the message, focus on disease or treatment details without prognostic interpretation, or express uncertainty without referencing the “big picture” context during discussions about equivocal disease status, even in the setting of anticipated poor prognosis. This phenomenon of “kicking the proverbial can down the road” likely has multifactorial origins. For example, oncologists historically self-report fears that discussing uncertainty may harm therapeutic alliance or steal hope from patients and families. [38,39,40,41,42,43] Contrary to this assumption, however, patients and parents who received honest information about poor prognosis were more likely to report feeling peace of mind, trust in the physician, and hope, [26, 27, 44,45,46] suggesting that some of these fears may be unfounded. At the same time, the impact of uncertainty on patients’ and parents’ experiences of prognostic communication remains understudied.
Oncologists’ personal values and attributes also may influence their communication approaches. An oncologist’s own tolerance for uncertainty has been shown to be significantly associated with willingness to discuss an uncertain prognosis with patients and families. [18] Additionally, oncologists describe awareness of “collusion” as a common phenomenon where stakeholders avoid direct conversation about prognosis as part of an unspoken dance. [47] The premise of this phenomenon, however, rests upon an assumption that the patient and family share the same understanding of prognosis as the oncologist. Counter to this, previous studies demonstrate that concordance in prognostic understanding between oncologists and parents of children with advanced cancer is often poor. [6, 48, 49] Collusion, by definition, is only possible in settings in which both parties know and understand the prognosis.
Patients’ and families’ preferences for discussing prognosis in the setting of uncertainty and equivocal disease reevaluation data are not well understood, although preliminary work suggests that families recognize the challenges and benefits of having direct conversation about prognostic uncertainty. [13] Notably, most adolescents with cancer and parents of children with cancer want to hear direct, truthful, individualized, and regular communication about prognosis across the illness course [21, 44, 50,51,52] and seek support in applying population-level prognostic information to their child’s specific trajectory. [52] For personal or cultural reasons, some families prefer for prognostic information to be withheld from the patient; yet data suggest that, when asked directly, patients often express a preference for their physician to be honest, even as families strive to protect them from stressful information. [53].
Presently, few communication guidelines exist to support pediatric oncologists in disclosing uncertain or equivocal disease reevaluation findings. Resources and training should emphasize the value of person-centered communication, with awareness of the importance of empowering adolescent and young adult patients to participate in conversations and decision-making in alignment with their preferences and values. [54, 55] Two communication tools used frequently in palliative medicine practice and pedagogy may be useful in guiding conversations conveying equivocal information to patients of varying ages and their families: “this means” and the “3Ws.” In Fig. 1, we illustrate how the patterns of prognostic communication used by oncologists in this study might be reframed using “this means” and the “3Ws” (“I wish…”, “I worry…”, “I wonder…”) statements to help navigate communication during uncertain timepoints in the setting of an anticipated poor prognosis. In particular, “I worry” statements offer an effective strategy for “seed planting” [6] to help clinicians broach difficult prognostic communication in a gentle, step-wise approach across time.
Applying communication strategies for prognostic communication in settings of equivocal news. Patterns of prognostic communication in disease reevaluation conversations conveying equivocal news are specified with recommendations for re-stating news using two communication strategies: (1) “this means” and (2) the “3Ws” (e.g., “I wish, I worry, I wonder”). Suggestions for alternative phrasings are based on the authors’ collective clinical experiences
We also recognize that meaningful gaps exist between what is known (and visible on disease surveillance imaging) and what is perceived, interpreted, or discerned based on the oncologist’s deep experience and knowledge. Oncologists may describe discussions as “equivocal” because the concrete evidence before them does not afford sufficient certainty to label the discussion as “bad news.” We advocate for increasing emphasis on communication training for oncologists to help them navigate the space between the visible and the invisible to communicate uncertainty with care and intentionality. Experiential learning [56] and role-play with standardized patients and/or bereaved parent educators [57] allows clinicians (e.g., oncologists, other physicians, and members of interprofessional teams, including social workers, psychologists, nurse practitioners, nurses, and others) to practice strategies for communicating about prognosis when disease progression is ambiguous, yet overall survival is unlikely.
Study findings should be interpreted in the context of limitations. Single-site design limits generalizability; however, qualitative research inherently does not aim for generalizability, and sample size was adequate for saturation of concepts. Sampling bias should be considered, as the study was conducted at a pediatric cancer center that recruits for phase I/II trials, and oncologist communication approaches could be influenced by a focus on cancer-directed treatments. Despite purposive sampling, racial and ethnic diversity was limited, which necessitates prioritization in future work. For example, Black patients comprised 11% of participants in this study, but they comprise 15% of the institution’s patient population and 16% of the state population. Similarly, purposive sampling was not successful in increasing Hispanic/Latino participation, due in part to eligibility criteria that precluded non-English speaking dyads. Similarly, lack of diversity in oncology faculty at the institution limited ability to represent different perspectives in oncology participants. Subsequent research protocols building upon these data have taken steps to proactively increase diversity across recruitment.
Rarely, discussions were not recorded due to logistical issues or at the request of the participating patient or parent; missing data could influence inductive analysis, although a few missing timepoints in the context of thousands of recorded minutes are less likely to influence data synthesis. Our analysis focused more on oncologist communication about prognosis, rather than on parent responses or questions; we underscore that patient/parent questions can change how an oncologist communicates about prognosis, and examination of patient/parent language to prompt or shape oncologist responses is an important consideration to inform future research. We did not conduct analyses stratified by patient age to explore potential variances in communication patterns influenced by age, and this query deserves future investigation. Finally, the phenomenon where oncologists defined a conversation as “equivocal” (instead of labeling it as “bad news”), yet dialogue frequently was coded as “disease changing for the worse,” is deserving of further attention. In this analysis, parsing out these nuances would have required extensive reading “between the lines,” as oncologists did not directly verbalize this discrepancy and researchers did not attempt to elicit or probe it in real time since the finding was identified during later targeted analyses. We advocate for purposeful exploration of this discrepancy in future work.
During conversations about equivocal disease reevaluation findings, pediatric oncologists rarely discussed prognosis directly with patients and families. Given that equivocal findings occurred frequently for pediatric patients with high-risk cancer, formal guidance is needed to better support oncologists in navigating uncertainty while sharing honest, person- and family-centered information about prognosis. Patient, parent, and oncologist perspectives and preferences should inform the design and evaluation of clinical communication tools to support prognostic communication across the illness course.
Data availability
Deidentified selections of the datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Innes S, Payne S. Advanced cancer patients’ prognostic information preferences: A review. Palliat Med. 2009;23(1):29–39. https://doi.org/10.1177/0269216308098799.
Mitchison D, Butow P, Sze M, et al. Prognostic communication preferences of migrant patients and their relatives. Psychooncology. 2012;21(5):496–504. https://doi.org/10.1002/pon.1923.
Mauri E, Vegni E, Lozza E, Parker PA, Moja EA. An exploratory study on the Italian patients’ preferences regarding how they would like to be told about their cancer. Support Care Cancer. 2009;17(12):1523–30. https://doi.org/10.1007/s00520-009-0621-7.
Mack JW, Joffe S. Communicating about prognosis: Ethical responsibilities of pediatricians and parents. Pediatrics. 2014;133(SUPPL. 1):S24-30. https://doi.org/10.1542/peds.2013-3608E.
Cortez D, Maynard DW, Campbell TC. Creating space to discuss end-of-life issues in cancer care. Patient Educ Couns. 2019;102(2):216–22. https://doi.org/10.1016/j.pec.2018.07.002.
Kaye EC, Stall M, Woods C, et al. Prognostic communication between oncologists and parents of children with advanced cancer. Pediatrics. 2021;147(6):e2020044503. https://doi.org/10.1542/peds.2020-044503.
Surbone A, Ritossa C, Spagnolo AG. Evolution of truth-telling attitudes and practices in italy. Crit Rev Oncol Hematol. 2004;52(3):165–72. https://doi.org/10.1016/j.critrevonc.2004.09.002.
Kazdaglis GA, Arnaoutoglou C, Karypidis D, Memekidou G, Spanos G, Papadopoulos O. Disclosing the truth to terminal cancer patients: A discussion of ethical and cultural issues. East Mediterr Heal J. 2010;16(4):442–7. https://doi.org/10.26719/2010.16.4.442.
Khalil RB. Attitudes, beliefs and perceptions regarding truth disclosure of cancer-related information in the middle east: A review. Palliat Support Care. 2013;11(1):69–78. https://doi.org/10.1017/S1478951512000107.
Shahidi J. Not telling the truth: Circumstances leading to concealment of diagnosis and prognosis from cancer patients. Eur J Cancer Care (Engl). 2010;19(5):589–93. https://doi.org/10.1111/j.1365-2354.2009.01100.x.
Lee A, Wu HY. Diagnosis disclosure in cancer patients - When the family says “no!”. Singap Med J. 2002;43(10):533–8.
Parsons SK, Saiki-Craighill S, Mayer DK, et al. Telling children and adolescents about their cancer diagnosis: cross-cultural comparisons between pediatric oncologists in the US and Japan. Psychooncology. 2007;16(1):60–8. https://doi.org/10.1002/pon.1048.
Krawczyk M, Gallagher R. Communicating prognostic uncertainty in potential end-of-life contexts: experiences of family members. BMC Palliat Care. 2016;15(1):59. https://doi.org/10.1186/s12904-016-0133-4.
Simpkin AL, Armstrong KA. Communicating uncertainty: A narrative review and framework for future research. J Gen Intern Med. 2019;34(11):2586–91. https://doi.org/10.1007/s11606-019-04860-8.
LeBlanc TW, Temel JS, Helft PR. “How Much Time Do I Have?”: communicating prognosis in the era of exceptional responders. Am Soc Clin Oncol Educ B. 2018;(38):787–94. https://doi.org/10.1200/edbk_201211.
Tulsky JA, Fischer GS, Rose MR, Arnold RM. Opening the black box: How do physicians communicate about advance directives? Ann Intern Med. 1998;129(6):441–9. https://doi.org/10.7326/0003-4819-129-6-199809150-00003.
Habib A, Zhang Z, Tanasijevic A, et al. Prevalence of prognostic uncertainty and impact on prognostic discussions in thoracic oncology. J Clin Oncol. 2019;37(31_suppl):33–3. https://doi.org/10.1200/jco.2019.37.31_suppl.33.
Habib AR, Chen R, Magnavita ES, et al. Prevalence and tolerance of prognostic uncertainty among thoracic oncologists. Oncologist. 2021;26(8):e1480–2. https://doi.org/10.1002/onco.13788.
Smith AK, White DB, Arnold RM. Uncertainty — The other side of prognosis. N Engl J Med. 2013;368(26):2448–50. https://doi.org/10.1056/nejmp1303295.
Hill DL, Walter JK, Szymczak JE, DiDomenico C, Parikh S, Feudtner C. Seven types of uncertainty when clinicians care for pediatric patients with advanced cancer. J Pain Symptom Manage. 2020;59(1):86–94. https://doi.org/10.1016/j.jpainsymman.2019.08.010.
Sisk BA, Friedrich AB, DuBois J, Mack JW. Characteristics of uncertainty in advanced pediatric cancer conversations. Patient Educ Couns. 2021;104(5):1066–74. https://doi.org/10.1016/j.pec.2020.10.006.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Heal Care. 2007;19(6):349–57. https://doi.org/10.1093/intqhc/mzm042.
Kaye EC, Woods C, Kennedy K, et al. Communication around palliative care principles and advance care planning between oncologists, children with advancing cancer and families. Br J Cancer. 2021;(April):1–11. https://doi.org/10.1038/s41416-021-01512-9.
Kaye EC, Woods C, Velrajan S, Lemmon ME, Baker JN, Mack JW. Broaching goals-of-care conversations in advancing pediatric cancer. Pediatr Blood Cancer. 2021;68(10):1–4. https://doi.org/10.1002/pbc.29270.
Kaye EC, Rockwell S, Woods C, et al. Facilitators associated with building and sustaining therapeutic alliance in advanced pediatric cancer. JAMA Netw Open. 2021;4(8):e2120925. https://doi.org/10.1001/jamanetworkopen.2021.20925.
Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. J Clin Oncol. 2006;24(33):5265–70. https://doi.org/10.1200/JCO.2006.06.5326.
Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Hope and prognostic disclosure. J Clin Oncol. 2007;25(35):5636–42. https://doi.org/10.1200/JCO.2007.12.6110.
Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25(11):1357–62. https://doi.org/10.1200/JCO.2006.08.3170.
Mack JW, Joffe S, Hilden JM, et al. Parents’ views of cancer-directed therapy for children with no realistic chance for cure. J Clin Oncol. 2008;26(29):4759–64. https://doi.org/10.1200/JCO.2007.15.6059.
Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES—A six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302–11. https://doi.org/10.1634/theoncologist.5-4-302.
Kaplan M. SPIKES: A framework for breaking bad news to patients with cancer. Clin J Oncol Nurs. 2010;14(4):514–6. https://doi.org/10.1188/10.CJON.514-516.
Sisk BA, Mack JW, Ashworth R, DuBois J. Communication in pediatric oncology: state of the field and research agenda. Pediatr Blood Cancer. 2018;65(1). https://doi.org/10.1002/pbc.26727.
Fenton JJ, Duberstein PR, Kravitz RL, et al. Impact of prognostic discussions on the patient-Physician relationship: prospective cohort study. J Clin Oncol. 2018;36(3):225–30. https://doi.org/10.1200/JCO.2017.75.6288.
Korstjens I, Moser A. Series. Practical guidance to qualitative research. Part 4: Trustworthiness and publishing. Eur J Gen Pract. 2018;24(1):120–4. https://doi.org/10.1080/13814788.2017.1375092.
Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–907. https://doi.org/10.1007/s11135-017-0574-8.
Schönfelder W. CAQDAS and Qualitative Syllogism Logic — NVivo 8 and MAXQDA 10 Compared. Qual Sozialforsch / Forum Qual Soc Res. 2011;12(1):Art. 21.
Birks M, Chapman Y, Francis K. Memoing in qualitative research: probing data and processes. J Res Nurs. 2008;13(1):68–75. https://doi.org/10.1177/1744987107081254.
Han PKJ, Gutheil C, Hutchinson RN, LaChance JA. Cause or Effect? The role of prognostic uncertainty in the fear of cancer recurrence. Front Psychol. 2021;11(January):1–12. https://doi.org/10.3389/fpsyg.2020.626038.
Mack JW, Smith TJ. Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–7. https://doi.org/10.1200/JCO.2012.42.4564.
Smith TJ, Dow LA, Virago E, Khatcheressian J, Lyckholm LJ, Matsuyama R. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24(6):521–5.
Smith TJ, Dow LA, Virago EA, Khatcheressian J, Matsuyama R, Lyckholm LJ. A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer. J Support Oncol. 2011;9(2):79–86. https://doi.org/10.1016/j.suponc.2010.12.005.
Helft PR. Necessary collusion: prognostic communication with advanced cancer patients. J Clin Oncol. 2005;23(13):3146–50. https://doi.org/10.1200/JCO.2005.07.003.
Ruddick W. Hope and deception. Bioethics. 1999;13(3–4):343–57. https://doi.org/10.1111/1467-8519.00162.
Mack JW, Fasciano KM, Block SD. Communication about prognosis with adolescent and young adult patients with cancer: information needs, prognostic awareness, and outcomes of disclosure. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(18):1861–7. https://doi.org/10.1200/JCO.2018.78.2128.
Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Peace of mind and sense of purpose as core existential issues among parents of children with cancer. Arch Pediatr Adolesc Med. 2009;163(6):519–24. https://doi.org/10.1001/archpediatrics.2009.57.
Marron JM, Cronin AM, Kang TI, Mack JW. Intended and unintended consequences: ethics, communication, and prognostic disclosure in pediatric oncology. Cancer. 2018;124(6):1232–41. https://doi.org/10.1002/cncr.31194.
The AM, Hak T, Koeter G, Van der Wal G. Collusion in doctor-patient communication about imminent death: An ethnographic study. Br Med J. 2000;321(7273):1376–81. https://doi.org/10.1136/bmj.321.7273.1376.
Rosenberg AR, Orellana L, Kang TI, et al. Differences in parent-provider concordance regarding prognosis and goals of care among children with advanced cancer. J Clin Oncol. 2014;32(27):3005–11. https://doi.org/10.1200/JCO.2014.55.4659.
Ullrich CK, Dussel V, Hilden JM, Sheaffer JW, Lehmann L, Wolfe J. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood. 2010;115(19):3879–85. https://doi.org/10.1182/blood-2009-10-250225.
Greenzang KA, Cronin AM, Kang T, Mack JW. Parent understanding of the risk of future limitations secondary to pediatric cancer treatment. Pediatr Blood Cancer. 2018;65(7):139–48. https://doi.org/10.1002/pbc.27020.
Sisk BA, Kang TI, Mack JW. Prognostic disclosures over time: parental preferences and physician practices. Cancer. 2017;123(20):4031–8. https://doi.org/10.1002/cncr.30716.
Bluebond-Langner M, Hall N, Vincent K, et al. Parents’ responses to prognostic disclosure at diagnosis of a child with a high-risk brain tumor: Analysis of clinician-parent interactions and implications for clinical practice. Pediatr Blood Cancer. 2021;68(3). https://doi.org/10.1002/pbc.28802.
Wu J, Wang Y, Jiao X, Wang J, Ye X, Wang B. Differences in practice and preferences associated with truth-telling to cancer patients. Nurs Ethics. 2021;28(2):272–81. https://doi.org/10.1177/0969733020945754.
Wiener L, Zadeh S, Battles H, et al. Allowing adolescents and young adults to plan their end-of-life care. Pediatrics. 2012;130(5):897–905. https://doi.org/10.1542/peds.2012-0663.
Wiener L, Bedoya S, Battles H, et al. Voicing their choices: Advance care planning with adolescents and young adults with cancer and other serious conditions. Palliat Support Care. 2021;20(4):462–70. https://doi.org/10.1017/S1478951521001462.
Wiener L, Shaw Weaver M, Sansom Daly UM, Bell CJ. Threading the cloak: palliative care education for care providers of adolescents and young adults with cancer. Clin Oncol Adolesc Young Adults. 2015;5:1. https://doi.org/10.2147/coaya.s49176.
Snaman JM, Kaye EC, Spraker-Perlman H, et al. Incorporating bereaved parents as faculty facilitators and educators in teaching principles of palliative and end-of-life care. Am J Hosp Palliat Med. 2018;35(12):1518–25. https://doi.org/10.1177/1049909118786875.
Acknowledgements
The authors acknowledge and thank the Quality of Life Research Division for its support of this study; the Bereaved Parent Steering Council for reviewing study design, methods, and materials and for providing feedback; and Melanie Gattas, CRA-RN, and Marian Shaw, CRA-RN, for administrative support of research processes.
Funding
This work is supported by Dr. Kaye’s Career Development Award from the National Palliative Care Research Center and the by ALSAC.
Author information
Authors and Affiliations
Contributions
AP directed the analysis, drafted the initial manuscript, and revised the manuscript. CW assisted with data collection, assisted with data analysis, and critically reviewed the manuscript. MS and SV assisted with data analysis and critically reviewed the manuscript. JB assisted with study design, assisted with data analysis, and critically reviewed the manuscript. JM assisted with study design, assisted with data synthesis, and critically reviewed the manuscript. EK conceptualized and designed the study, supervised data collection, supervised the analysis, and reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the St. Jude Children’s Hospital Institutional Review Board [U-CHAT (Pro00006473); approval date: 7/12/2016]. Informed consent was obtained from all subjects and/or their legal guardian(s), per Institutional Review Board guidelines. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
No individual person’s data containing identifiable information are included in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Porter, A.S., Woods, C., Stall, M. et al. Oncologist approaches to communicating uncertain disease status in pediatric cancer: a qualitative study. BMC Cancer 22, 1109 (2022). https://doi.org/10.1186/s12885-022-10190-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10190-6