Abstract
Background
Local recurrence is common after curative resections for rectal cancer. Surgical intervention is among the best treatment choices. However, achieving a negative resection margin often requires extensive pelvic organ resections; thus, the postoperative complication rate is quite high. Recent studies have reported that the inflammatory index could predict postoperative complications. This study aimed to validate the correlation between clinical factors, including inflammatory markers, and severe complications after surgery for local recurrent rectal cancer.
Methods
This retrospective study included 99 patients that underwent radical resections for local recurrences of rectal cancer. Postoperative complications were graded according to the Clavien-Dindo classification. Grades ≥3 were defined as severe complications. Risk factors for severe complications were identified with univariate and multivariate logistic regression models and assessed with receiver-operating characteristic curves.
Results
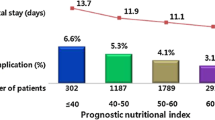
Severe postoperative complications occurred in 38 patients (38.4%). Analyses of correlations between inflammatory markers and severe postoperative complications revealed that the strongest correlation was found between the prognostic nutrition index and severe postoperative complications. The receiver-operating characteristic analysis showed that the optimal prognostic nutrition index cut-off value was 42.2 (sensitivity: 0.790, specificity: 0.508). In univariate and multivariate analyses, a prognostic nutrition index ≤44.2 (Odds ratio: 3.007, 95%CI:1.171–8.255, p = 0.02) and a blood loss ≥2850 mL (Odds ratio: 2.545, 95%CI: 1.044–6.367, p = 0.04) were associated with a significantly higher incidence of severe postoperative complications.
Conclusions
We found that a low preoperative prognostic nutrition index and excessive intraoperative blood loss were risk factors for severe complications after surgery for local recurrent rectal cancer.
Similar content being viewed by others
Background
Colorectal cancer is one of the most common cancers in the world [1]. The 5-year survival rate for colorectal cancer has improved in the last 30 years, due to advancements in surgical and drug treatments. However, recurrences occur frequently after colorectal cancer surgery. In particular, the local recurrence of rectal cancer (LRRC) after a curative resection occurs at rates of 5.6 to 11% [2,3,4].
LRRC can be cured with radical resection. The reported 5-year survival rate is 43 to 70% after a curative resection for LRRC [5,6,7]. Local re-recurrences after LRRC surgery are often observed; therefore, a radical resection for LRRC often requires a highly invasive procedure, such as a total pelvic exenteration (TPE) combined with a sacral resection [8]. Consequently, postoperative complications occur frequently in LRRC at rates of 24 to 68% [9,10,11,12].
Recent studies have shown that inflammatory markers, such as the neutrophil/lymphocyte ratio (NLR) and the platelet/lymphocyte ratio (PLR), were useful for predicting postoperative complications and prognosis [13, 14]. These markers are calculated based on a complete blood count, which makes them simple and versatile. In addition, the prognostic nutritional index (PNI, based on serum albumin and lymphocyte counts) and the serum C-reactive protein (CRP)/serum albumin ratio (CAR) were shown to be effective predictors of postoperative complications after colorectal cancer surgery [15, 16]. However, no study has clarified the association between inflammatory markers and postoperative complications in LRRC.
The purpose of this study was to clarify the correlation between severe postoperative complications and various clinical factors, including inflammatory markers, in LRRC.
Methods
Study population
This retrospective cohort study was performed in Osaka University Hospital. We collected information for patients that had undergone curative resections for LRRC between January 2000 and January 2020. A total of 99 patients were eligible for this study.
Patient and tumor characteristics
We acquired information on patient characteristics, including age at surgery, sex, body mass index (BMI), and the preoperative physical state, assessed with the American Society of Anesthesiologists (ASA) physical status classification. In addition, we examined the location and TNM stage of the primary tumor (based on the Union for International Cancer Control, 8th edition), the postoperative adjuvant therapy given after the initial treatment, and the preoperative treatment for LRRC surgery. We also included data on intraoperative details, including the surgical procedures, surgical approaches, combined resected organs, operation time, blood loss volume, and residual tumor (R status). We assessed the severity of postoperative complications with the Clavien-Dindo (CD) classification. Severe postoperative complications were defined as CD grade ≥ 3.
Inflammatory markers
The latest laboratory data processed before the LRRC surgery was collected, including the complete blood count and the serum albumin and CRP levels. Serum CRP levels under 0.04 mg/L were below the detection limit of our measuring instrument; therefore, values under 0.04 mg/L were set to 0.04 mg/L for this analysis. These data were used to calculate the inflammatory markers, NLR, PLR, lymphocyte/CRP ratio (LCR), CAR, and PNI.
Statistical analysis
All categorical data were presented as number of cases and percentages, while continuous data were shown as median and interquartile range (IQR). We performed logistic regression analyses to assess correlations between the incidence of severe postoperative complications and each inflammatory marker. We then created receiver operating characteristic (ROC) curves and compared areas under the ROC curves to determine the strongest effective marker of severe postoperative complications. We performed multiple logistic regression analyses to assess the correlation between severe postoperative complications and clinical factors.
All data were processed and analyzed with JMP Pro 14.0 (SAS Institute Inc., Cary NC). Two-tailed p-values < 0.05 were considered statistically significant. We calculated the exact 95% confidence intervals (95% CIs) for absolute differences and odds ratios (ORs).
This study was performed in accordance with the Declaration of Helsinki (1975, and revised in 2008). The study protocol was approved by the Ethics Committee of Osaka University Hospital. All study participants provided written informed consent.
Results
Clinical characteristics of the study population
Table 1 and Supplemental Table 1 show patient and primary tumor characteristics for the 99 patients. The cohort included 57 males and 42 females. The median age was 61 years [IQR 54.5–68]. The median BMI was 22.2 kg/m2 [IQR 20.2–24.1]. The majority of the patients were class 2 of preoperative physical state (n = 71; 71.7%). A total of 63 patients (63.6%) received neo-adjuvant therapy for LRRC. More than a half of the patients (n = 53; 53.5%) had chemoradiotherapy, whereas smaller numbers had chemotherapy (n = 9; 9.1%) or radiotherapy alone (n = 1; 1.0%). About primary tumor characteristics, more than 40% of the primary tumors were located in the lower rectum (n = 45; 45.6%). Regarding the T stage of the primary tumors, the majority of patients was T3 or T4 (T3, n = 48; 48.5%, T4, n = 35; 35.4%). About half of the patients had lymph node metastasis in the primary cancers (N1, n = 26; 26.3%, N2, n = 21; 21.2%). Adjuvant chemotherapy for the primary cancer was given to more than half of the patients (n = 56; 56.6%).
Preoperative laboratory data and inflammatory makers of the study population
Table 2 shows the preoperative laboratory data results for the 99 patients. Regarding complete blood count, the median numbers of neutrophils, lymphocytes and platelets were within normal range. The median level of serum albumin (g/dL) was lower than normal value (value = 3.8 [IQR 3.4–4.1]). The median level of CRP was within normal range (value = 0.09 [IQR 0.04–0.46]. The inflammatory markers were calculated from the blood test data and each median value was as follow: NLR, 3.1, PLR, 232.5; CAR, 0.026; LCR, 11417.3; and PNI, 42.8.
Operative details of the study population
Table 3 displays the intraoperative data for the 99 patients. The majority of the patients (n = 79; 79.8%) underwent a laparotomy and the rest of 20 patients (20.2%) underwent laparoscopic surgery. Regarding the type of surgery, the most common procedure was a tumorectomy or lateral lymph node dissection (n = 44; 44.4%) and a TPE was performed in 22 patients (22.2%). More than 40% of the patients (n = 40; 40.4%) underwent a combined sacral resection. The median operation time was 680 min [IQR 438–871], and the median intraoperative blood loss was 2850 mL [IQR 530–5240]. In histopathological outcome, the complete resection rate (R0) was 93.9% (n = 93).
Postoperative complications of the study population
The incidence of postoperative complications is shown in Table 4. Postoperative complications occurred in 79 patients (79.8%). Complications included a pelvic abscess in 36 patients (36.4%), perineal wound infections in 37 patients (37.4%), abdominal wound infections in 16 patients (16.2%), bleeding in 8 patients (8.1%), bowel obstruction or ileus in 20 patients (20.2%), urinary tract infections in 21 patients (21.2%), and venous thromboembolism (VTE) in 5 patients (5.1%). The postoperative complications were classified as follows, 41 patients (41.4%) had CD grade I or II, 36 patients (36.4%) had CD grade III or IV, and 2 patients (2.0%) had CD grade V.
Correlation between inflammatory markers and severe postoperative complications
To identify correlations between inflammatory markers and severe postoperative complications in LRRC, we performed univariate analyses. Then, we created ROC curves to analyze the sensitivity and specificity of each inflammatory marker that was correlated to severe postoperative complications (Fig. 1). The area under the curve (AUC) and p-value for each inflammatory marker are shown in each panel. The PNI showed the strongest correlation with severe postoperative complications in LRRC. The PNI cutoff value for indicating severe complications was 44.2, and it showed a sensitivity of 0.790 and a specificity of 0.508.
Receiver operating characteristic curves analysis to evaluate the predictive value of each inflammatory marker for postoperative complications in patients with local recurrent rectal cancer (LRRC). Preoperative prognostic nutrition index showed the highest accuracy for the prediction of postoperative complications compared with neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-CRP ratio (LCR) and CRP-albumin ratio (CAR) in patients with LRRC
Risk factors for severe postoperative complications
Potential risk factors for severe postoperative complications in LRRC were analyzed with univariate and multivariate analyses based on logistic regression. As shown in Table 5, the univariate analysis showed that the PNI and intraoperative blood loss were significantly correlated with severe postoperative complications. The multivariate analysis of these factors revealed that a PNI < 44.2 (OR: 3.007, 95%CI: 1.171–8.255, p = 0.02) and blood loss ≥2850 mL (OR: 2.545, 95%CI: 1.044–6.367, p = 0.04) were independent risk factors (Table 6).
Discussion
Postoperative complications are known to be associated with a poor prognosis in colorectal cancer [17]. We found a postoperative complication rate of 79.8% in LRRC, and the rate of CD ≥ III complications was 38.4%. To avoid postoperative complications, it is important to know the risk factors and provide a presurgical therapeutic intervention to prevent them. We identified two independent risk factors for complications after LRRC surgery in this study: a preoperative PNI < 44.2 and intraoperative blood loss ≥2850 mL. These findings indicated that the preoperative nutritional status and surgical invasiveness could be related to severe postoperative complications in patients with LRRC.
The PNI is a simple nutritional index, based on the serum albumin level and the lymphocyte count. It has been associated with perioperative complications in various carcinomas [18, 19]. Albumin is an index of nutritional capacity, and lymphocytes are an index of nutrition and immune capacity. Thus, the PNI reflects nutrition and immune status. Accordingly, patients with low PNIs are expected to have low wound healing ability and low immune function.
One might ask why did severe complications after LRRC surgery show a stronger correlation with PNI than with other inflammatory markers? One explanation might be that the NLR and PLR values after chemotherapy did not reflect the nutrition and immune status correctly. Chemotherapy reduces blood cell counts by suppressing bone marrow activity; therefore, the NLR and PLR might be affected, because they are calculated from blood cell counts. In the present study, preoperative chemotherapy for LRRC was performed in 62 patients (62.6%). As a result, the NLR and PLR might be less sensitive to severe postoperative complications than expected. Another potential explanation might be the limited accuracy of our blood test equipment. Because we could not assess CRP levels below 0.04, CRP levels less than 0.04 were treated as 0.04 in this study. Therefore, we could not accurately assess the CAR and LCR values, which required the CRP level.
The relationship between blood loss and postoperative complications was previously reported in colorectal cancer surgery [20, 21]. Heavy bleeding can change the hemodynamics and impair organs, particularly the kidneys and liver, and it also affects coagulation. These changes can lead to several postoperative complications, such as VTE and bleeding. It has been reported that VTE and bleeding at or greater than CD grade III after curative resection of primary colorectal cancer occurs at rates of 0 to 0.16% and 0.2 to 0.81%, respectively [22,23,24]. Compared with these data, in the present study, VTE and bleeding events more frequently occurred (Table 4).
Some previous studies have reported that preoperative nutritional interventions were effective for postoperative outcomes in various types of cancer. Indeed, preoperative exercise and nutritional support improved the postoperative outcome in patients with gastric cancer [25]. Despite concern that nutritional interventions might delay surgery, preoperative treatments are often performed in patients with LRRC; therefore, there should be sufficient time to improve the patient’s nutritional status.
We previously reported that laparoscopic surgery was safe and useful for LRRC [26]. The magnifying effect of the laparoscope is highly effective in surgery for pelvic organ cancers, where it is difficult to expand the field of view. Additionally, the carbon dioxide insufflation used to create a working space in the abdomen can provide pressure, which reduces bleeding. Indeed, patients with rectal cancer that underwent laparoscopic surgery had less intraoperative blood loss than patients that underwent a laparotomy [27, 28]. Although laparoscopic surgery for LRRC could potentially prevent intraoperative and postoperative complications, the number of patients that underwent laparoscopic surgery in this study was insufficient to assess the correlation between the surgical approach and complications.
This study had some limitations. First, the study was retrospective, and data were from a single center. Second, the cohort was relatively small. Although 99 patients represented a relatively large cohort in clinical research for LRRC, it was insufficient to clarify the risk factors for postoperative complications.
Conclusions
In conclusion, we found that a PNI < 44.2 and blood loss ≥2850 mL were independent risk factors for severe complications after LRRC surgery. Preoperative interventions that improve the nutritional status and an approach that reduces surgical invasiveness could potentially reduce the risk of severe complications after LRRC surgery.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- LRRC:
-
Local recurrence of rectal cancer
- TPE:
-
Total pelvic exenteration
- NLR:
-
Neutrophil/lymphocyte ratio
- PLR:
-
Platelet/lymphocyte ratio
- PNI:
-
Prognostic nutritional index
- CRP:
-
C-reactive protein
- CAR:
-
CRP/serum albumin ratio
- BMI:
-
Body mass index
- ASA:
-
American Society of Anesthesiologists
- CD:
-
Clavien-Dindo
- LCR:
-
Lymphocyte/CRP ratio
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- 95% CI:
-
95% confidence interval
- AUC:
-
Area under curve
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210.
Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701. https://doi.org/10.1097/01.sla.0000257358.56863.ce.
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82. https://doi.org/10.1016/S1470-2045(11)70097-3.
Beppu N, Kimura F, Aihara T, Doi H, Tomita N, Yanagi H, et al. Patterns of local recurrence and oncologic outcomes in T3 low rectal cancer (≤5 cm from the anal verge) treated with short-course radiotherapy with delayed surgery : outcomes in T3 low rectal cancer treated with short-course radiotherapy with delayed surgery. Ann Surg Oncol. 2017;24(1):219–26. https://doi.org/10.1245/s10434-016-5604-6.
Pacelli F, Tortorelli AP, Rosa F, Bossola M, Sanchez AM, Papa V, et al. Locally recurrent rectal cancer: prognostic factors and long-term outcomes of multimodal therapy. Ann Surg Oncol. 2010;17(1):152–62. https://doi.org/10.1245/s10434-009-0737-5.
Kanemitsu Y, Hirai T, Komori K, Kato T. Prediction of residual disease or distant metastasis after resection of locally recurrent rectal cancer. Dis Colon Rectum. 2010;53(5):779–89. https://doi.org/10.1007/DCR.0b013e3181cf7609.
Harris CA, Solomon MJ, Heriot AG, Sagar PM, Tekkis PP, Dixon L, et al. The outcomes and patterns of treatment failure after surgery for locally recurrent rectal Cancer. Ann Surg. 2016;264(2):323–9. https://doi.org/10.1097/SLA.0000000000001524.
Uemura M, Ikeda M, Yamamoto H, Kitani K, Tokuoka M, Matsuda K, et al. Clinicopathological assessment of locally recurrent rectal cancer and relation to local re-recurrence. Ann Surg Oncol. 2011;18(4):1015–22. https://doi.org/10.1245/s10434-010-1435-z.
Dresen RC, Gosens MJ, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol. 2008;15(7):1937–47. https://doi.org/10.1245/s10434-008-9896-z.
Asoglu O, Karanlik H, Muslumanoglu M, Igci A, Emek E, Ozmen V, et al. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33(10):1199–206. https://doi.org/10.1016/j.ejso.2007.02.026.
Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14(2):447–54. https://doi.org/10.1245/s10434-006-9256-9.
Uemura M, Ikeda M, Sekimoto M, Haraguchi N, Mizushima T, Yamamoto H, et al. Prevention of severe pelvic abscess formation following extended radical surgery for locally recurrent rectal cancer. Ann Surg Oncol. 2009;16(8):2204–10. https://doi.org/10.1245/s10434-009-0505-6.
Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2014;23(7):1204–12. https://doi.org/10.1158/1055-9965.EPI-14-0146.
Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S603–13.
Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36(3):995–1001.
Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–92. https://doi.org/10.1007/s00268-013-2156-9.
Aoyama T, Oba K, Honda M, Sadahiro S, Hamada C, Mayanagi S, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Med. 2017;6(7):1573–80. https://doi.org/10.1002/cam4.1126.
Okada S, Shimada J, Teramukai S, Kato D, Tsunezuka H, Miyata N, et al. Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. 2018;25(5):1254–61. https://doi.org/10.1245/s10434-018-6368-y.
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–74. https://doi.org/10.1002/bjs.7305.
Egenvall M, Mörner M, Påhlman L, Gunnarsson U. Degree of blood loss during surgery for rectal cancer: a population-based epidemiologic study of surgical complications and survival. Color Dis. 2014;16(9):696–702. https://doi.org/10.1111/codi.12630.
Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. 2012;255(6):1126–8. https://doi.org/10.1097/SLA.0b013e3182512df0.
Pak J, Ikeda M, Uemura M, Miyake M, Nishikawa K, Miyamoto A, et al. Risk factors for bleeding in patients receiving fondaparinux after colorectal cancer surgery. J Anus Rectum Colon. 2018;1(4):131–5. https://doi.org/10.23922/jarc.2017-022.
Yamaoka Y, Ikeda M, Ikenaga M, Haraguchi N, Miyake M, Sekimoto M. Safety and efficacy of fondaparinux for prophylaxis of venous thromboembolism after colorectal cancer resection: a propensity score matched analysis. Dig Surg. 2015;32(3):190–5. https://doi.org/10.1159/000381034.
Hata K, Kimura T, Tsuzuki S, Ishii G, Kido M, Yamamoto T, et al. Safety of fondaparinux for prevention of postoperative venous thromboembolism in urological malignancy: a prospective randomized clinical trial. Int J Urol. 2016;23(11):923–8. https://doi.org/10.1111/iju.13189.
Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao M, Nishikawa K, Miyamoto A, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. 2017;20(5):913–8. https://doi.org/10.1007/s10120-016-0683-4.
Uemura M, Ikeda M, Kawai K, Nishimura J, Takemasa I, Mizushima T, et al. Laparoscopic surgery using a Gigli wire saw for locally recurrent rectal cancer with concomitant intraperitoneal sacrectomy. Asian J Endosc Surg. 2018;11(1):83–6. https://doi.org/10.1111/ases.12407.
Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol. 2014;32(17):1804–11. https://doi.org/10.1200/JCO.2013.54.3694.
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–8. https://doi.org/10.1016/S1470-2045(13)70016-0.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conception and design of the study, MP, MU and TM; analysis and interpretation of data, MP, MU, NM and HT; acquisition of data, MP, MU, MK, SF and TO; drafting of manuscript, MP, MU, HY and TM; critical revision of manuscript, MP, MU, TM and YD; final approval of the article: HE. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Osaka University Hospital. All study participants provided written informed consent.
Consent for publication
Consent for publication was obtained from the all study participants.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
Primary tumor characteristics of the patients in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paku, M., Uemura, M., Kitakaze, M. et al. Impact of the preoperative prognostic nutritional index as a predictor for postoperative complications after resection of locally recurrent rectal cancer. BMC Cancer 21, 435 (2021). https://doi.org/10.1186/s12885-021-08160-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08160-5