Abstract
Background
Breast cancer is the most common cancer in women and the first cancer concerning mortality. Metastatic breast cancer remains a disease with a poor prognosis and about 30% of women diagnosed with an early stage will have a secondary progression. Metastatic breast cancer is an incurable disease despite significant therapeutic advances in both supportive cares and targeted specific therapies. In the management of a metastatic patient, each clinician follows a highly complex and strictly personal decision making process. It is based on a number of objective and subjective parameters which guides therapeutic choice in the most individualized or adapted manner.
Methods/design
The main objective is to integrate massive and heterogeneous data concerning the patient’s environment, personal and familial history, clinical and biological data, imaging, histological results (with multi-omics data), and microbiota analysis. These characteristics are multiple and in dynamic interaction overtime. With the help of mathematical units with biological competences and scientific collaborations, our project is to improve the comprehension of treatment response, based on health clinical and molecular heterogeneous big data investigation.
Discussion
Our project is to prove feasibility of creation of a clinico-biological database prospectively by collecting epidemiological, socio-economic, clinical, biological, pathological, multi-omic data and to identify characteristics related to the overall survival status before treatment and within 15 years after treatment start from a cohort of 300 patients with a metastatic breast cancer treated in the institution.
Trial registration
ClinicalTrials.gov identifier (NCT number): NCT03958136. Registration 21st of May, 2019; retrospectively registered.
Similar content being viewed by others
Background
Disease background
Breast cancer is the most common cancer in women with 58,459 new cases in France in 2018. It is the first cancer concerning mortality with 12,146 deaths in 2018, but mortality rate is decreasing in France since the last 15 years. This decreasing rate is in relation with early detection, screening and adjuvant therapies [1].
Metastatic breast cancer remains a disease with a poor prognosis with a 5-year survival less than 20%, and a median-survival of 24 to 30 months after metastasis diagnosis. Each year 5 to 10% of new breast cancers are diagnosed with a metastatic staging. About 30% of women diagnosed with an early stage will have a secondary progression. Metastatic breast cancer is an incurable disease despite significant therapeutic advances in both supportive cares and targeted specific therapies (anti-HER2, anti-estrogenic) and cytotoxic molecules [2,3,4,5]. This therapeutic arsenal improves clearly quality of life of patients, and sometimes a gain in terms of overall survival.
General management of therapies in metastatic breast cancer
In the management of a metastatic patient, each clinician builds his own decision algorithm. It is based on a number of objective and subjective parameters which allow the therapeutic decision making process to become the most individualized or adapted:
-
Extrinsic objectives parameters are currently based on EBM (evidence-based-medicine): the age of the patient, the aggressiveness of the disease, previous therapies (neoadjuvant, metastatic), relapse time to initial diagnosis, hormone receptor (HR) expression, estrogen (ER +) and / or progesterone (PR +), overexpression of the oncogene HER2 (HER2 +), mutation of PIK3CA, ESR1or BCRA1/2, expression of PDL1 and previous clinical trial results (overall survival, time to progression).
-
Intrinsic subjective parameters are taken into account in decision-making: parameters that are linked to the oncologist’s assumptions, such as, for example, the sensitivity to the theoretical efficacy of treatments and the definition of sensitivity. From the point of view of the patient, the choice is influenced by her more or less pregnant social life, the experience of a previous treatment, her age, her psychological state, her symptoms and the survival hoped gain.
Current therapeutic strategies
Currently, the clinician rationalizes these therapeutic indications according to the prediction of the treatment response from the “phenotypic classification” [6,7,8]. This immunohistochemistry (IHC)-based classification includes three subtypes: breast cancers defined as luminal by HR positivity, HER2 + cancers and triple-negative cancers (HR and HER2 -). The targeting of oncogenic addictive pathways by anti-estrogenic therapies (SERM - Selective Estrogen Receptor Modulators, SERD - Selective Estrogen Receptor Degradation and aromatase inhibitors) or HER2 inhibitory approaches (trastuzumab, pertuzumab, TDM-1,lapatinib, neratinib) induces mitigate signals of death, survival, and cell proliferation [9,10,11]. However, initially, the signals of death and cellular arrest are predominant and then they reverse under therapeutic pressure. The tumor escapes by adapting to its new environment induced by the treatment.
The identification of resistance or adaptive pathways led to the development of additive strategies. This strategy with a strong EBM literature has been shown to be effective in both ER + (CDK, mTOR and PI3Kinase inhibitors) and HER2 + patients (pertuzumab, lapatinib, TDM-1, neratinib) [12]. This strategy derives directly from the first strategy, via the identification (by DNA sequencing technique) of anomalies for which there is a specific therapy [13,14,15]. However, the decision algorithms, described and based on a target-one treatment, are not optimal and it is now necessary to define a new therapeutic strategy based on a systemic approach for a complex disease.
Research hypothesis
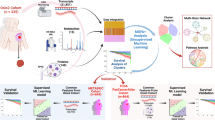
As explained above, current therapeutic strategies are based on a reductionist approach, and they do not meet the expected success. Cancer is a complex disease relying on multiple parameters in dynamic, organized and evolving interactions, and analysis of a complex system requires a systemic approach (Fig. 1).
Thus, we need to evolve from a reductionist, disjunctive, analytical view of the characterization of cell components (genes, transcripts, proteins, etc.) to a global, systemic, conjunctive and organizational vision: distinct datasets are linked and we need to unravel these underlying links.
Massive data
In our current and modern clinical practice with new innovative and numeric tools, physicians collect massive data relative to the patient. Multi-omics approach is now described in literature [16,17,18].
In a global approach it seems important to collect the most exhaustive global information about the patient and not only the biological characteristics. However, these data are usually heterogeneous, quantitative versus qualitative, possibly censored or missing.
To our knowledge, little literature exists about the exploitation of such massive and heterogeneous data in metastatic breast cancer field.
We thus intend to integrate a massive and curated database with dynamic data overtime that will allow us to model the metastatic cancer during its various stages of progression, and will help us to understand it and better individualize the treatments.
Nevertheless, the heterogeneity, the censured character of the data, and above all, the very large number of variables with respect to the number of patients involve the use of statistical methods which have the ability to remain efficient despite these constraints (see the mathematical section below for details). As a consequence, it seems of first importance to associate the expertise of several teams in order to provide a satisfying method to decide which treatment process is the most adapted to each patient.
Rationale for conducting this study
Resistance to treatment in metastatic breast cancer remain poorly understood. The hypothesis on the multifactorial mechanisms of resistance must include tumor datas, patients and environment datas and need to be prospectively studied. This hypothesis explains the building of this prospective database concerning metastatic breast cancer patients.
This database contains epidemiological, socio-economic, clinical, biological, imaging, pathological and multi-omics data in order to take into account this multifactorial hypothesis.
With this project, we want to demonstrate the ability to exploit complex data in healthcare and in particular in cancer management. We chose a specific metastatic breast cancer model with no literature available for mathematical development in this application field. By sharing dynamic expertise in massive data and mathematics with different units, we want to enhance therapeutic management in the actual metastatic breast cancer example chosen.
Justification for this study is based on the following 3 points:
-
Prediction and new modelling of breast cancer outcome from complex data sources
-
Creation of algorithms and expertise to use massive data in cancer management
-
Interdisciplinary databases and co-working for data collection and analysis.
Study objectives
Short term and main purpose
To prove feasibility of creation of a clinico-biological database prospectively by collecting epidemiological, socio-economic, clinical, biological, imaging, pathological and multi-omics data before treatment and within 15 years after treatment start.
At the end of feasibility period, this database will contain a complete view for 300 patients. Once proved the feasibility, further prospective inclusions will permit in the mid-term to identify with sufficient statistical power the independent prognostic parameters for 15 yr-overall survival among the environmental, clinical, biological, imaging, bio-pathological and omic collected characteristics of patients with metastatic breast cancer.
The secondary objectives are
-
To describe response to treatment for each therapeutic sequence and identify new prognostic and predictive factors related to treatment response (assessed by multi-omic biological and tumor tissue analysis).
-
-To evaluate progression free survival (PFS) for each therapeutic sequence.
-
To identify the predictive factors of resistance to treatment.
-
To identify new prognostic and predictive factors related to treatment response.
-
To describe socio demographic profile, quality of life, Emotional Vulnerability, physical activity practice, nutritional assessment before and after treatments.
Exploratory objective
We will use in silico methods to integrate together complex data (epidemiological characteristics, clinical, biological, imaging, bio-pathological, and microbiota characteristics of each patient [19]) from this cohort in order to define an algorithm of individual decision for the prediction of the treatment response with needs to develop new statistical and modeling tools.
Design
Study design
This is a prospective uncontrolled cohort study of patients with metastatic breast cancer (Fig. 2).
Patients are followed in the institution (ICO cancer center, Nantes and Angers) with the usual therapeutic care and additional samples for 15 years.
Study population
Three phenotypic groups are identified on IHC done at inclusion: on metastatic sites or breast tumor if local recurrence, usual treatment protocols are often guided by the following groups:
-
Group 1: Patients HR + (ER+ and/or PR+ and HER2-)
. patients with history of adjuvant therapy
. patients with de novo metastatic disease
-
Group 2: Patients HER2 + with or without HR+
. patients with history of adjuvant therapy
. patients with de novo metastatic disease
-
Group 3: Patients triple negative (HR- and HER2-)
- patients with history of adjuvant therapy.
- patients with de novo metastatic disease.
For statistical analysis we will define a specific subgroup of BRCA mutation patients studied to highlight specific elements according to the main objective.
Inclusion criteria
-
1.
Written informed consent obtained from the patient prior to performing any protocol-related procedures, including screening biopsy, blood sample, faeces and questionnaires
-
2.
Men or women > 18 years old at time of written consent
-
3.
Patient with histologically confirmed breast cancer
-
4.
Breast cancer metastatic disease or locally advanced not eligible for local curative treatment intent with or without personal history of adjuvant therapy for this cancer (chemotherapy, radiotherapy, surgery …)
-
5.
Patient with metastases that can be biopsied.
-
6.
Performance status ≤2 (according to WHO criteria)
-
7.
Indication of any systemic therapeutic strategy can be performed alongside this current cohort in accordance with national and / or international recommendations.
-
8.
HR and HER2 status on metastatic sites or breast tumor if local recurrence
-
9.
Menopausal status: as per the institutional standard of care
-
10.
Patient is willing and able to comply with the protocol for the duration of the study including undergoing treatment and scheduled visits and examinations including follow up.
-
11.
Patient must be affiliated to a Social Health Insurance
Non-inclusion criteria
-
1.
Other malignancy treated within the last 5 years (except non-melanoma skin cancer or in situ carcinoma of the cervix)
-
2.
Coagulopathy or other pathology that contraindicates biopsy procedures
-
3.
Prior systemic treatment in metastatic setting
-
4.
Patients with exclusive brain metastasis not available for surgery
-
5.
Pregnant or nursing patient
-
6.
Individual deprived of liberty or placed under the authority of a tutor
-
7.
Impossibility to submit to the medical follow-up of this clinical trial for geographical, social or psychological reasons
Study visits
Further evaluation visits (Table 1):
For group1 without chemotherapy treatment: to be done every 4 months for 2 years, every 6 months for 3 years and once a year afterwards.
After end of chemotherapy: For group1, group2 group 3 receiving chemotherapy treatment: evaluation visits are to be done every 4 months afterwards.
-
1.
Hematology: Hematocrit, hemoglobin, platelet counts, red blood cell counts, white blood cell counts, and white blood cell differential, Coagulation parameters: PT, PTT, INR, Chemistry: Albumin, Alkaline phosphatase, Alanine aminotransferase (ALT), Amylase, Phosphorus, Aspartate aminotransferase (AST), Bicarbonate, Calcium, Chloride, Creatinine, creatinine clearance, Gamma GT, Glucose, Lactate dehydrogenase (LDH), Lipase, Magnesium, Total protein, Potassium, Sodium, Total bilirubin, Direct Bilirubin, Conjugated Bilirubin, Uric acid, Total Cholesterol, Triglycerides, HDL, LDL, CRP, albumin, pré-albumin, orosomucoïde, Iron status: iron, ferritin, soluble transferrin receptor, Thyroid function: TSH and fT3 and fT4.
-
2.
Disease-specific tumor markers: CA 15–3, ACE.
-
3.
Urine dipstick analysis: Bilirubin, pH, Blood, Protein, Glucose, Specific gravity, Ketones, Colour and appearance (Microscopy should be used as appropriate to investigate white blood cells and use the high-power field for red blood cells).
Data collection (Table 2)
Data management
For clinical data management the platform used to collect and manage the database will be centralized and hosted with the entire control of the institution.
All access to all data (entry, modification or simple consultation) is only possible with a password and is plotted in the database.
According to the recommendations of regulatory authorities, procedures have been defined and implemented to ensure the physical and computer security of the data:
-
Access is protected
-
Equipment hosting the database are dedicated and deposited in a private bay of the secure data center.
-
Backup of the computer system
-
Measures ensure the safeguarding of the computer system
-
Measures ensure the confidentiality of the data during the development of the computer application
-
Measures ensure the confidentiality of data during the maintenance of software or equipment
-
Authentication / Identification of the persons authorized to access the application
Sample size and statistical analysis
Determination of sample size: the primary endpoint is to detect predictive factors (profile), based on clinical and molecular analyses, and associated with 5 years-overall survival.
With experience of observational studies in our institution like ESME [21], the accrual rate of patients meeting inclusion criteria is 165 patients per year. According to observed events (OS) occurring within 60 months of follow-up, the proportion of patients alive at 60 months is 15%.
To provide a power of 80% to detect a clinico- biological profile that reduced OS with a hazard ratio equal to 1.5 and to concede a 5% first species error rate alpha, we plan to include 300 patients. Indeed, the number of event would be around 254, which allow analysing 20 profiles.
For BRCA mutation we will collect this information to define a sub-group for specific analysis.
Statistical analysis process: Table 3
Discussion
The EPICURE study aims to prove feasibility of creation of a dynamic and longitudinal clinico-biological database prospectively by collecting epidemiological, socio-economic, clinical, biological, pathological, multi-omic data. It offers a systemic and “more exhaustive possible” approach to collect all data available without “a priori” on its interests and with large variety of data in the longitudinal way of real-life. Enrollment started in December 2018.
This cohort and its databases serve different research programs:
SIRIC-ILIAD project (Imaging and Longitudinal Investigations to Ameliorate Decision-making in Multiple Myeloma and Breast Cancer) on imaging and biological research approaches; program supported by INCA DGOS and INSERM.
FEDER program on molecular imaging technological approaches and data integration specific questions.
Several specific scientific projects; based yet on one or several clinical, biological or omic compartiment data.
Availability of data and materials
There are no data available as this is a study protocol.
Abbreviations
- CNRS:
-
Centre National pour la Recherche Scientifique;
- CRCINA:
-
Centre de Recherche en Cancérologie Nantes Angers;
- INSERM:
-
Institut national de la Santé et recherché Médicale
- BC:
-
Breast Cancer
- QoL:
-
Quality of life
- INCA:
-
Institut National du Cancer
- DGOS:
-
Direction Générale de l’Offre de Soins
- IHC:
-
Immunohistochemistry
References
Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. JAMA. 2018;319(2):154–64.
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9.
Johnston SRD. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(7):1979–87.
Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero J-M, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(22):2718–24.
Campone M, Juin P, André F, Bachelot T. Resistance to HER2 inhibitors: is addition better than substitution? Rationale for the hypothetical concept of drug sedimentation. Crit Rev Oncol Hematol. 2011;78(3):195–205. https://doi.org/10.1016/j.critrevonc.2010.04.012.
Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29. https://doi.org/10.1007/s10549-018-05125-4.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. https://doi.org/10.1016/S1470-2045(14)71159-3.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S-A, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39.
Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(21):2585–92.
Le Tourneau C, Delord J-P, Gonçalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–34. https://doi.org/10.1016/S1470-2045(15)00188-6.
Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(6):811–21. https://doi.org/10.1016/S1470-2045(16)00106-6.
Baselga J, Im S-A, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904–16. https://doi.org/10.1016/S1470-2045(17)30376-5.
Reigosa A, Hardisson D, Sanzi F, Caleiras E, Saldivia F, Fernández A. Subclassification of the molecular types of breast cancer based on the expression of immunohistochemical markers and evolution. Invest Clin. 2016;57(2):187–216.
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83.
Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J mars. 2018;9(1):77–102. https://doi.org/10.1007/s13167-018-0128-8.
Chakraborty S, Hosen MI, Ahmed M, Shekhar HU. Onco-multi-OMICS approach: a new frontier in Cancer research. Biomed Res Int. 2018;2018:9836256.
Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67(4):326–44.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158(3):200–7.
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24.
Acknowledgements
Not Applicable.
Funding
This protocol is supported by:
- FEDER fundings: European Commission fundings managed by the Région Pays de Loire: « Fonds européen de développement régional » / European fundings for regional development https://projet-epicure.fr/
- AstraZeneca France: financial support in the bioclinical collection constitution (clinical trial).
- Lilly France: financial support in the bioclinical collection constitution (clinical trial).
EPICURE is integrated in the national French program SIRIC and will benefit from the multidisciplinary expertise of physicians and researchers teams. It will also benefit from the existing technological platforms.
Author information
Authors and Affiliations
Contributions
MCa and MCo lead the project. MCa MCo JSF FB and VS designed the study. PJ and VS provided statistic expertise. MCo MCa PJ and MR managed the study, data analysis and funding. MCo wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Each patient gives his written consent before inclusion in the study.
Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM): Date of authorization: 18/06/2018
Ethic committee: PROTECTION COMMITTEE Ile de France – Paris - Date of approval: 19/06/2018 Ref. CPP: CPPIDF1–2018-ND40-cat. 1
ClinicalTrials.gov identifier (NCT number): NCT03958136, registration 21st of May, 2019; retrospectively registered: https://clinicaltrials.gov/ct2/show/NCT03958136?term=NCT03958136&draw=2&rank=1
Consent for publication
Not Applicable.
Competing interests
The authors declare to have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Colombié, M., Jézéquel, P., Rubeaux, M. et al. The EPICURE study: a pilot prospective cohort study of heterogeneous and massive data integration in metastatic breast cancer patients. BMC Cancer 21, 333 (2021). https://doi.org/10.1186/s12885-021-08060-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08060-8