Abstract
Background
The identification of factors responsible for false negative (FN) rate at 18F- Fluorodeoxyglucose (FDG) Positron Emission Tomography /Computed Tomography (PET/CT) in para-aortic (PA) lymph nodes in the presurgical staging of patients with locally advanced cervical cancer (LACC) is challenging. The aim of this study was to evaluate the impact of PET/CT technology.
Methods
A total of 240 consecutive patients with LACC (International Federation of Gynecology and Obstetrics, FIGO, stage IB2-IVA) and negative Magnetic Resonance Imaging (MRI) and/or Computed Tomography (CT) and negative 18F-FDG PET/CT in the PA region, undergoing laparoscopic PA lymphadenectomy before chemoradiotherapy were included. The FN rate in patients studied with Time of flight (TOF) PET/CT (TOF PET) or non-Time of flight PET/CT (no-TOF PET) technology was retrospectively compared.
Results
Patients presented with FIGO stage IB (n = 78), stage IIA-B (n = 134), stage III (n = 18) and stage IVa (n = 10), squamous cell carcinoma (n = 191) and adenocarcinoma (n = 49). 141/240 patients were evaluated with no-TOF PET/CT and 99/240 with TOF PET/CT. Twenty-two patients (9%) had PA nodal involvement at histological analysis and considered PET/CT FN findings. The FN rate was 8.5% for no-TOF PET and 10% for TOF PET subgroup respectively (p = 0.98). Ninety patients (38%) presented with pelvic node uptakes at PET/CT. The FN rate in the PA region was 18% (16/90) and 4% (6/150) in patients with and without pelvic node involvement at PET/CT respectively (19 vs 3% for no-TOF PET and 17 vs 5% for TOF PET subgroup).
Conclusions
In LACC, FN rate in PA lymph nodes detection is a clinical issue even for modern PET/CT, especially in patients with pelvic uptake. Surgical lymphadenectomy should be performed in case of negative PET/CT at PA level in these patients, while it could be discussed in the absence of pelvic uptake.
Similar content being viewed by others
Background
The therapeutic approach in locally advanced cervical cancer (LACC) is a challenge and based on tumor volume, nodal metastasis, and clinical stage [1]. In LACC (i.e. FIGO stage IB2 to IVA) chemoradiation in association with pelvic External Beam Radiation Therapy (EBRT) is recommended in case of metastatic pelvic but negative Para-Aortic (PA) lymph nodes; in case of metastatic PA lymph nodes, an extended radiation field to PA region is applied [1, 2].
Therefore, imaging techniques with high diagnostic accuracy are fundamental for disease staging. Diagnostic imaging in cervical cancer includes abdomino-pelvic Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan and 18F-Fluorodeoxyglucose (FDG) Positron Emission Tomography /Computed Tomography (PET/CT) 18F-FDG PET/CT [3,4,5,6].
18F-FDG PET/CT may help to rule out loco-regional lymph nodes and distant metastasis through a whole-body examination. Indeed, recent guidelines recommend PET/CT over CT in initial staging of LACC to detect extra pelvic disease and for EBRT plan delineation [1]. However, in patients with LACC, high false negative rate for PET/CT in detecting metastatic PA lymph nodes has been reported in several published studies [7,8,9,10,11]. For this reason, surgical staging is still considered in patients with negative presurgical 18F-FDG PET/CT at PA level [12]. During the last years, we assisted to a rapid development of PET technology. In particular, the introduction of Time of Flight (TOF) technique using crystal materials with relatively high time resolution should theoretically increase the sensitivity and consequently lesion detectability [13,14,15].
The aim of this study is to determine the impact of TOF PET/CT vs no-TOF PET/CT technology on false negative rate in PA lymph node detection in the presurgical staging of patients with LACC.
Methods
Study design and patient enrolment
All consecutive patients with locally advanced cervical cancer (LACC) according to FIGO classification (stage IB2-IVA) treated between 2007 and 2015 at Gustave Roussy (GR), with negative morphological imaging (abdomino-pelvic MRI in most cases or CT scan and pelvic MRI) and negative 18F-FDG PET/CT in the para-aortic area (PA), undergoing laparoscopic PA lymphadenectomy before chemoradiotherapy were included. Patients with a poor prognosis according to histological subtype or peritoneal carcinomatosis were excluded. Institutional review board of Gustave Roussy approved the study and waived the need to obtain informed consent.
The surgery included the removal of PA nodes from the aortic bifurcation to the left renal vein. PA above and below the inferior mesenteric artery, preaortic, superficial intercavoaortic and precaval groups were removed. Pelvic nodes were not resected because included in the radiotherapy field.
All patients were treated with pelvic external beam radiation therapy (45 to 50 Gy) associated to a concomitant cisplatin-based chemotherapy protocol after surgery. Patients with histologically proven PA node metastasis after staging laparoscopic surgery were treated with pelvic and extended to PA region EBRT (45 to 60 Gy) with concomitant cisplatin chemotherapy.
A total of 258 patients were initially evaluated. Eighteen patients were excluded from analysis because of a final IB1 staging with involved pelvic nodes (n = 9), small cell carcinoma histology (n = 1), discovery of peritoneal carcinomatosis during laparoscopy (n = 5), ovarian metastasis (n = 1) and dubious findings for lymph node metastases in the para-aortic region at PET/CT (n = 2) (Fig. 1). In the end, a total of 240 patients were considered for the analysis and two subgroups of patients were retrospectively identified according to the PET/CT technology used: patients studied with TOF PET/CT and patients studied with no-TOF PET/CT.
18F-FDG pet/CT
All the patients underwent a 18F-FDG PET/CT before surgery. One hundred and eighteen patients underwent 18F-FDG PET/CT at Gustave Roussy and the remaining 122 in others different Nuclear Medicine Services in France according to guidelines [16]. After 6 h of fasting, patients performed images acquisition 60 min after the administration of a median activity of 254 MBq of 18F-FDG (range: 137–591 MBq). Median glycaemia before PET/CT was 4.6 mmol/L (range:0.74–10.4).
On the basis of constructor characteristics and reconstruction algorithms, PET/CT scanners were divided into two categories: TOF PET/CT (TOF PET) using 3D coincidence algorithms for image reconstruction and including TOF system (PET/CT Gemini-TF Philips, PET/CT Biograph mCT20-mCT40 Siemens, PET/CT Discovery 690 General Electric Healthcare, PET/CT Discovery 710 General Electric Healthcare) and no-TOF PET/CT (no-TOF PET) with 2D-3D coincidence algorithms for image reconstruction and not provided with TOF system (PET/CT Biograph 6-Siemens, PET/CT Gemini GXL).
Statistical analyses
Patient characteristics were reported as relative/absolute frequencies for categorical covariates and as median (range) for continuous ones. Their potential differences, when stratified as TOF-PET vs. no-TOF PET, were inferred by the Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous ones. All reported p-values were obtained by the two-sided exact method, at the conventional 5% significance level. Data were analysed by R 3.6.3 (R Foundation for Statistical Computing, Vienna-A, http://www.R-project.org).
Results
Patient characteristics
The median age was 45 years (21–68). Among 240 patients, 78/240 presented with stage IB, 134/240 with stage IIA-B, 18/240 with stage III and 10/240 with stage IVa of the disease respectively. One hundred ninety-one patients (n = 191) had squamous cell carcinoma and 49 had adenocarcinoma. Patient Body Mass Index (BMI) ranged from 14 to 43 Kg/m2 with a median of 23 Kg/m2.
Before surgery, 18F-FDG PET showed uptake in pelvic lymph nodes in 90/240 (38%) patients. The uptake was unilateral in 57/90 (63%) patients, located in the right (45%) and in the left region (51%) respectively (3.5% missing data). All patients with unilateral uptake presented with ≤2 positive lymph nodes at 18FDG PET/CT. Pelvic uptake was bilateral in 33/90 (37%) of patients (Fig. 2). Thirty patients presented with ≤2 positive lymph nodes at 18FDG PET/CT by side, while the remaining 3 three patients presented with > 2 positive lymph nodes (5 + 2 in 1 case, 2 + 3 in 1 case and 1 + 3 in 1 case in the left and right region respectively). In the remaining 150/240 (62%) there was no uptake in the pelvic region. In regard to surgical laparoscopy techniques, 235 patients underwent a retroperitoneal approach and five a transperitoneal approach. One patient had conversion to laparotomy. The mean delay between 18F-FDG PET/CT and surgery was 15.5 days (range: 1–56). The median number of removed PA lymph nodes per patient was 15 (range: 2–40). Twenty-two patients (9%) had para-aortic nodal involvement at histological analysis considered as FN PET findings with a median of 2 metastatic lymph nodes per-patient (range: 1–8). Twenty-one patients had squamous carcinoma and 1/22 had a clear cell adenocarcinoma subtype. In 13/22 (59%) the size of the biggest PA involved node was > 5 mm while in the remaining 9/22 (41%) the biggest size was ≤5 mm. Among the 22 patients with PA metastases, 16 presented pelvic 18F-FDG uptake at PET/CT while the remaining 6 patients did not. The FN rate in the PA region was 18% (16/90) and 4% (6/150) in patients with and without pelvic lymph node uptake respectively. The FN rate was 16% (9/57) and 21% (7/33) in patients with unilateral and bilateral pelvic uptake respectively. Patient population characteristics are summarized in Table 1.
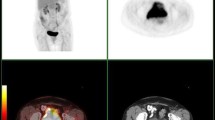
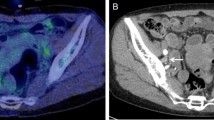
This is a case (a) of a patient with stage IIB cervical cancer (b blu arrow) studied with TOF PET/CT presenting pelvic pathological lymph nodes (red arrow) located in right (b, c red arrow: 2 lymph nodes), in the left pelvic region (c red arrow: 1 lymph node) and in the common iliac region. No significant uptake was present in the para-aortic region at. A single macrometastase in the para-aortic region was detected at final histology
TOF vs no-TOF PET/CT analysis
In relation to PET tomograph technology, 141/240 patients were evaluated with no-TOF PET/CT (73 at GR and 68 in other centers) and 99/240 with TOF PET/CT (45 at GR and the remaining 54 in other centers). Patient population characteristics of the two groups are summarized in Table 2. Patients studied with TOF PET were slightly older than no-TOF PET group (median age 48 vs 45 years; p = 0.009). The majority of patients were classified as stage IIb, 73/141 (51.8%) compared to 48/99 (48.5%) in no-TOF and TOF PET group respectively (p = 0.36). Before surgery, 18F-FDG PET showed uptake in pelvic lymph nodes in 48/141 (34%) and 42/99 (42%) patients evaluated with no-TOF and TOF PET/CT scan respectively (p = 0.50). The median number of PA lymph nodes removed was 15 (range: 2–39) and 16 (range: 3–40) in the group of patients evaluated with no-TOF and TOF PET/CT (p = 0.25) respectively. Amongst the first group, 12/141 (8.5%) presented with metastatic PA lymph nodes at histology compared to 10/99 (10%) in the second group (p = 0.98). The maximum size of metastatic PA lymph nodes was > 5 mm in 7/12 (58.3%) and in 6/10 (60%) patients and ≤ 5 mm in 5/12 (41.7%) and 4/10 (40%) respectively in patients evaluated with no-TOF and TOF PET/CT (p = 0.48). The FN rate was 19% (9/48) and 17% (7/42) in patients with pelvic uptake and 3% (3/93) and 5% (3/57) in patients without pelvic uptake in the no-TOF and TOF PET group. FN rate was 16% (5/32) and 16% (4/25) in cases of unilateral pelvic uptake vs 25% (4/16) and 18% (3/17) in cases of bilateral uptake in the no-TOF and TOF PET group (Table 3).
Discussion
PA lymph node metastases occur in 10–25% of patients with LACC and are correlated with pelvic, common iliac nodes involvement and larger primary tumor size [17, 18]. The detection of metastatic PA lymph nodes is a fundamental step to define the correct therapeutic approach in LACC and to improve patient outcome [19,20,21,22]. False negative rates for 18F-FDG PET and PET/CT in PA areas have been reported in several studies ranging from 5 to 17% [7, 11].
The identification of factors responsible for false negative PET/CT findings in PA region in LACC is challenging. In the first studies on this topic, false negative rate was mainly related to lymph node size < 5 mm considered beyond the machine resolution. In the study by Roh et al. including patients with stages IA to IVA PET sensitivity was 38% increasing to 52% in case of lymph nodes > 5 mm and 65% in the case of lymph nodes > 10 mm [7].
The use of hybrid machines such as PET/CT has greatly improved the accuracy of the technique allowing a better localisation and characterisation of abdominal FDG uptakes [23]. However, results among the studies are variable and even in more recent studies false negative rate of 18F-FDG PET/CT remains high up to 22%, in particular in early-stage disease [24,25,26,27]. The retrospective study by Leblanc et al. including 125 patients with cervical cancer in stage IB2 –IIA with negative pre-operative CT scans or MRI, FDG PET or PET/CT showed a sensitivity of 33% in PA lymph nodes detection with false negative rate of 67% [10]. A recent metanalysis including 29 studies focused on 18F-FDG PET alone and PET/CT reported a pooled sensitivity of 0.72 in detecting both pelvic and PA lymph nodes, 0.83 in case of locally advanced disease and 0.41 in early-stage disease [6]. Another recent recent metanalysis from Yu et al. including 14 studies evaluated specifically the diagnostic performance of PET/CT for the detection of PA lymph nodes [28]. The authors reported a pooled sensitivity and a pooled specificity of 0.71 (95% CI: 0.54–0.83) and 0.97 (95% CI: 0.93–0.98) respectively and a pooled positive likelihood ratio (PLR) and negative likelihood ratio NLR (NLR) of 21.53 and 0.30, respectively. The authors concluded that 18F-FDG PET is an important imaging method for the detection of PA lymph nodes but underline the limitations of the included studies, mostly retrospective and with low sample size. The ACRIN6671 trial reported a sensitivity for 18F-FDG PET with contrast enhanced CT of 0.83 in the detection of pelvic lymph nodes decreasing to 0.50 for PA lymph nodes [29].
In a previous multicentric prospective study evaluating 237 patients in the same setting, our group demonstrated 18F-FDG PET/CT false negative rate of 12% and the results supported the conclusion that PA lymph node dissection should be performed in LACC in case of negative 18F-FDG PET/CT in PA region for a proper patient staging [11].
The impact of PET device has never been taken into account in order to evaluate and to compare accuracy in this scenario. The aim of this study was to evaluate the impact of PET/CT technology on false negative rate in PA lymph node detection in LACC staging. In the last decades there have been multiple advances in PET technology with improvement in cancer imaging [30]. The introduction of Time of Flight (TOF) technique using crystal materials with relatively high time resolution should increase the sensitivity and (consequently) lesion detectability especially in overweight patients [31, 32]. This technology is useful in clinical practice, allowing shorter examinations, reduction of the radiation dose to the patient, successful scanning of overweight patients, clearer characterisation of low uptake areas and visualization of smaller lesions [31, 32].
Our data show that the false negative rate in PA lymph node detection in patients evaluated by PET/CT scan without TOF system integration compared to PET/CT with TOF integration is comparable (8.5 vs 10%; p = 0.98). These findings suggest that even if the sensitivity or lesion detectability is supposed to be higher in modern PET/CT scanner the limitation of the technique to assess PA lymph node is still a clinical issue in this specific clinical setting. The resolution of the PET/CT technology with TOF is considered between 4 and 6 mm [13, 31, 32]. Considering lesion size, in our study 59% of the metastatic lymph nodes detected at histology presented with a diameter > 5 mm with a similar distribution in the two groups. In our opinion, the size cannot be considered the only cause of the false negative rates of 18F-FDG PET/CT. Some studies suggest that 18F-FDG uptake can be influenced by tumoral histotype in primary and metastatic lesions, with higher 18F-FDG uptake in squamous cell compared to non-squamous cell tumors. In the study of Lin et al. nodal metastasis was less likely to be detected by 18F-FDG-PET in patients with early-stage adenocarcinoma compared to squamous carcinoma [33]. Nevertheless, in our study almost 80% of the patients and 21/22 patients with false negative 18F FDG PET/CT presented with squamous cell carcinoma. The para-aortic lymph nodes are close to the urinary tract and 18F-FDG physiological urinary elimination can hide small 18F-FDG avid lesions. In this case, the use of hybrid imaging such as PET/MRI and contrast enhanced diagnostic CT coupled with PET might better differentiate between pathological and physiological uptake, but well-designed trials are warranted to confirm this assumption [34]. Patient BMI should be also taken into account because in overweight patients the quality of images can be affected and the detection rate reduced, but the BMI in the two groups of our study was similar.
Finally, patients with pelvic pathological nodes metastases, compared to patients without pathological pelvic nodes, have a higher risk to develop PA metastases [17, 18]. In our study the percentage of pelvic positive lymph nodes in TOF PET compared to no-TOF PET group (42% vs 34%) was not statistically different (p = 0.5). FN rate in PA region was higher in case of pelvic uptake at 18F-FDG PET/CT, especially when bilateral, compared to negative pelvic uptake cases. These data are confirmed in both no-TOF and TOF PET groups with FN rate of 19% vs 17 and 3% vs 5% respectively according to pelvic vs no pelvic uptake status.
A very recent retrospective study (ONCO-GF) reported a PET/CT sensitivity in identifying PA lymph nodes metastases of 23.5%. Among 151 patients with negative PA lymph node at PET/CT, 26 (17,2%) had histological proven metastases including 21 with macroscopic lesions and the percentage increased considering patients with positive pelvic uptake (18,1%) confirming our observation. The authors conclude that para-aortic surgical staging contributes significantly to individualise the radiation treatment plan especially in patients with positive pelvic uptake [35]. FRANCOGYN study including 647 patients with LACC treated by concurrent chemoradiation therapy (CRT) and no evidence of para-aortic metastasis on pre-operative imaging work-up, confirmed in multivariate model analysis surgical staging as an independent prognostic factor for Disease Free Survival [36]. On the other hand, a retrospective study from Mayo Clinic registry including 148 patients did not find differences comparing surgical versus radiological staging (mainly based on CT, MRI and PET combination) in 5-year PFS (HR 1.11, 95% CI 0.54–2.30, p = 0.77) and 5-year OS (HR 1.02, 95% CI 0.46–2.29, p = 0.96). The presence of PA lymph nodes metastasis significantly predicted unfavourable PFS (HR 2.76, 95% CI 1.23–6.18, p = 0.01) and OS (HR 3.46, 95% CI 1.40–8.55 [37]. More consistent data will be forthcoming in large trial of surgical vs radiological staging. A prospective study, including 600 patients with stage IB2- IVA cervical cancer, is ongoing in order to evaluate the impact on survival of standard chemoradiotherapy treatment based only on 18F-FDG PET/CT staging compared to an adapted treatment based on PA lymph node dissection staging [38]. However, considering our results and previous published data, surgical lymph node dissection could be discussed in patients without involved pelvic lymph nodes on 18F-FDG PET/CT, considering the very low false negative rate in this case. On the other hand, in case of 18F-FDG PET/CT uptake in the pelvic region, in particular if bilateral, the risk of FN findings in PA lymph node is higher and surgical dissection could be preferred.
Our study presents several limitations, in particular the retrospective nature, the heterogeneity of the PET/CT scanners employed and the lack of a standardized protocol for images acquisition. Furthermore, for strength conclusions on the impact of TOF technique a direct comparison of images derived from the same patient with and without TOF reconstruction method should be performed through a proper trial. On the other hand, the impact of PET/CT device has been tested in a real clinical setting, in a wide sample size population with histological confirmation, and in comparable patient populations. At this moment the increasing availability of Digital PET scanner will probably change the scenario and a direct comparison among different reconstruction methods will be the next step to evaluate the impact of technological development in this clinical setting. Finally, patients with positive PA at 18F-FDG PET/CT have not been included in the study and the analysis of true or false positive findings in the PA region has not been performed because a reliable confirmation by histology would not have been available being the patient directly sent to extended EBRT protocol.
Conclusions
The FN rate in PA lymph nodes detection in LACC is high, independently from PET/CT technology. Surgical staging should be preferred in case of negative pre-surgical imaging in the PA region, in particular in case of 18F-FDG PET/CT abnormal uptakes in pelvic lymph nodes. However, the FN rate in PA lymph nodes detection decreases dramatically in case of negative PET/CT in pelvic region and surgical staging could be discussed in this case. Prospective trials are mandatory to validate this approach.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due privacy rules but are available from the first author on reasonable request at the following email address Sebastien.GOUY@gustaveroussy.fr.
Abbreviations
- 18F-FDG:
-
18F- Fluorodeoxyglucose (FDG)
- PET/CT:
-
Positron Emission Tomography /Computed Tomography
- CT:
-
Computed Tomography
- MRI:
-
Magnetic Resonance Imaging
- FIGO:
-
International Federation of Gynecology and Obstetrics
- LACC:
-
Locally advanced cervical cancer
- PA:
-
Para-aortic
- FN:
-
False Negative
- TOF:
-
Time of Flight
- EBRT:
-
External Beam Radiation Therapy
- GR:
-
Gustave Roussy
- BMI:
-
Body Mass Index
- CONSORT:
-
Consolidated standards of reporting Trials
- SUV:
-
Standardized Uptake Value
References
Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17(1):64–84.
Bhatla N, Berek JS, Cuello Fredes MDenny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–35.
Bellomi M, Bonomo G, Landoni F, Villa G, Leon ME, Bocciolone L, et al. Accuracy of computed tomography and magnetic resonance imaging in the detection of lymph node involvement in cervix carcinoma. Eur Radiol. 2005;15(12):2469–74.
Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, Grigsby PW. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010;28(12):2108–13.
Balleyguier C, Sala E, Da Cunha T, Bergman A, Brkljacic B, Danza F, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21(5):1102–10.
Liu B, Gao S, Li S. A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical Cancer: a meta-analysis based on 67 studies. Gynecol Obstet Investig. 2017;82(3):209–22.
Roh JW, Seo SS, Lee S, et al. Role of positron emission tomography in pretreatment lymph node staging of uterine cervical cancer: a prospective surgicopathologic correlation study. Eur J Cancer. 2005;41(14):2086–92.
Boughanim M, Leboulleux S, Rey A, Pham CT, Zafrani Y, Duvillard P, et al. Histologic results of para-aortic lymphadenectomy in patients treated for stage IB2/II cervical cancer with negative [18F] fluorodeoxyglucose positron emission tomography scans in the para-aortic area. J Clin Oncol. 2008;26(15):2558–61.
Mortier DG, Stroobants S, Amant F, Neven P, VAN Limbergen E, Vergote I. Laparoscopic Para-aortic lymphadenectomy and positron emission tomography scan as staging procedures in patients with cervical carcinoma stage IB2-IIIB. Int J Gynecol Cancer. 2008;18(4):723–9.
Leblanc E, Gauthier H, Querleu D, Ferron G, Zerdoud S, Morice P, et al. Accuracy of 18-fluoro-2-deoxy-D-glucose positron emission tomography in the pretherapeutic detection of occult Para-aortic node involvement in patients with a locally advanced cervical carcinoma. Ann Surg Oncol. 2011;18(8):2302–9.
Gouy S, Morice P, Narducci F, Uzan C, Martinez A, Rey A, et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic Para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol. 2013;31(24):3026–33.
Gouy S, Morice P, Narducci F, Uzan C, Gilmore J. Kolesnikov-Gauthier H, et al nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol. 2012;13(5):e212–20.
Moses WW. Recent advances and future advances in time-of-flight PET. Nucl Instrum Methods Phys Res A. 2007;580:919–24.
Karp JS, Suleman S, Daube-Witherspoon ME, Muehllehner G. Benefit of time-of-flight in PET: experimental and clinical results. J Nucl Med. 2008;49:462–7.
Suljic A, Tomse P, Jensterle L, Skrk D. The impact of reconstruction algorithms and time of flight information on PET/CT image quality. Radiol Oncol. 2015;49(3):227–33.
Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200.
Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999 Apr 1;85(7):1547–54.
Huang H, Liu J, Li Y, Wan T, Feng Y, Li Z, Huang Q. Metastasis to deep obturator and Para-aortic lymph nodes in 649 patients with cervical carcinoma. Eur J Surg Oncol. 2011 Nov;37(11):978–83.
Gold MA, Tian C, Whitney CW, Rose PG, Lanciano R. Surgical versus radiographic determination of para-aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: a Gynecologic Oncology Group Study. Cancer. 2008;112(9):1954–63.
Marnitz S, Schram J, Budach V, Sackerer I, Vercellino GF, Sehouli J, Köhler C. Extended field chemoradiation for cervical cancer patients with histologically proven Para-aortic lymph node metastases after laparaoscopic lymphadenectomy. Strahlenther Onkol. 2015;191(5):421–8.
Pomel C, Martinez A, Bourgin C, Beguinot M, Benoit C, Naik R, et al. Survival effect of laparoscopic Para-aortic staging in locally advanced cervical cancer: a retrospective cohort analysis. BJOG. 2017;124(7):1089–94.
Sapienza LG, Gomes MJL, Calsavara VF, Leitao MM Jr, Baiocchi G. Does Para-aortic irradiation reduce the risk of distant metastasis in advanced cervical cancer? A systematic review and meta-analysis of randomized clinical trials. Gynecol Oncol. 2017;144(2):312–7.
Leseur J, Devillers A, Williaume D, Le Prisé E, Fougerou C, Bouriel C, et al. ((18) F)-fluorodeoxyglucose PET/CT in cervix cancer: lymph node assessment and prognostic/predictive value of primary tumour analysis. Cancer Radiother. 2011;15(8):699–708.
Ramirez PT, Jhingran A, Macapinlac HA, Euscher ED, Munsell MF, Coleman RL, et al. Laparoscopic extraperitoneal Para-aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117(9):1928–34.
Dong Y, Wang X, Wang Y, Liu Y, Zhang J, Qian W, Wuet S. Validity of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for pretreatment evaluation of patients with cervical carcinoma: a retrospective pathology-matched study. Int J Gynecol Cancer. 2014;24(9):1642–7.
Driscoll DO, Halpenny D, Johnston C, Sheehy N, Keogan M. 18F-FDG-PET/CT is of limited value in primary staging of early stage cervical cancer. Abdom Imaging. 2015;40(1):127–33.
Nogami Y, Banno K, Irie H, Iida M, Kisu I, Masugi Y, et al. The efficacy of preoperative positron emission tomography-computed tomography (PET-CT) for detection of lymph node metastasis in cervical and endometrial cancer: clinical and pathological factors influencing it. Jpn J Clin Oncol. 2015;45(1):26–34.
Yu W, Kou C, Bai W, Yu X, Duan R, Zhu B, Li Y, Hua W, Ren X, Yang Y. The diagnostic performance of PET/CT scans for the detection of Para-aortic metastatic lymph nodes in patients with cervical cancer: a meta-analysis. PLoS One. 2019;14(7):e0220080.
Atri M, Zhang Z, Dehdashti F, Lee SI, Ali S, Marques H, et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: results of ACRIN6671/GOG0233 trial. Gynecol Oncol. 2016;142(3):413–9.
van der Vos CS, Koopman D, Rijnsdorp S, Arends AJ, Boellaard R, van Dalen JA, et al. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):4–16.
El Fakhri G, Surti S, Trott CM, Scheuermann J, Karp JS. Improvement in lesion detection with whole-body oncologic time-of-flight PET. J Nucl Med. 2011;52(3):347–53.
Surti S, Scheuermann J, El Fakhri G, Daube-Witherspoon ME, Lim R, Abi-Hatem N, et al. Impact of time-of-flight PET on whole-body oncologic studies: a human observer lesion detection and localization study. J Nucl Med. 2011;52(5):712–9.
Lin AJ, Wright JD, Dehdashti F, Siegel BA, Markovina S, Schwarz J, et al. Impact of tumor histology on detection of pelvic and Para-aortic nodal metastasis with 18 F-fluorodeoxyglucose-positron emission tomography in stage IB cervical cancer. Int J Gynecol Cancer. 2019;29(9):1351–4.
Kitajima K, Suenaga Y, Ueno Y, Kanda T, Maeda T, Deguch M, et al. Fusion of PET and MRI for staging of uterine cervical cancer: comparison with contrast-enhanced (18) F-FDG PET/CT and pelvic MRI. Clin Imaging. 2014;38(4):464–9.
De Cuypere M, Lovinfosse P, Goffin F, Gennigens C, Rovira R, Duch J, et al. Added value of Para-aortic surgical staging compared to 18F-FDG PET/CT on the external beam radiation field for patients with locally advanced cervical cancer: an ONCO-GF study. Eur J Surg Oncol. 2020;46(5):883–7.
Dabi Y, Simon V, Carcopino X, Bendifallah S, Ouldamer L, Lavoue V, Groupe de Recherche FRANCOGYN, et al. Therapeutic value of surgical paraaortic staging in locally advanced cervical cancer: a multicenter cohort analysis from the FRANCOGYN study group. J Transl Med. 2018;16(1):326.
Yang J, Delara R, Magrina J, Magtibay P, Yi J, Langstraat C, et al. Comparing survival outcomes between surgical and radiographic lymph node assessment in locally advanced cervical cancer: a propensity score-matched analysis. Gynecol Oncol. 2020;156(2):320–7.
Frumovitz M, Querleu D, Gil-Moreno A, Morice P, Jhingran A, Munsellet MF. al. Lymphadenectomy in locally advanced cervical cancer study (LiLACS): phase III clinical trial comparing surgical with radiologic staging in patients with stages IB2-IVA cervical cancer. J Minim Invasive Gynecol. 2014;21(1):3–8.
Acknowledgments
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
S.G. and D. D did the literature research, conceived and designed the study and analysed the data. S. G., V.S., A.I., A. M. and P.M. were responsible for all the surgical interventions. C.C. was responsible for patient’s radiotherapy planning and treatment. C. G. was responsible for all histological analysis. P. P. and A. L. were responsible for oncological patient management. D. D, M.T., S.G. were responsible for all PET scans revision. All the authors significantly contributed to the development and approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board of Gustave Roussy approved the study and due to the retrospective nature of the study the need to obtain informed consent was waived.
Consent for publication
Not applicable.
Competing interests
S.G. received speaker honorarium from ROCHE, outside the submitted work.
The other authors have nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gouy, S., Seebacher, V., Chargari, C. et al. False negative rate at 18F-FDG PET/CT in para-aortic lymphnode involvement in patients with locally advanced cervical cancer: impact of PET technology. BMC Cancer 21, 135 (2021). https://doi.org/10.1186/s12885-021-07821-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-07821-9