Abstract
Background
Epidemiological studies have clarified the potential associations between regular aspirin use and cancers. However, it remains controversial on whether aspirin use decreases the risk of cancers risks. Therefore, we conducted an updated meta-analysis to assess the associations between aspirin use and cancers.
Methods
The PubMed, Embase, and Web of Science databases were systematically searched up to March 2017 to identify relevant studies. Relative risks (RRs) with 95% confidence intervals (CIs) were used to assess the strength of associations.
Results
A total of 218 studies with 309 reports were eligible for this meta-analysis. Aspirin use was associated with a significant decrease in the risk of overall cancer (RR = 0.89, 95% CI: 0.87–0.91), and gastric (RR = 0.75, 95% CI: 0.65–0.86), esophageal (RR = 0.75, 95% CI: 0.62–0.89), colorectal (RR = 0.79, 95% CI: 0.74–0.85), pancreatic (RR = 0.80, 95% CI: 0.68–0.93), ovarian (RR = 0.89, 95% CI: 0.83–0.95), endometrial (RR = 0.92, 95% CI: 0.85–0.99), breast (RR = 0.92, 95% CI: 0.88–0.96), and prostate (RR = 0.94, 95% CI: 0.90–0.99) cancers, as well as small intestine neuroendocrine tumors (RR = 0.17, 95% CI: 0.05–0.58).
Conclusions
These findings suggest that aspirin use is associated with a reduced risk of gastric, esophageal, colorectal, pancreatic, ovarian, endometrial, breast, and prostate cancers, and small intestine neuroendocrine tumors.
Similar content being viewed by others
Background
Aspirin has been used as an analgesic and in the prevention of cardiovascular diseases events in the past decades and is one of the most commonly used drugs worldwide [1, 2]. Clinical and epidemiological studies reported that the rates of aspirin usage in different populations across different countries ranging from 11% to 54% [3,4,5]. Since the 1970s, many researchers started to focus on the effects of aspirin on cancers [6, 7]. However, these original studies were not comprehensive, and the effects on some cancers were controversial [8, 9].
Although several meta-analyses have been conducted to assess the associations between aspirin use and the risk of cancers(e.g., gastric, esophageal, pancreatic, lung, squamous cell carcinoma, breast, ovarian, and prostate cancers) [10,11,12,13,14,15,16,17,18], most of these studies were restricted to certain types of cancers, and some types such as hepatobiliary and cervical cancer could not be investigated. In addition, 70 new studies have been published since 2012. Therefore, this comprehensive systematic review and updated meta-analysis was conducted to explore the reliability of risk estimates between aspirin usage and most types of cancers and provide a landscape of aspirin use and cancer incidence.
Methods
Search strategy
This systematic review was conducted in accordance with the checklist proposed by the Meta-analysis of Observational Studies in Epidemiology group [19]. We searched multiple electronic bibliographic databases to identify studies published from database inception till March 2017, including PubMed, Embase, and Web of Science databases, with the following search terms: (“cancer” OR “neoplasm” OR “carcinoma”) AND (“aspirin” OR “acetylsalicylic acid” OR “non-steroidal anti-inflammatory drugs” OR “NSAIDs”). We restricted our search to human studies and published in English. In addition, reference lists from relevant reviews and retrieved articles were searched for qualifying studies.
Inclusion criteria
The inclusion criteria were: 1) case-control or cohort studies; 2) studies that evaluated the relationships between the use of aspirin and the risk of cancers; 3) studies that reported risk estimates with 95% confidence interval (CI) or provided information that enabled us to calculate them. The exclusion criteria were: 1) studies that used other combinations of NSAIDs, which prevented the determination of the specific effect of aspirin, and 2) studies involving patients with specific diseases (e.g., Barrett’s esophagus, Crohn’s disease, or ulcerative colitis). Only the latest or the most informative study was included when multiple studies were published on the same study population.
Data extraction
The following information was obtained from each study: first author name, year of publication, study period, study location, study design, number of cases, number of participants, gender, definition of aspirin exposure, as curtained methods of exposure, odds ratios (ORs), hazard ratios (HRs) or relative risks (RRs) with their corresponding 95% CIs, and confounding factors adjusted in the analysis. The most fully-adjusted risk estimates with its corresponding 95% CIs (when available) were preferentially extracted. Data extraction was conducted independently by two authors (Y.Q. and T.T.Y.), and discrepancies were resolved by discussion with a third investigator (Z.X.L.).
Quality assessment
Quality assessment of eligible studies was performed independently by two reviewers (Y.Q. and T.T.Y.) according to the Newcastle-Ottawa Quality Assessment Scale [20]. This scale allocates a maximum of nine points based on the selection (0–4 points), comparability (0–2 points), and exposure/outcome of the study participants (0–3 points). Scores of 0–3, 4–6, and 7–9 were classified as low, moderate, and high-quality studies respectively.
Statistical analysis
RRs were used as the common measurement of the associations between aspirin use and the risk of cancer. Because cancer is a rare event in general, we could generally ignore the distinctions among the various measures of relative risk (e.g., odds ratios, rate ratios, and risk ratios) [21], and considered that ORs and HRs were similar to RRs. When risk estimates for different durations of aspirin use or different levels of aspirin utilization were available, the study-specific RRs were subsequently recalculated in the primary analysis by pooling the risk estimates compared with the reference group. A random effects model was selected to estimate the pooled RRs (95% CI) for the associations between aspirin use and the risk of cancer if the risk estimates for different subtypes of cancer were available. Summary estimates were derived from meta-analyses using random effects models. Studies involving different populations or different types of cancers were treated as independent studies.
To assess the heterogeneity in results of individual studies, I2 statistic (values of 25%, 50%, and 75% represented cutoff points for low, moderate, and high degrees of heterogeneity, respectively) were used [22]. Publication bias was assessed with Funnel plots, the Begg’s rank correlations and Egger’s regression model. Subgroup analyses for study design, study location, gender, exposure assessment, quality assessment, duration of aspirin use (years), and frequency of aspirin use (tablets/week) were conducted to explore the potential heterogeneity among studies. Subgroup analysis was not conducted for strata with less than five studies. Because time-related biases are common in observational studies of medications and are often responsible for apparent protective effects of drugs, we conducted analyses both including and excluding studies with immortal time bias (bias because of the inclusion of follow-up time during which events cannot occur) [23]. Statistical analyses were performed with Stata version 12.0. (College Station, TX, USA). All reported probabilities (P values) were two-tailed with a significance level of 0.05.
Results
Literature search and study characteristic
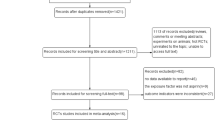
Figure 1 shows the process for the identification of eligible studies. A total of 28,683 studies were identified and 298 studies remained in the analysis after assessing the titles and abstracts according to the criteria mentioned above. In total, 307 potentially relevant articles were reviewed in their entirety. Among them, 89 articles were further excluded due to the following reasons: 26 articles were not observational design, 11 articles defined exposure combined with other NSAIDs, 8 articles evaluated cancer mortality, 39 articles were duplicate publications on the same subject population, and 5 articles (1 for Crohn’s disease [24], 1 for ulcerative colitis [25], 3 for Barrett’s esophagus [26,27,28]) included patients with specific diseases. Ultimately, 218 studies with 309 independent reports were included in the present meta-analysis.
The main characteristics of the 218 eligible articles published between 1985 and 2016 are summarized in Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 and 21. Results were presented according to study design. This study altogether included 161 cohort studies and 148 case-control studies. Among them, 135 studies were conducted in North America, 12 in Asia, 61 in Europe, 8 in Oceania, and 2 were multi-country studies. Overall, the summarized RR was 0.89(95%CI: 0.87–0.91), indicating a decreased risk of cancer associated with the use of aspirin. The combined RRs were 0.82 (95% CI: 0.79–0.85) for the case-control studies and 0.94 (95% CI: 0.92–0.97) for the cohort studies. We also observed a apparent beneficial effect of aspirin use when excluding 41 studies deemed to be prone to immortal time bias (RR = 0.87, 95%CI:0.85–0.89) in the meta-analysis.
Aspirin use and the risk of cancers
Figures 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and 18 and Additional file 1: Table S1 shows the RRs for the 21 separate cancer sites that we assessed and that of the total cancers. The use of aspirin was associated with a reduced cancer risk for ten specific sites: gastric cancer (RR =0.75, 95%CI:0.65–0.86), esophagus cancer (RR = 0.75, 95%CI:0.62–0.89), colorectal cancer(RR = 0.79, 95%CI:0.74–0.85), pancreatic cancer (RR = 0.80, 95%CI:0.68–0.93), breast cancer (RR = 0.92, 95%CI:0.88–0.96), ovarian cancer (RR = 0.89, 95%CI:0.83–0.95), endometrial cancer (RR = 0.92, 95%CI:0.85–0.99), prostate cancer (RR = 0.94, 95%CI:0.90–0.99), and small intestine neuroendocrine tumors (RR = 0.17, 95%CI:0.05–0.58). However, there was no significant association between aspirin use and the risk of some cancers, including hepato-biliary, lung, cervical uterus, renal, renal pelvis and ureter, bladder, brain, head and neck, thyroid, and skin cancers, as well as lymphoma and leukemia.
Additional file 1: Tables S1–S18 shows the RRs for cancers at 17 sites, in subgroups of studies defined by their design, study location, gender, exposure assessment, quality assessment, duration of aspirin use, and frequency of aspirin use.
We conducted a subgroup analysis stratified by questionnaires and medical records, and found a lower risk in medical records with most cancers (gastric, esophageal, colorectal, hepato-biliary, and pancreatic cancers), however, significant heterogeneity of effects was noted for those subgroups (Additional file 1: Tables S2–S18). As we expected, the decreased risk of colorectal cancer (RRs = 0.76, 95%CI: 0.66–0.87 for ≥5 years), pancreatic cancer (RRs = 0.75, 95%CI: 0.57–0.99 for ≥5 years), ovarian cancer (RRs = 0.77, 95%CI: 0.63–0.93 for ≥5 years), and brain cancer (RRs = 0.65, 95%CI: 0.43–0.97 for ≥5 years) were more pronounced with longer duration of aspirin use. However, the aspirin-associated RR for 21 specific cancers did not vary significantly by other characteristics (gender, quality assessment and frequency of aspirin use).
Publication bias
The funnel plot showed asymmetry (Fig. 19). In addition, the Begg’s test and Egger’s test provided evidence of publication bias among the included studies (Begg’s test Z = 4.34, P < 0.001; Egger’s test Z = − 5.27, P < 0.001).
Discussion
The results of our meta-analysis supported the presence of inverse associations between aspirin use and the risk of overall cancer, gastric, esophageal, colorectal, pancreatic, breast, ovarian, endometrial, and prostate cancers, as well as small intestine neuroendocrine tumors. However, no significant associations were observed between the use of aspirin and the risk of other cancers, including hepato-biliary, lung, cervical uterus, renal, renal pelvis and ureter, bladder, brain, head and neck, thyroid, and skin cancers, as well as lymphoma, and leukemia.
There are several potential biological mechanisms through which aspirin could reduce the risk of cancer. First, aspirin and other NSAIDs have been proven to inhibit the activity of the enzyme cyclooxygenase 2 (COX-2), which is responsible for the synthesis of prostaglandins [29]. COX-2 has been reported to be overexpressed in many cancers and participates in key cellular activities, including cell proliferation, apoptosis, angiogenesis, and metastasis [30,31,32]. Second, aspirin could activate the NF-kappa B (NF-κB) signaling pathway, which triggers apoptosis in neoplasia [33, 34]. In addition, some studies showed that aspirin might induce gene selection and modulate mitochondrial voltage dependent anion channels (VDACs) to reduce the risk of cancer progression and metastasis [35, 36].
The results of this meta-analysis indicated that utilization of aspirin had different protective effects on the development of cancer. This difference may be attributed to the different expression levels of COX in various cancers [37]. Furthermore, Zumwalt et al. [38] reported that the effectiveness of aspirin was primarily determined by specific genetic variants. Aspirin inhibited cell growth in all cancer cell lines regardless of mutational background, however, the effects were exacerbated in cells with PIK3CA mutations, which might explain the different effects of aspirin on cancers.
The decreased risk of gastric, esophageal, pancreatic, lung, breast, and ovarian cancers was observed in the case-control studies but not in the cohort studies. One possible explanation for the difference might be that cases in the case-control studies might have a recall bias and tended to overestimate the risk of cancer by aspirin use. Another possible explanation is that misclassification or measurement errors for aspirin use in the cohort studies might have distorted the association because most of our analyses were based on baseline data, and there might be a discrepancy between initial recruitment and subsequent aspirin consumption.
The longer those who had used aspirin, the lower their risk of cancer was, with longer duration of use associated with an RR of 0.90 (95% CI 0.89–0.74), based on 118 studies that reported associations with longer (≥5 years) duration of aspirin use and 105 studies that reported associations with shorter (< 5 years) duration of aspirin use. For most cancers (colorectal, pancreatic, ovarian, and brain cancers), risk reductions were more pronounced with longer duration of use, and these results agree with those of previous studies [39,40,41]. In addition, the United States Preventive Services Task Force (USPSTF) indicated that cancer prevention was a significant aspect in the overall health benefit of aspirin, but this benefit was not apparent until several years after the initiation of aspirin therapy [42, 43]. It is of note that a significant inverse association with prostate cancer was observed in the patients who took aspirin for less than 5 years. Indeed, after the study that relied on the General Practice Research Database [44] was excluded, the discrepancy disappeared. Considering that aspirin use was off-prescription in the United Kingdom, misclassification was likely to occur in this study because many commonly used aspirins do not require a prescription. Therefore, it can be deduced that the patients who used aspirin for at least 5 years were more likely to realize the potential cancer prevention benefit.
There was no statistically significant difference between the pooled RRs for the frequency of aspirin in most studies. Given that a few studies were included in the subgroup analysis on the basis of the frequency of aspirin use and most studies lacked information on this variable, the results on the risks associated with the frequency of aspirin use should be interpreted with caution. Further studies that explore the associations between the frequency of aspirin use and cancer risk are necessary to elucidate the effects of aspirin.
In addition, our results indicated that the strongest reduction in the risk of most cancers associated with aspirin was found in North American countries. However, two-thirds of the included studies were performed in North America and a few studies were performed in Asian and European countries, which might distort the accuracy of the results. Therefore, more studies are necessary to examine the discrepancies among the different countries and regions.
Comparison with other studies
Bosetti et al. (2011) [45] conducted a meta-analysis on aspirin and 12 selected cancer sites based on 139 observational studies and 187,167 cases. Our study included 218 studies involving 737,409 cases and examined the correlation between aspirin use and the risk of skin, head and neck, hepatobiliary, thyroid, cervical uterus, renal pelvis, ureter, and brain cancers, lymphoma, small intestine neuroendocrine tumors, and leukemia, thereby providing more comprehensive and reliable evidence for this correlation. More importantly, this study was the first meta-analysis to evaluate the association between aspirin use and the risk of hepatobiliary cancer and we found a non-significant effect of aspirin on the risk of hepatobiliary cancer (OR = 0.64, 95% CI: 0.40–1.02).
Algra and Rothwell (2012) [46] conducted a meta-analysis on the association between aspirin use and the risk of cancer based on 195 studies and 215,211 cases. Compared with their review, our meta-analysis have added approximately 70 new articles published since 2012, with a total of 737,409 cases, which significantly enhanced the statistical power to determine this potential association. In addition, the exposure in the previous review was inconsistent, which may mislead the estimation. Many studies defined aspirin as the exposure but only a few studies defined NSAIDs as the exposure, and thus the specific effect of aspirin on cancers was not defined. The exposure to aspirin in our meta-analysis was consistent and ensured the reliability of the findings.
Strengths and limitations
This study is the most up-to-date comprehensive review of the effect of aspirin use on the risk of all types of cancers, and the large sample size provides reliable results with greater precision and power. The potential limitations of this study should be noted. First, there was substantial heterogeneity across the included studies, which was likely due to differences in the definitions of exposure, units, assessment methods, and the adjusted variables across different studies. Second, misclassification or measurement errors for aspirin use might distort the association because our analyses were based on baseline data, and changes in the exposure to aspirin were not updated during the follow-up period. Third, the visual inspection of a funnel plot showed asymmetry, and the Begg’s test and Egger’s test also identified evidence of publication bias among the studies included in our meta-analysis.
Our meta-analysis indicated a beneficial role for aspirin for overall cancers; however, the results should be interpreted with caution. Considering that most evaluated studies were based on secondary prevention rather than on primary prevention, the totality of evidence for the high-risk population was incomplete, and it is appropriate to let the beneficial role remain uncertain. At present, we should accept the uncertainties, and future chemoprevention trials should clarify the extent to which aspirin decreases cancers incidence.
Conclusions and implications
Evidence from observational studies indicates that utilization of aspirin is associated with reduced risk of gastric, colorectal, esophageal, pancreatic, ovarian, endometrial, breast, and prostate cancers, in addition to small intestine neuroendocrine tumors. A stronger protective effect was observed in the North American populations and patients who used aspirin for at least 5 years. It is important to address immortal time bias not only to ensure the integrity of the meta-analysis, but also to ensure the integrity of pharmacoepidemiological studies. Moreover, given the confidence limits of the evaluated studies, adequately powered mechanistic studies should help elucidate the mechanisms underlying this correlation.
Abbreviations
- CI:
-
Confidence interval
- COX-2:
-
Cyclooxygenase 2
- HRs:
-
Hazard ratios
- NF-κB:
-
NF-kappa B
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- ORs:
-
Odds ratios
- RRs:
-
Relative risks
- USPSTF:
-
United States Preventive Services Task Force
- VDACs:
-
Voltage dependent anion channels
References
Oldenburg NC, Duval S, Luepker RV, et al. A 16-month community-based intervention to increase aspirin use for primary prevention of cardiovascular disease. Prev Chronic Dis. 2014;11:E83.
Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27.
Pignone M, Anderson GK, Binns K, Tilson HH, Weisman SM. Aspirin use among adults aged 40 and older in the United States: results of a national survey. Am J Prev Med. 2007;32:403–7.
Rodondi N, Cornuz J, Marques-Vidal P, et al. Aspirin use for the primary prevention of coronary heart disease: a population-based study in Switzerland. Prev Med. 2008;46:137–44.
VanWormer JJ, Greenlee RT, McBride PE, et al. Aspirin for primary prevention of CVD: are the right people using it? J Fam Pract. 2012;61:525–33.
Gilles M, Skyring A. Gastric ulcer, duodenal ulcer and gastric carcinoma: a case-control study of certain social and environmental factors. Med J Aust. 1968;2:1132–6.
Oleske D, Golomb HM, Farber MD, Levy PS. A case-control inquiry into the etiology of hairy cell leukemia. Am J Epidemiol. 1985;121:675–83.
Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–9.
Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77–85.
Zhong S, Chen L, Zhang X, Yu D, Tang J, Zhao J. Aspirin use and risk of breast cancer: systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomark Prev. 2015;24:1645–55.
Cui XJ, He Q, Zhang JM, Fan HJ, Wen ZF, Qin YR. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43:135–40.
Oh SW, Myung SK, Park JY, Lee CM, Kwon HT. Aspirin use and risk for lung cancer: a meta-analysis. Ann Oncol. 2011;22:2456–65.
Huang TB, Yan Y, Guo ZF, et al. Aspirin use and the risk of prostate cancer: a meta-analysis of 24 epidemiologic studies. Int Urol Nephrol. 2014;46(9):1715–28.
Zhang YP, Wan YD, Sun YL, Li J, Zhu RT. Aspirin might reduce the incidence of pancreatic cancer: a meta-analysis of observational studies. Sci Rep. 2015;5:15460.
Muranushi C, Olsen CM, Pandeya N, Green AC. Aspirin and nonsteroidal anti-inflammatory drugs can prevent cutaneous squamous cell carcinoma: a systematic review and meta-analysis. J Invest Dermatol. 2015;135:975–83.
Sivarasan N, Smith G. Role of aspirin in chemoprevention of esophageal adenocarcinoma: a meta-analysis. J Dig Dis. 2013;14:222–30.
Zhang D, Bai B, Xi Y, Wang T, Zhao Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol Oncol. 2016;142:368–77.
Yang P, Zhou Y, Chen B, et al. Aspirin use and the risk of gastric cancer: a meta-analysis. Dig Dis Sci. 2010;55:1533–9.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Canada: Hospital Research Institute, Inc.; 2009.
Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–9.
Chan SS, Luben R, Bergmann MM, Boeing H, Olsen A, Tjonneland A, Overvad K, Kaaks R, Kennedy H, Khaw KT, Riboli E, Hart AR. Aspirin in the aetiology of Crohn's disease and ulcerative colitis:a European prospective cohort study. Aliment Pharmacol Ther. 2011;34(6):649–55.
Velayos FS, Loftus EV Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case-control study. Gastroenterology. 2006;130(7):1941–9.
Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol. 2005;6(12):945–52.
Masclee GM, Coloma PM, Spaander MC, Kuipers EJ, Sturkenboom MC. NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett's oesophagus: a population-based case-control study. BMJ Open. 2015;5(1):e006640.
Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett's oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2012;24(8):917–23.
Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–52.
Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20.
Jana NR. NSAIDs and apoptosis. Cell Mol Life Sci. 2008;65:1295–301.
Hung WC. Anti-metastatic action of non-steroidal anti-inflammatory drugs. Kaohsiung J Med Sci. 2008;24:392–7.
Stark LA, Reid K, Sansom OJ, et al. Aspirin activates the NF-kappa B signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–76.
Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br J Cancer. 2004;91:381–8.
Ruschoff J, Wallinger S, Dietmaier W, et al. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci U S A. 1998;95:11301–6.
Tewari D, Majumdar D, Vallabhaneni S, Bera AK. Aspirin induces cell death by directly modulating mitochondrial voltage-dependent anion channel (VDAC). Sci Rep. 2017;7:45184.
Dore M. Cyclooxygenase-2 expression in animal cancers. Vet Pathol. 2011;48:254–65.
Zumwalt TJ, Wodarz D, Komarova NL, et al. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev Res (Phila). 2017;10:208–18.
Ye X, Fu J, Yang Y, Chen S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PLoS One. 2013;8:e57578.
Huang XZ, Chen Y, Wu J, et al. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: a dose-response meta-analysis. Oncotarget. 2017;8:4781–95.
Egan KM, Nabors LB, Thompson ZJ, et al. Analgesic use and the risk of primary adult brain tumor. Eur J Epidemiol. 2016;31:917–25.
Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, Anderson ML. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. preventive services task force. Ann Intern Med. 2016;164:814–25.
Chubak J, Kamineni A, Buist DSM, Anderson ML, EP W. Aspirin use for the prevention of colorectal cancer: an updated systematic evidence review for the U.S. preventive services task force. US: Agency for Healthcare Research and Quality, Inc.; 2015.
Garcia Rodriguez LA, Gonzalez PA. Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomark Prev. 2004;13:649–53.
Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27.
Iqbal U, Yang HC, Jian WS, Yen Y, Li YJ. Does aspirin use reduce the risk for cancer? J Investig Med. 2017;65(2):391–2.
Wang Y, Shen C, Ge J, Duan H. Regular aspirin use and stomach cancer risk in China. Eur J Surg Oncol. 2015;41(6):801–4.
Gong EJ, Ahn JY, Jung HY, Lim H, Choi KS, Lee JH, Kim DH, Choi KD, Song HJ, Lee GH, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014;29(2):301–9.
Bertuccio P, Bravi F, Bosetti C, Negri E, La Vecchia C. Aspirin and gastric cancer risk. Eur J Cancer Prev. 2010;19(6):426–7.
Figueroa JD, Terry MB, Gammon MD, Vaughan TL, Risch HA, Zhang FF, Kleiner DE, Bennett WP, Howe CL, Dubrow R, et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control. 2009;20(3):361–8.
Duan L, Wu AH, Sullivan-Halley J, Bernstein L. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles County. Cancer Epidemiol Biomark Prev. 2008;17(1):126–34.
Fortuny J, Johnson CC, Bohlke K, Chow WH, Hart G, Kucera G, Mujumdar U, Ownby D, Wells K, Yood MU, et al. Use of anti-inflammatory drugs and lower esophageal sphincter-relaxing drugs and risk of esophageal and gastric cancers. Clin Gastroenterol Hepatol. 2007;5(10):1154–9. e1153
Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer. 2001;84(7):965–8.
Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomark Prev. 2000;9(1):119–23.
Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Aspirin protects against gastric cancer: results of a case-control study from Moscow, Russia. Int J Cancer. 1999;82(4):473–6.
Kim YI, Kim SY, Kim JH, Lee JH, Kim YW, Ryu KW, Park JH, Choi IJ. Long-term low-dose aspirin use reduces gastric cancer incidence: a Nationwide cohort study. Cancer Res Treat. 2016;48(2):798–805.
Lee J, Lee SH, Hur KY, Woo SY, Kim SW, Kang WK. Statins and the risk of gastric cancer in diabetes patients. BMC Cancer. 2012;12:596.
Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100(3):551–7.
Epplein M, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am J Epidemiol. 2009;170(4):507–14.
Lindblad M, Lagergren J, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomark Prev. 2005;14(2):444–50.
Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88(5):684–8.
Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5(2):138–46.
Sadeghi S, Bain CJ, Pandeya N, Webb PM, Green AC, Whiteman DC, Study AC. Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidem Biomark Prev. 2008;17(5):1169–78.
Ranka S, Gee JM, Johnson IT, Skinner J, Hart AR, Rhodes M. Non-steroidal anti-inflammatory drugs, lower oesophageal sphincter-relaxing drugs and oesophageal cancer. A case-control study. Digestion. 2006;74(2):109–15.
Anderson LA, Johnston BT, Watson RG, Murphy SJ, Ferguson HR, Comber H, McGuigan J, Reynolds JV, Murray LJ. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66(9):4975–82.
Jayaprakash V, Menezes RJ, Javle MM, McCann SE, Baker JA, Reid ME, Natarajan N, Moysich KB. Regular aspirin use and esophageal cancer risk. Int J Cancer. 2006;119(1):202–7.
Sharp L, Chilvers CE, Cheng KK, McKinney PA, Logan RF, Cook-Mozaffari P, Ahmed A, Day NE. Risk factors for squamous cell carcinoma of the oesophagus in women: a case-control study. Br J Cancer. 2001;85(11):1667–70.
Macfarlane TV, Lefevre K, Watson MC. Aspirin and non-steroidal anti-inflammatory drug use and the risk of upper aerodigestive tract cancer. Br J Cancer. 2014;111(9):1852–9.
Friis S, Riis AH, Erichsen R, Baron JA, Sorensen HT. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163(5):347–55.
Rennert G, Rennert HS, Pinchev M, Gruber SB. A case-control study of levothyroxine and the risk of colorectal cancer. J Natl Cancer Inst. 2010;102(8):568–72.
Din FVN, Theodoratou E, Farrington SM, Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME, Campbell H, Dunlop MG. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59(12):1670–U1114.
Harris RE, Beebe-Donk J, Alshafie GA. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2008;8:237.
Kim S, Martin C, Galanko J, Woosley JT, Schroeder JC, Keku TO, Satia JA, Halabi S, Sandler RS. Use of nonsteroidal Antiinflammatory drugs and distal large bowel cancer in whites and African Americans. Am J Epidemiol. 2008;168(11):1292–300.
Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer. 2007;121(6):1325–30.
Slattery ML, Curtin K, Wolff R, Ma KN, Sweeney C, Murtaugh M, Potter JD, Levin TR, Samowitz W. PPAR gamma and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States). Cancer Causes Control. 2006;17(3):239–49.
Macarthur M, Sharp L, Hold GL, Little J, El-Omar EM. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidem Biomark Prev. 2005;14(7):1613–8.
Juarranz M, Calle-Puron ME, Gonzalez-Navarro A, Regidor-Poyatos E, Soriano T, Martinez-Hernandez D, Rojas VD, Guinee VF. Physical exercise, use of Plantago ovata and aspirin, and reduced risk of colon cancer. Eur J Cancer Prev. 2002;11(5):465–72.
Evans RC, Fear S, Ashby D, Hackett A, Williams E, Van der Vliet M, Dunstan FDJ, Rhodes JM. Diet and colorectal cancer: an investigation of the lectin/galactose hypothesis. Gastroenterology. 2002;122(7):1784–92.
Neugut AI, Rosenberg DJ, Ahsan H, Jacobson JS, Wahid N, Hagan M, Rahman MI, Khan ZR, Chen L, Pablos-Mendez A, et al. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidem Biomark Prev. 1998;7(10):869–73.
Rosenberg L, Louik C, Shapiro S. Nonsteroidal antiinflammatory drug use and reduced risk of large bowel carcinoma. Cancer. 1998;82(12):2326–33.
LaVecchia C, Negri E, Franceschi S, Conti E, Montella M, Giacosa A, Falcini F, Decarli A. Aspirin and colorectal cancer. Br J Cancer. 1997;76(5):675–7.
Reeves MJ, Newcomb PA, Trentham-Dietz A, Storer BE, Remington PL. Nonsteroidal anti-inflammatory drug use and protection against colorectal cancer in women. Cancer Epidemiol Biomark Prev. 1996;5(12):955–60.
Suh O, Mettlin C, Petrelli NJ. Aspirin use, cancer, and polyps of the large bowel. Cancer. 1993;72(4):1171–7.
Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne colorectal cancer study. Cancer Res. 1988;48(15):4399–404.
Park SY, Wilkens LR, Kolonel LN, Monroe KR, Haiman CA, Marchand LL. Exploring differences in the aspirin-colorectal cancer association by sex and race/ethnicity: the multiethnic cohort study. Cancer Epidemiol Biomark Prev. 2017;26(2):162–9.
Kim C, Zhang X, Chan AT, Sesso HD, Rifai N, Stampfer MJ, Ma J. Inflammatory biomarkers, aspirin, and risk of colorectal cancer: findings from the physicians’ health study. Cancer Epidemiol. 2016;44:65–70.
Soriano LC, Soriano-Gabarro M, Rodriguez LAG. The protective effect of low-dose aspirin against colorectal cancer is unlikely explained by selection bias: results from three different study designs in clinical practice. PLoS One. 2016;11(7):e0159179.
Vaughan LE, Prizment A, Blair CK, Thomas W, Anderson KE. Aspirin use and the incidence of breast, colon, ovarian, and pancreatic cancers in elderly women in the Iowa Women's health study. Cancer Causes Control. 2016;27(11):1395–402.
Lin CC, Lai MS, Shau WY. Can aspirin reduce the risk of colorectal cancer in people with diabetes? A population-based cohort study. Diabet Med. 2015;32(3):324–31.
Hollestein LM, van Herk-Sukel MPP, Ruiter R, de Vries E, Mathijssen RHJ, Wiemer EAC, Stijnen T, Coebergh JWW, Lemmens VEPP, Herings RMC, et al. Incident cancer risk after the start of aspirin use: results from a Dutch population-based cohort study of low dose aspirin users. Int J Cancer. 2014;135(1):157–65.
Brasky TM, Liu JM, White E, Peters U, Potter JD, Walter RB, Baik CS, Lane DS, Manson JE, Vitolins MZ, et al. Non-steroidal anti-inflammatory drugs and cancer risk in women: results from the Women's Health Initiative. Int J Cancer. 2014;135(8):1869–83.
Brasky TM, Potter JD, Kristal AR, Patterson RE, Peters U, Asgari MM, Thornquist MD, White E. Non-steroidal anti-inflammatory drugs and cancer incidence by sex in the VITamins and lifestyle (VITAL) cohort. Cancer Causes Control. 2012;23(3):431–44.
Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106(7):1340–50.
Friis S, Poulsen AH, Sorensen HT, Tjonneland A, Overvad K, Vogel U, McLaughlin JK, Blot WJ, Olsen JH. Aspirin and other non-steroidal anti-inflammatory drugs and risk of colorectal cancer: a Danish cohort study. Cancer Causes Control. 2009;20(5):731–40.
Siemes C, Visser LE, Coebergh JWW, Hofman A, Uitterfnden AG, Stricker BHC. Protective effect of NSAIDs on cancer and influence of COX-2 C(−765)G genotype. Curr Cancer Drug Targets. 2008;8(8):753–64.
Vinogradova Y, Hippisley-Cox J, Coupland C, Logan RF. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133(2):393–402.
Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99(8):608–15.
Larsson SC, Giovannucci E, Wolk A. Long-term aspirin use and colorectal cancer risk: a cohort study in Sweden. Br J Cancer. 2006;95(9):1277–9.
Muscat JE, Dyer AM, Rosenbaum RE, Rigas B. Nitric oxide-releasing medications and colorectal cancer risk: the Framingham study. Anticancer Res. 2005;25(6c):4471–4.
Rahme E, Barkun AN, Toubouti Y, Bardou M. The cyclooxygenase-2-selective inhibitors rofecoxib and celecoxib prevent colorectal neoplasia occurrence and recurrence. Gastroenterology. 2003;125(2):404–12.
Rodriguez LAG, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12(1):88–93.
Paganini-Hill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases: a cohort study of the elderly. BMJ. 1989;299(6710):1247–50.
Choi JG, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, Therneau TM, Olson JE, Chaiteerakij R, Roberts LR. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64(3):785–96.
Yang BY, Petrick JL, Chen J, Hagberg KW, Sahasrabuddhe VV, Graubard BI, Jick S, McGlynn KA. Associations of NSAID and paracetamol use with risk of primary liver cancer in the clinical practice research datalink. Cancer Epidemiol. 2016;43:105–11.
Burr NE, Talboys RJ, Savva S, Clark A, Phillips M, Metcalfe M, Dennison A, Robinson R, Lewis MP, Rhodes M, et al. Aspirin may prevent cholangiocarcinoma: a case-control study from the United Kingdom. Dig Dis Sci. 2014;59(7):1567–72.
Kim G, Jang SY, Han E, Lee YH, Park SY, Nam CM, Kang ES. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer. 2017;140(4):798–806.
Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, Beane-Freeman LE, Buring JE, Chen J, Chong DQ, Freedman ND, Fuchs CS, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res. 2015;8(12):1156–62.
Liu EJ, Sakoda LC, Gao YT, Rashid A, Shen MC, Wang BS, Deng J, Han TQ, Zhang BH, Fraumeni JF, et al. Aspirin use and risk of biliary tract cancer: a population-based study in shanghai, China. Cancer Epidem Biomark Prev. 2005;14(5):1315–8.
Risch HA, Lu LG, Streicher SA, Wang J, Zhang W, Ni QX, Kidd MS, Yu H, Gao YT. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidem Biomark Prev. 2017;26(1):68–74.
Kho PF, Fawcett J, Fritschi L, Risch H, Webb PM, Whiteman DC, Neale RE. Nonsteroidal anti-inflammatory drugs, statins, and pancreatic cancer risk: a population-based case-control study. Cancer Causes Control. 2016;27(12):1457–64.
Streicher SA, Yu H, Lu LG, Kidd MS, Risch HA. Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidem Biomark Prev. 2014;23(7):1254–63.
Tan XL, Lombardo KMR, Bamlet WR, Oberg AL, Robinson DP, Anderson KE, Petersen GM. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res. 2011;4(11):1835–41.
Pugh TFG, Little M, Carey F, Metcalfe M, Robinson R, Clark A, Ndokera R, Ing H, Dennison A, Hart A. Aspirin, Nsaids, Calcium-Channel blockers and statins in the Aetiology of pancreatic cancer: preliminary results from a case-control study in two Centres in the Uk. Gut. 2011;60:A81.
Bonifazi M, Gallus S, Bosetti C, Polesel J, Serraino D, Talamini R, Negri E, La Vecchia C. Aspirin use and pancreatic cancer risk. Eur J Cancer Prev. 2010;19(5):352–4.
Menezes RJ, Huber KR, Mahoney MC, Moysich KB. Regular use of aspirin and pancreatic cancer risk. BMC Public Health. 2002;2:18.
Bradley MC, Hughes CM, Cantwell MM, Napolitano G, Murray LJ. Non-steroidal anti-inflammatory drugs and pancreatic cancer risk: a nested case-control study. Br J Cancer. 2010;102(9):1415–21.
Anderson KE, Johnson TW, Lazovich D, Folsom AR. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst. 2002;94(15):1168–71.
Lim WY, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, Ng A, Poh WT, Tee A, Teh M, et al. Aspirin and non-aspirin non-steroidal anti-inflammatory drug use and risk of lung cancer. Lung Cancer. 2012;77(2):246–51.
McCormack VA, Hung RJ, Brenner DR, Bickeboller H, Rosenberger A, Muscat JE, Lazarus P, Tjonneland A, Friis S, Christiani DC, et al. Aspirin and NSAID use and lung cancer risk: a pooled analysis in the international lung cancer consortium (ILCCO). Cancer Causes Control. 2011;22(12):1709–20.
Kelly JP, Coogan P, Strom BL, Rosenberg L. Lung cancer and regular use of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs. Pharmacoepidem Drug Saf. 2008;17(4):322–7.
Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidem Biomark Prev. 2008;17(1):148–57.
Harris RE, Beebe-Donk J, Alshafie GA. Reduced risk of human lung cancer by selective cyclooxygenase 2 (COX-2) blockade: results of a case control study. Int J Biol Sci. 2007;3(5):328–34.
Muscat JE, Chen SQ, Richie JP Jr, Altorki NK, Citron M, Olson S, Neugut AI, Stellman SD. Risk of lung carcinoma among users of nonsteroidal antiinflammatory drugs. Cancer. 2003;97(7):1732–6.
Moysich KB, Menezes RJ, Ronsani A, Swede H, Reid ME, Cummings KM, Falkner KL, Loewen GM, Bepler G. Regular aspirin use and lung cancer risk. BMC Cancer. 2002;2:31.
Baik CS, Brasky TM, Pettinger M, Luo JH, Gong ZH, Wactawski-Wende J, Prentice RL. Nonsteroidal anti-inflammatory drug and aspirin use in relation to lung cancer risk among postmenopausal women. Cancer Epidem Biomark Prev. 2015;24(5):790–7.
Brasky TM, Baik CS, Slatore CG, Potter JD, White E. Non-steroidal anti-inflammatory drugs and small cell lung cancer risk in the VITAL study. Lung Cancer. 2012;77(2):260–4.
Olsen JH, Friis S, Poulsen AH, Fryzek J, Harving H, Tjonneland A, Sorensen HT, Blot W. Use of NSAIDs, smoking and lung cancer risk. Br J Cancer. 2008;98(1):232–7.
Hernandez-Diaz S, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer. 2007;120(7):1565–72.
Hayes JH, Anderson KE, Folsom AR. Association between nonsteroidal anti-inflammatory drug use and the incidence of lung cancer in the Iowa women's health study. Cancer Epidem Biomark Prev. 2006;15(11):2226–31.
Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Koenig KL, Shore RE. Aspirin and lung cancer in women. Br J Cancer. 2002;87(1):49–53.
Dierssen-Sotos T, Gomez-Acebo I, de Pedro M, Perez-Gomez B, Servitja S, Moreno V, Amiano P, Fernandez-Villa T, Barricarte A, Tardon A, et al. Use of non-steroidal anti-inflammatory drugs and risk of breast cancer: the Spanish multi-case-control (MCC) study. BMC Cancer. 2016;16(1):660.
Cui Y, Deming-Halverson SL, Shrubsole MJ, Beeghly-Fadiel A, Cai H, Fair AM, Shu XO, Zheng W. Use of nonsteroidal anti-inflammatory drugs and reduced breast cancer risk among overweight women. Breast Cancer Res Treat. 2014;146(2):439–46.
Brasky TM, Bonner MR, Moysich KB, Ambrosone CB, Nie J, Tao MH, Edge SB, Kallakury BV, Marian C, Trevisan M, et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the western New York exposures and breast cancer (WEB) study. Cancer Causes Control. 2010;21(9):1503–12.
Cronin-Fenton DP, Pedersen L, Lash TL, Friis S, Baron JA, Sorensen HT. Prescriptions for selective cyclooxygenase-2 inhibitors, non-selective non-steroidal anti-inflammatory drugs, and risk of breast cancer in a population-based case-control study. Breast Cancer Res. 2010;12(2):R15.
Slattery ML, Curtin K, Baumgartner R, Sweeney C, Byers T, Giuliano AR, Baumgartner KB, Wolff RR. IL6, aspirin, nonsteroidal anti-inflammatory drugs, and breast cancer risk in women living in the southwestern United States. Cancer Epidemiol Biomark Prev. 2007;16(4):747–55.
Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27.
Swede H, Mirand AL, Menezes RJ, Moysich KB. Association of regular aspirin use and breast cancer risk. Oncology. 2005;68(1):40–7.
Zhang YQ, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of nonsteroidal antiinflammatory drugs and risk of breast cancer: the case-control surveillance study revisited. Am J Epidemiol. 2005;162(2):165–70.
Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291(20):2433–40.
Moorman PG, Grubber JM, Millikan RC, Newman B. Association between non-steroidal anti-inflammatory drugs (NSAIDs) and invasive breast cancer and carcinoma in situ of the breast. Cancer Causes Control. 2003;14(10):915–22.
Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidem Biomark Prev. 2001;10(11):1213–7.
Kim S, Shore DL, Wilson LE, Sanniez EI, Kim JH, Taylor JA, Sandler DP. Lifetime use of nonsteroidal anti-inflammatory drugs and breast cancer risk: results from a prospective study of women with a sister with breast cancer. BMC Cancer. 2015;15:960.
Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat. 2011;126(1):149–55.
Bosco JL, Palmer JR, Boggs DA, Hatch EE, Rosenberg L. Regular aspirin use and breast cancer risk in US black women. Cancer Causes Control. 2011;22(11):1553–61.
Eliassen AH, Chen WY, Spiegelman D, Willett WC, Hunter DJ, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and risk of breast cancer among premenopausal women in the Nurses’ health study II. Arch Intern Med. 2009;169(2):115–21.
Friis S, Thomassen L, Sorensen HT, Tjonneland A, Overvad K, Cronin-Fenton DR, Vogel U, McLaughlin JK, Blot WJ, Olsen JH. Nonsteroidal anti-inflammatory drug use and breast cancer risk: a Danish cohort study. Eur J Cancer Prev. 2008;17(2):88–96.
Gierach GL, Lacey JV Jr, Schatzkin A, Leitzmann MF, Richesson D, Hollenbeck AR, Brinton LA. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health-AARP diet and health study. Breast Cancer Res. 2008;10(2):R38.
Ready A, Velicer CM, McTiernan A, White E. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat. 2008;109(3):533–43.
Gill JK, Maskarinec G, Wilkens LR, Pike MC, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and breast cancer risk - the multiethnic cohort. Am J Epidemiol. 2007;166(10):1150–8.
Gallicchio L, Visvanathan K, Burke A, Hoffman SC, Helzlsouer KJ. Nonsteroidal anti-inflammatory drugs and the risk of developing breast cancer in a population-based prospective cohort study in Washington County, MD. Int J Cancer. 2007;121(1):211–5.
Marshall SF, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross PL, Mohrenweiser H, Peel D, Pinder R, Purdie DM, Reynolds P, et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97(11):805–12.
Rahme E, Ghosn J, Dasgupta K, Rajan R, Hudson M. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer. 2005;5:159.
Rodriguez LAG, Gonzalez-Perez A. Risk of breast cancer among users of aspirin and other antiinflammatory drugs. Br J Cancer. 2004;91(3):525–9.
Harris RE, Kasbari S, Farrar WB. Prospective study of nonsteroidal anti-inflammatory drugs and breast cancer. Oncol Rep. 1999;6(1):71–3.
Peres LC, Camacho F, Abbott SE, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, Cote ML, Crankshaw S, Funkhouser E, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114(7):819–25.
Baandrup L. Drugs with potential chemopreventive properties in relation to epithelial ovarian cancer--a nationwide case-control study. Dan Med J. 2015;62(7).
Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23(2):311–9.
Ammundsen HB, Faber MT, Jensen A, Hogdall E, Blaakaer J, Hogdall C, Kjaer SK. Use of analgesic drugs and risk of ovarian cancer: results from a Danish case-control study. Acta Obstet Gynecol Scand. 2012;91(9):1094–102.
Pinheiro SP, Gates MA, De Vivo I, Rosner BA, Tworoger SS, Titus-Ernstoff L, Hankinson SE, Cramer DW. Interaction between use of non-steroidal anti-inflammatory drugs and selected genetic polymorphisms in ovarian cancer risk. Int J Mol Epidemiol Genet. 2010;1(4):320–31.
Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124(6):1409–15.
Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM. Inverse association of NSAID use and ovarian cancer in relation to oral contraceptive use and parity. Br J Cancer. 2008;98(11):1781–3.
Merritt MA, Green AC, Nagle CM, Webb PM, Australian Cancer S, Australian Ovarian Cancer Study G. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170–6.
Schildkraut JM, Moorman PG, Halabi S, Calingaert B, Marks JR, Berchuck A. Analgesic drug use and risk of ovarian cancer. Epidemiology. 2006;17(1):104–7.
Moysich KB, Mettlin C, Piver MS, Natarajan N, Menezes RJ, Swede H. Regular use of analgesic drugs and ovarian cancer risk. Cancer Epidemiol Biomark Prev. 2001;10(8):903–6.
Rosenberg L, Palmer JR, Rao RS, Coogan PF, Strom BL, Zauber AG, Stolley PD, Shapiro S. A case-control study of analgesic use and ovarian cancer. Cancer Epidemiol Biomark Prev. 2000;9(9):933–7.
Tavani A, Gallus S, La Vecchia C, Conti E, Montella M, Franceschi S. Aspirin and ovarian cancer: an Italian case-control study. Ann Oncol. 2000;11(9):1171–3.
Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351(9096):104–7.
Setiawan VW, Matsuno RK, Lurie G, Wilkens LR, Carney ME, Henderson BE, Kolonel LN, Goodman MT. Use of nonsteroidal anti-inflammatory drugs and risk of ovarian and endometrial cancer: the multiethnic cohort. Cancer Epidemiol Biomark Prev. 2012;21(9):1441–9.
Murphy MA, Trabert B, Yang HP, Park Y, Brinton LA, Hartge P, Sherman ME, Hollenbeck A, Wentzensen N. Non-steroidal anti-inflammatory drug use and ovarian cancer risk: findings from the NIH-AARP diet and health study and systematic review. Cancer Causes Control. 2012;23(11):1839–52.
Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's health study. Cancer Epidemiol Biomark Prev. 2010;19(2):435–42.
Lacey JV, Sherman ME, Hartge P, Schatzkin A, Schairer C. Medication use and risk of ovarian carcinoma: a prospective study. Int J Cancer. 2004;108(2):281–6.
Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Kato I, Koenig KL, Shore RE. Aspirin and epithelial ovarian cancer. Prev Med. 2001;33(6):682–7.
Brons N, Baandrup L, Dehlendorff C, Kjaer SK. Use of nonsteroidal anti-inflammatory drugs and risk of endometrial cancer: a nationwide case-control study. Cancer Causes Control. 2015;26(7):973–81.
Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, Australian National Endometrial Cancer Study G. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. Int J Cancer. 2013;132(5):1146–55.
Bosetti C, Bravi F, Talamini R, Montella M, Negri E, La Vecchia C. Aspirin and risk of endometrial cancer: a case-control study from Italy. Eur J Cancer Prev. 2010;19(5):401–3.
Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, Zauber AG, Olson SH. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomark Prev. 2009;18(5):1448–56.
Bodelon C, Doherty JA, Chen C, Rossing MA, Weiss NS. Use of nonsteroidal antiinflammatory drugs and risk of endometrial cancer. Am J Epidemiol. 2009;170(12):1512–7.
Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomark Prev. 2005;14(12):2923–8.
Brasky TM, Moysich KB, Cohn DE, White E. Non-steroidal anti-inflammatory drugs and endometrial cancer risk in the VITamins and lifestyle (VITAL) cohort. Gynecol Oncol. 2013;128(1):113–9.
Danforth KN, Gierach GL, Brinton LA, Hollenbeck AR, Katki HA, Leitzmann MF, Schatzkin A, Lacey JV Jr. Nonsteroidal anti-inflammatory drug use and endometrial cancer risk in the NIH-AARP diet and health study. Cancer Prev Res (Phila). 2009;2(5):466–72.
Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res. 2008;68(7):2507–13.
Friel G, Liu CS, Kolomeyevskaya NV, Hampras SS, Kruszka B, Schmitt K, Cannioto RA, Lele SB, Odunsi KO, Moysich KB. Aspirin and acetaminophen use and the risk of cervical cancer. J Low Genit Tract Dis. 2015;19(3):189–93.
Wilson JC, O'Rorke MA, Cooper JA, Murray LJ, Hughes CM, Gormley GJ, Anderson LA. Non-steroidal anti-inflammatory drug use and cervical cancer risk: a case-control study using the clinical practice research datalink. Cancer Epidemiol. 2013;37(6):897–904.
Skriver C, Dehlendorff C, Borre M, Brasso K, Sorensen HT, Hallas J, Larsen SB, Tjonneland A, Friis S. Low-dose aspirin or other nonsteroidal anti-inflammatory drug use and prostate cancer risk: a nationwide study. Cancer Causes Control. 2016;27(9):1067–79.
Veitonmaki T, Tammela TLJ, Auvinen A, Murtola TJ. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer. 2013;49(4):938–45.
Murad AS, Down L, Smith GD, Donovan JL, Lane JA, Hamdy FC, Neal DE, Martin RM. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer. 2011;128(6):1442–8.
Salinas CA, Kwon EM, FitzGerald LM, Feng ZD, Nelson PS, Ostrander EA, Peters U, Stanford JL. Use of aspirin and other nonsteroidal Antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172(5):578–90.
Harris RE, Beebe-Donk J, Alshafie GA. Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade: results of case control studies. Subcell Biochem. 2007;42:193–212.
Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C. Aspirin and the risk of prostate cancer. Eur J Cancer Prev. 2006;15(1):43–5.
Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006;12(2):130–5.
Liu X, Plummer SJ, Nock NL, Casey G, Witte JS. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol. 2006;164(10):984–9.
Menezes RJ, Swede H, Niles R, Moysich KB. Regular use of aspirin and prostate cancer risk (United States). Cancer Causes Control. 2006;17(3):251–6.
Perron L, Bairati I, Moore L, Meyer F. Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer. 2003;106(3):409–15.
Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer. 1998;77(4):511–5.
Lapi F, Levi M, Simonetti M, Cancian M, Parretti D, Cricelli I, Sobrero A, Cricelli C. Risk of prostate cancer in low-dose aspirin users: a retrospective cohort study. Int J Cancer. 2016;139(1):205–11.
Nordstrom T, Clements M, Karlsson R, Adolfsson J, Gronberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer. 2015;51(6):725–33.
Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, Grubb R, Church TR, Chia D, Zhou C, Chu LW, et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer. 2012;107(1):207–14.
Mahmud SM, Franco EL, Turner D, Platt RW, Beck P, Skarsgard D, Tonita J, Sharpe C, Aprikian AG. Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case-control study. PLoS One. 2011;6(1):e16412.
Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E. Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the VITamins and lifestyle (VITAL) cohort. Cancer Epidem Biomark Prev. 2010;19(12):3185–8.
Platz EA, Rohrmann S, Pearson JD, Corrada MM, Watson DJ, De Marzo AM, Landis PK, Metter EJ, Carter HB. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore longitudinal study of aging. Cancer Epidem Biomark Prev. 2005;14(2):390–6.
Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control. 2002;13(5):427–34.
Karami S, Daughtery SE, Schwartz K, Davis FG, Ruterbusch JJ, Wacholder S, Graubard BI, Berndt SI, Hofmann JN, Purdue MP, et al. Analgesic use and risk of renal cell carcinoma: a case-control, cohort and meta-analytic assessment. Int J Cancer. 2016;139(3):584–92.
Tavani A, Scotti L, Bosetti C, Dal Maso L, Montella M, Ramazzotti V, Negri E, Franceschi S, La Vecchia C. Aspirin and risk of renal cell cancer in Italy. Eur J Cancer Prev. 2010;19(4):272–4.
Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Regular use of analgesics is a risk factor for renal cell carcinoma. Br J Cancer. 1999;81(3):542–8.
Chow WH, McLaughlin JK, Linet MS, Niwa S, Mandel JS. Use of analgesics and risk of renal cell cancer. Int J Cancer. 1994;59(4):467–70.
McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the kidney and renal pelvis. Int J Cancer. 1993;53(2):245–9.
McCredie M, Ford JM, Stewart JH. Risk factors for cancer of the renal parenchyma. Int J Cancer. 1988;42(1):13–6.
Liu W, Park Y, Purdue MP, Giovannucci E, Cho E. A large cohort study of nonsteroidal anti-inflammatory drugs and renal cell carcinoma incidence in the National Institutes of Health-AARP diet and health study. Cancer Causes Control. 2013;24(10):1865–73.
Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, Choueiri TK. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med. 2011;171(16):1487–93.
Linet MS, Chow WH, McLaughlin JK, Wacholder S, Yu MC, Schoenberg JB, Lynch C, Fraumeni JF Jr. Analgesics and cancers of the renal pelvis and ureter. Int J Cancer. 1995;62(1):15–8.
Ross RK, Paganini-Hill A, Landolph J, Gerkins V, Henderson BE. Analgesics, cigarette smoking, and other risk factors for cancer of the renal pelvis and ureter. Cancer Res. 1989;49(4):1045–8.
Jensen OM, Knudsen JB, Tomasson H, Sorensen BL. The Copenhagen case-control study of renal pelvis and ureter cancer: role of analgesics. Int J Cancer. 1989;44(6):965–8.
Baris D, Karagas MR, Koutros S, Colt JS, Johnson A, Schwenn M, Fischer AH, Figueroa JD, Berndt SI, Han S, et al. Nonsteroidal anti-inflammatory drugs and other analgesic use and bladder cancer in northern New England. Int J Cancer. 2013;132(1):162–73.
Fortuny J, Kogevinas M, Zens MS, Schned A, Andrew AS, Heaney J, Kelsey KT, Karagas MR. Analgesic and anti-inflammatory drug use and risk of bladder cancer: a population based case control study. BMC Urol. 2007;7:13.
Fortuny J, Kogevinas M, Garcia-Closas M, Real FX, Tardon A, Garcia-Closas R, Serra C, Carrato A, Lloreta J, Rothman N, et al. Use of analgesics and nonsteroidal anti-inflammatory drugs, genetic predisposition, and bladder cancer risk in Spain. Cancer Epidem Biomark Prev. 2006;15(9):1696–702.
Castelao JE, Yuan JM, Gago-Dominguez M, Yu MC, Ross RK. Non-steroidal anti-inflammatory drugs and bladder cancer prevention. Br J Cancer. 2000;82(7):1364–9.
Steineck G, Wiholm BE, Gerhardsson de Verdier M. Acetaminophen, some other drugs, some diseases and the risk of transitional cell carcinoma. A population-based case-control study. Acta Oncol. 1995;34(6):741–8.
Shih C, Hotaling JM, Wright JL, White E. Long-term NSAID use and incident urothelial cell carcinoma in the VITamins and lifestyle (VITAL) study. Urol Oncol. 2013;31(8):1689–95.
Daugherty SE, Pfeiffer RM, Sigurdson AJ, Hayes RB, Leitzmann M, Schatzkin A, Hollenbeck AR, Silverman DT. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am J Epidemiol. 2011;173(7):721–30.
Genkinger JM, De Vivo I, Stampfer MJ, Giovannucci E, Michaud DS. Nonsteroidal antiinflammatory drug use and risk of bladder cancer in the health professionals follow-up study. Int J Cancer. 2007;120(10):2221–5.
Gaist D, Garcia-Rodriguez LA, Sorensen HT, Hallas J, Friis S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: a case-control study. Br J Cancer. 2013;108(5):1189–94.
Ferris JS, McCoy L, Neugut AI, Wrensch M, Lai R. HMG CoA reductase inhibitors, NSAIDs and risk of glioma. Int J Cancer. 2012;131(6):E1031–7.
Bannon FJ, O'Rorke MA, Murray LJ, Hughes CM, Gavin AT, Fleming SJ, Cardwell CR. Non-steroidal anti-inflammatory drug use and brain tumour risk: a case-control study within the clinical practice research datalink. Cancer Causes Control. 2013;24(11):2027–34.
Daugherty SE, Moore SC, Pfeiffer RM, Inskip PD, Park Y, Hollenbeck A, Rajaraman P. Nonsteroidal anti-inflammatory drugs and glioma in the NIH-AARP diet and health study cohort. Cancer Prev Res (Phila). 2011;4(12):2027–34.
Di Maso M, Bosetti C, La Vecchia C, Garavello W, Montella M, Libra M, Serraino D, Polesel J. Regular aspirin use and nasopharyngeal cancer risk: a case-control study in Italy. Cancer Epidemiol. 2015;39(4):545–7.
Becker C, Wilson JC, Jick SS, Meier CR. Non-steroidal anti-inflammatory drugs and the risk of head and neck cancer: a case-control analysis. Int J Cancer. 2015;137(10):2424–31.
Macfarlane TV, Macfarlane GJ, Thakker NS, Benhamou S, Bouchardy C, Ahrens W, Pohlabeln H, Lagiou P, Lagiou A, Castellsague X, et al. Role of medical history and medication use in the aetiology of upper aerodigestive tract cancers in Europe: the ARCAGE study. Ann Oncol. 2012;23(4):1053–60.
Ahmadi N, Goldman R, Seillier-Moiseiwitsch F, Noone AM, Kosti O, Davidson BJ. Decreased risk of squamous cell carcinoma of the head and neck in users of nonsteroidal anti-inflammatory drugs. Int J Otolaryngol. 2010;2010:424161.
Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, Menezes RJ, Reid ME. Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg. 2006;132(11):1231–6.
Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125(12):1327–36.
Bosetti C, Talamini R, Franceschi S, Negri E, Garavello W, La Vecchia C. Aspirin use and cancers of the upper aerodigestive tract. Br J Cancer. 2003;88(5):672–4.
Wilson JC, Murray LJ, Hughes CM, Black A, Anderson LA. Non-steroidal anti-inflammatory drug and aspirin use and the risk of head and neck cancer. Br J Cancer. 2013;108(5):1178–81.
Patel D, Kitahara CM, Park Y, Liao LM, Linet M, Kebebew E, Nilubol N. Thyroid cancer and nonsteroidal anti-inflammatory drug use: a pooled analysis of patients older than 40 years of age. Thyroid. 2015;25(12):1355–62.
Reinau D, Surber C, Jick SS, Meier CR. Nonsteroidal anti-inflammatory drugs and the risk of nonmelanoma skin cancer. Int J Cancer. 2015;137(1):144–53.
Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer. 2012;118(19):4768–76.
Torti DC, Christensen BC, Storm CA, Fortuny J, Perry AE, Zens MS, Stukel T, Spencer SK, Nelson HH, Karagas MR. Analgesic and nonsteroidal anti-inflammatory use in relation to nonmelanoma skin cancer: a population-based case-control study. J Am Acad Dermatol. 2011;65(2):304–12.
Curiel-Lewandrowski C, Nijsten T, Gomez ML, Hollestein LM, Atkins MB, Stern RS. Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma: results of a United States case-control study. J Invest Dermatol. 2011;131(7):1460–8.
Jeter JM, Bonner JD, Johnson TM, Gruber SB. Nonsteroidal anti-inflammatory drugs and risk of melanoma. J Skin Cancer. 2011;2011:598571.
Asgari MM, Chren MM, Warton EM, Friedman GD, White E. Association between nonsteroidal anti-inflammatory drug use and cutaneous squamous cell carcinoma. Arch Dermatol. 2010;146(4):388–95.
Wysong A, Ally MS, Gamba CS, Desai M, Swetter SM, Seiffert-Sinha K, Sinha AA, Stefanick ML, Tang JY. Non-melanoma skin cancer and NSAID use in women with a history of skin cancer in the Women's Health Initiative. Prev Med. 2014;69:8–12.
Jeter JM, Han J, Martinez ME, Alberts DS, Qureshi AA, Feskanich D. Non-steroidal anti-inflammatory drugs, acetaminophen, and risk of skin cancer in the Nurses’ health study. Cancer Causes Control. 2012;23(9):1451–61.
Cahoon EK, Rajaraman P, Alexander BH, Doody MM, Linet MS, Freedman DM. Use of nonsteroidal anti-inflammatory drugs and risk of basal cell carcinoma in the United States radiologic technologists study. Int J Cancer. 2012;130(12):2939–48.
Asgari MM, Maruti SS, White E. A large cohort study of nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst. 2008;100(13):967–71.
Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundstrom C, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692–701.
Zhang YQ, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Risk of non-Hodgkin lymphoma and use of non-steroidal anti-inflammatory drugs. Cancer Detect Prev. 2006;30(1):99–101.
Flick ED, Chan KA, Bracci PM, Holly EA. Use of nonsteroidal antinflammatory drugs and non-Hodgkin lymphoma: a population-based case-control study. Am J Epidemiol. 2006;164(5):497–504.
Baker JA, Weiss JR, Czuczman MS, Menezes RJ, Ambrosone CB, Moysich KB. Regular use of aspirin or acetaminophen and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2005;16(3):301–8.
Chang ET, Zheng TZ, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Aspirin and the risk of Hodgkin's lymphoma in a population-based case-control study. J Natl Cancer I. 2004;96(4):305–15.
Zhang YW, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, Zhang B, Zou KY, Flynn S, Tallini G, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419–28.
Birmann BM, Giovannucci EL, Rosner BA, Colditz GA. Regular aspirin use and risk of multiple myeloma: a prospective analysis in the health professionals follow-up study and Nurses’ health study. Cancer Prev Res. 2014;7(1):33–41.
Teras LR, Gapstur SM, Patel AV, Thun MJ, Diver WR, Zhai YS, Jacobs EJ. Aspirin and other nonsteroidal anti-inflammatory drugs and risk of non-Hodgkin lymphoma. Cancer Epidem Biomark Prev. 2013;22(3):422–8.
Chang ET, Froslev T, Sorensen HT, Pedersen L. A nationwide study of aspirin, other non-steroidal anti-inflammatory drugs, and Hodgkin lymphoma risk in Denmark. Br J Cancer. 2011;105(11):1776–82.
Walter RB, Milano F, Brasky TM, White E. Long-term use of acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of hematologic malignancies: results from the prospective vitamins and lifestyle (VITAL) study. J Clin Oncol. 2011;29(17):2424–31.
Erber E, Lim U, Maskarinec G, Kolonel LN. Common immune-related risk factors and incident non-Hodgkin lymphoma: the multiethnic cohort. Int J Cancer. 2009;125(6):1440–5.
Cerhan JR, Anderson KE, Janney CA, Vachon CM, Witzig TE, Habermann TM. Association of aspirin and other non-steroidal anti-inflammatory drug use with incidence of non-Hodgkin lymphoma. Int J Cancer. 2003;106(5):784–8.
Ross JA, Blair CK, Cerhan JR, Soler JT, Hirsch BA, Roesler MA, Higgins RR, Nguyen PL. Nonsteroidal anti-inflammatory drug and acetaminophen use and risk of adult myeloid leukemia. Cancer Epidemiol Biomark Prev. 2011;20(8):1741–50.
Weiss JR, Baker JA, Baer MR, Menezes RJ, Nowell S, Moysich KB. Opposing effects of aspirin and acetaminophen use on risk of adult acute leukemia. Leuk Res. 2006;30(2):164–9.
Kasum CM, Blair CK, Folsom AR, Ross JA. Non-steroidal anti-inflammatory drug use and risk of adult leukemia. Cancer Epidemiol Biomark Prev. 2003;12(6):534–7.
Rinzivillo M, Capurso G, Campana D, Fazio N, Panzuto F, Spada F, Cicchese N, Partelli S, Tomassetti P, Falconi M, et al. Risk and protective factors for small intestine neuroendocrine tumors: a prospective case-control study. Neuroendocrinology. 2016;103(5):531–7.
Acknowledgements
We would like to thank Xiaoxv Yin and Shiyi Cao for critical review of this manuscript. We are grateful to the members of the Professor Lu for their support and helpful discussions.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology, China(2016YXMS215). The funding body contributed to the design of the study and put forward some constructive suggestions for collection, analysis, and interpretation of data and the review and revision of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
YQ, YT, and ZL designed the study and were responsible for writing, analysis, interpretation and revision. YQ and YT carried out the data collection. YQ, YG and CW performed the statistical analyses. YQ, YT, and WL drafted the manuscript. ZL and YHG supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Summary table. Table S2. Subgroup analysis of relative risk of gastric cancer. Table S3. Subgroup analysis of relative risk of esophagus cancer. Table S4. Subgroup analysis of relative risk of colorectal cancer. Table S5. Subgroup analysis of relative risk of hepato-biliary cancer. Table S6. Subgroup analysis of relative risk of pancreatic cancer. Table S7. Subgroup analysis of relative risk of lung cancer. Table S8. Subgroup analysis of relative risk of breast cancer. Table S9. Subgroup analysis of relative risk of ovarian cancer. Table S10. Subgroup analysis of relative risk of endometrial cancer. Table S11. Subgroup analysis of relative risk of prostate cancer. Table S12. Subgroup analysis of relative risk of renal cancer. Table S13. Subgroup analysis of relative risk of bladder cancer. Table S14. Subgroup analysis of relative risk of brain tumor. Table S15. Subgroup analysis of relative risk of head and neck cancers. Table S16. Subgroup analysis of relative risk of skin cancer. Table S17. Subgroup analysis of relative risk of lymphoma. Table S18. Subgroup analysis of relative risk of leukemia. (DOC 549 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Qiao, Y., Yang, T., Gan, Y. et al. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer 18, 288 (2018). https://doi.org/10.1186/s12885-018-4156-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4156-5