Abstract
Background
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) readministration to lung cancer patients is common owing to the few options available. Impact of clinical factors on prognosis of EGFR-mutant non-small cell lung cancer (NSCLC) patients receiving EGFR-TKI readministration after first-line EGFR-TKI failure and a period of TKI holiday remains unclear. Through this retrospective study, we aimed to understand the impact of clinical factors in such patients.
Methods
Of 1386 cases diagnosed between December 2010 and December 2013, 80 EGFR-mutant NSCLC patients who were readministered TKIs after failure of first-line TKIs and intercalated with at least one cycle of cytotoxic agent were included. We evaluated clinical factors that may influence prognosis of TKI readministration as well as systemic inflammatory status in terms of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR).
Baseline NLR and LMR were estimated at the beginning of TKI readministration and trends of NLR and LMR were change amount from patients receiving first-Line TKIs to TKIs readministration.
Results
Median survival time since TKI readministration was 7.0 months. In the univariable analysis, progression free survival (PFS) of first-line TKIs, baseline NLR and LMR, and trend of LMR were prognostic factors in patients receiving TKIs readministration. In the multivariate analysis, only PFS of first-line TKIs (p < 0.001), baseline NLR (p = 0.037), and trend of LMR (p = 0.004) were prognostic factors.
Conclusion
Longer PFS of first-line TKIs, low baseline NLR, and high trend of LMR were good prognostic factors in EGFR-mutant NSCLC patients receiving TKI readministration.
Similar content being viewed by others
Background
Lung cancer is the leading cause of cancer-related deaths in Taiwan and worldwide [1, 2]. Although epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) are administered as standard first-line regimen for advanced EGFR-mutant non-small cell lung cancer (NSCLC) [3–5], the salvage treatment for cases with acquired resistance to EGFR-TKIs remains unclear. Owing to several barriers including difficulty of tumor re-biopsy, absence of EGFR T790m mutation or programmed death-ligand 1 expression, and high expenses, some patients do not have an opportunity to receive novel agents such as 3rd generation TKI [6] or immunotherapies [7, 8].
In patients with acquired resistance to EGFR-TKIs, readministration of first or second generation EGFR-TKIs has been proved to effectively increase patients’ survival time [9–11]. In non-selective patients, EGFR-TKI readministration has only modest efficacy with a progression free survival (PFS) of 2–4 months [10, 12]. However, in optimal selected patients, patients could have a PFS of more than 6 months [10]. Although several good prognostic factors for patients receiving TKI readministration have been reported, such as EGFR-TKI free holidays, better Eastern Cooperative Oncology Group performance status, and benefit from prior EGFR-TKI therapy [10–12], little is known about the correlation between systemic inflammatory markers and TKI readministration efficacies. In previous studies, several systemic inflammatory markers were found to be prognostic factors in lung cancer patients. NSCLC patients with higher blood neutrophil-to-lymphocyte ratio (NLR) had poor prognosis when treated with a combination of bevacizumab and cytotoxic agents [13]; those with higher lymphocyte-to-monocyte ratio (LMR) had better prognosis in EGFR-mutant NSCLC patients receiving first-line EGFR-TKIs [14].
Based on these aforementioned reasons we performed a retrospective study to understand the impact of clinical factors including NLR and LMR on EGFR-mutant NSCLC patients receiving TKI readministration. To decrease the impact of confounding factors, we only included EGFR-mutant NSCLC patients receiving TKI readministration as third or later line therapies after failure of first-line EGFR-TKIs and at least one cycle of intercalated chemotherapy.
Methods
Patients and clinical characteristics
We conducted a retrospective study between December 2010 and December 2013 at Kaohsiung Chang Gung Memorial Hospital in Taiwan. Patients were followed-up until November 2015. Adult patients aged ≥18 years with histologically or cytologically confirmed stage IIIB or IV NSCLC who had been treated with first line EGFR-TKIs and received TKIs readministration were included. Patients who had received a second TKI without intercalating with at least one cycle of cytotoxic chemotherapies were excluded.
Baseline assessments including clinical parameters, hematological variables, biochemistry, chest radiography, and chest computed tomography were performed within 4 weeks of initiation of TKI readministration.
Clinical parameters included length of TKI holiday and PFS of study patients receiving first line EGFR-TKIs. Data regarding hematological parameters were collected within 4 weeks of the initiation of first-line TKI therapy and also TKI readministration including neutrophil, lymphocyte, and monocyte counts. NLR was obtained by dividing the neutrophil count by the lymphocyte count, and LMR was obtained by dividing the lymphocyte count by the monocyte count. Baseline NLR and LMR were estimated at the beginning of TKI readministration and trends of NLR and LMR were obtained by dividing the data estimated at the beginning of TKI readministration with the data estimated at the beginning of first-line TKIs.
This study was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital. The need for informed consent was waived.
EGFR mutation testing
Tumor specimens were obtained by bronchoscopy CT-guided biopsy, pleural effusion cytology, or surgical procedures. The EGFR mutational analyses was performed using SCORPIONS and ARMS polymerase chain reaction using fragments amplified from genomic DNA extracted from paraffin-embedded tissues (QIAGEN EGFR RGQ PCR KIT). Exon 19 deletion and L858R mutations were defined as common mutations. Other mutations or compound mutations were defined as uncommon mutations.
Evaluation of response to EGFR-TKI readministration
Patients underwent routine chest radiography every 2–4 weeks and chest computed tomography every 2–3 months to evaluate tumor responses. PFS was defined as the time between the first day of EGFR-TKI administration and disease progression, death before documented progression, or the last visit during the follow-up period. Disease progression was determined by the clinician according to the Response Evaluation Criteria in Solid Tumors criteria 1.1 [15]. The endpoint was overall survival (OS), which was defined as the first day of EGFR-TKI readministration until death, or the last visit during the follow-up period.
Statistical analyses
Statistical analyses were performed using MedCalc (version 14.10.2). Receiver operating characteristic (ROC) curves with binary variable of OS longer or shorter than 7.0 months since readministration and Youden’s index were used to determine the best cut-off value for baseline values of and trends of NLR LMR as a prognostic factors. OS analyses were performed using the Kaplan-Meier method and the log-rank test. Cox proportional hazards regression test were used to evaluate independent factors. P value < 0.05 was considered significant in statistical tests.
Results
Patient characteristics
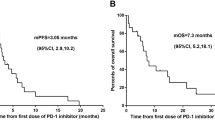
Between December 2010 and December 2013 1386 lung cancer cases were diagnosed. Of these, 269 patients had a positive EGFR mutation status and were treated with first-line EGFR-TKIs, and 80 patients were readministered TKIs with at least one cycle intercalated cytotoxic agent (Fig. 1). Lines and regimens of Intercalated chemotherapies were shown in Additional file 1: Table S1. The median follow-up time since readministration was 7.0 months the longest follow-up duration was 20.4 months. At the end of follow-up 78.8 % (63/80) patients showed disease progression under TKI readministration and 36.3 % (29/80) patients were alive. Baseline values and trends of hematological parameters were available for 78 and 77 patients, respectively. To evaluate baseline values and trends of NLR and LMR, using ROC curve analysis, we determined that the best cut-off values were 5.2, 1.1, 2.5, and 0.5, respectively.
Impact of clinical factors on overall survival of TKI readministration
Clinical factors found to be significant in the univariable analysis for poor OS since TKI readministration included shorter PFS of first-line TKI (p = 0.020) (Fig. 2) high baseline NLR (p < 0.001) (Fig. 3a), low baseline LMR (p = 0.006B), and low trend of LMR (p = 0.037) (Fig. 4) (Table 1).
Influence of baseline proinflammatory markers on overall survival (OS) of patients who were readministered with tyrosine kinase inhibitors (a) OS between patients with high and low baseline neutrophil-to-lymphocyte ratio (NLR); (b) OS between patients with high and low lymphocyte-to-monocyte ratio (LMR)
Length of TKI holiday changes in the TKI regimen, and first or second generation TKIs when TKI readministration, and trend of NLR did not significantly influence OS. In the multivariable analysis, independent prognostic factors for shorter OS were shorter first-line TKI PFS (p < 0.001), high baseline NLR (p = 0.037), and low trend of LMR (p = 0.004) (Table 1).
Discussion
Our retrospective observational study found that baseline NLR and trend of LMR as well as PFS of first-line EGFR-TKI treatment were prognostic factors in patients receiving TKI readministration. NLR was previously found to have a prognostic effect in different types of cancer like ovarian cancer, breast cancer, pancreatic cancer, and colorectal cancer, as well as in advanced NSCLC patients treated with first-line platinum-based chemotherapy [16–21]. LMR was found to be a prognostic factor in small cell lung cancer [22], in early-stage NSCLC patients post operation [23], in advanced lung cancer treated with cytotoxic chemotherapies [24], and in EGFR-mutant lung cancer patients treated with first-line EGFR-TKIs [14]. Several possible mechanisms may explain the prognostic effect of these pro-inflammatory markers. First, neutrophils release several pro-angiogenic factors and promote angiogenesis, which is essential for tumor progression. Second, lymphocytes play a pivotal role in tumor cell eradication [25], and tumor-associated macrophages promote tumor progression through remodeling of the tumor extracellular matrix [26, 27]. Based on the above pathophysiology, patients with high NLR and low LMR tend to have tumor progression and fewer T cells available for cancer cell eradication.
Previous studies have reported conflicting results regarding the influence of PFS of previous EGFR-TKI on the efficacy of TKI readministration. In one study that included all patients without TKI holidays longer PFS of previous TKI treatment paradoxically shortened the PFS of TKI readministration [11]. Another study in which 52 % of patients with a TKI holiday before TKI rechallenge revealed that PFS of previous TKI treatment was not related with the efficacy of TKI readministration [12]. By excluding patients without having TKIs holidays, our study revealed that patients with a longer PFS of previous TKI treatment have a longer OS of TKI readministration. In the first study, the authors speculated that in patients who received previous therapy for less than 12 months, the tumor may not yet have acquired the 790 M mutation. However, this concept was not supported by subsequent studies [28]. We speculated that when the disease progresses after the first TKI therapy, tumors have a dominant part of TKI-resistant clones and a minor part of TKI-sensitive clones.
After the TKI holidays and owing to intercalation with cytotoxic chemotherapies tumor redistribution occurred, which lead to TKI-sensitive clones increasing, and TKI-resistant clones decreasing. This redistribution was due to higher sensitivity to cytotoxic chemotherapies in TKI-resistant clones than that in TKI-sensitive clones. After tumor redistribution by the intercalated chemotherapies, tumor characteristics were more similar to those of TKI-naïve tumors than to TKI-resistant tumors.
This can explain at least partly, why PFS of previous TKIs has opposite influences in patients with or without TKI holidays. However, this concept should be proved with further studies.
Though several studies have reported on how clinical factors affect the efficacies of TKI readministration [10–12] patient heterogeneity is a confounding factor that cannot be neglected. One study included more than 70 % of patients without TKI holidays, whereas two other studies included 32 and 50 % patients with wild type EGFR mutation, respectively. We only included EGFR-mutant NSCLC patients receiving first-line EGFR-TKIs and at least one intercalated chemotherapy agent to decrease these confounding factors. To the best of our knowledge, this is the first study demonstrating that baseline NLR and trend of LMR are prognostic factors in patients receiving EGFR-TKI readministration. As a study aimed at patients receiving third and later line therapies, the number of patients is not small.
Our study had several limitations. First, data regarding the amount and pattern of inflammatory cell infiltration as well as the amount of tumor programmed death-ligand 1 expression in tumors were not available, which could have provided us further information about the immune condition in the tumor microenvironment [29]. Further studies are required to determine whether immunotherapy or anti-angiogenesis agents could prolong survival in those who were speculated to have poor prognosis to TKI readministration. Finally, our study was a retrospective study a prospective trial is needed to validate these results.
Conclusion
Longer PFS of first-line TKIs, low baseline NLR, and high trend of LMR were good prognostic factors in EGFR-mutant NSCLC patients receiving TKI readministration.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- LMR:
-
Lymphocyte-to-monocyte ratio
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- ROC:
-
Receiver operating characteristic
- TKI:
-
Tyrosine kinase inhibitor
References
Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA, Centers for Disease C, Prevention. Lung cancer incidence trends among men and women--United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2014;63(1):1–5.
Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in taiwan: a population-based cancer registry. J Thorac Oncol. 2013;8(9):1128–35.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26(9):1877–83.
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74.
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22.
Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–99.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–35.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Hata A, Katakami N, Kaji R, Fujita S, Imai Y. Does T790M disappear? Successful gefitinib rechallenge after T790M disappearance in a patient with EGFR-mutant non-small-cell lung cancer. J Thorac Oncol. 2013;8(3):e27–9.
Zhao ZR, Li W, Long H. Readministration of EGFR tyrosine kinase inhibitor in non-small cell lung cancer patients after initial failure, what affects its efficacy? Sci Rep. 2014;4:5996.
Asami K, Kawahara M, Atagi S, Kawaguchi T, Okishio K. Duration of prior gefitinib treatment predicts survival potential in patients with lung adenocarcinoma receiving subsequent erlotinib. Lung Cancer. 2011;73(2):211–6.
Hata A, Katakami N, Yoshioka H, Fujita S, Kunimasa K, Nanjo S, Otsuka K, Kaji R, Tomii K, Iwasaku M, et al. Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 2011;74(2):268–73.
Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14(6):469–75.
Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, Wang CC, Chung YH, Wang YH, Su MC, et al. Baseline and Trend of Lymphocyte-to-Monocyte Ratio as Prognostic Factors in Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer Patients Treated with First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PLoS One. 2015;10(8):e0136252.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132(3):542–50.
Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen K, Su F. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS One. 2015;10(11):e0143061.
Szkandera J, Stotz M, Eisner F, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Ress AL, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One. 2013;8(11):e78225.
Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015;5:11026.
Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260(2):287–92.
Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62(3):471–9.
Go SI, Kim RB, Song HN, Kang MH, Lee US, Choi HJ, Lee SJ, Cho YJ, Jeong YY, Kim HC, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31(12):323.
Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062.
Song YJ, Wang LX, Hong YQ, Lu ZH, Tong Q, Fang XZ, Tan J. Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumour Biol. 2016;37(4):5285–93.
Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73(8):2381–8.
Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31(2):248–58.
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–46.
Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31(8):1070–80.
Remark R, Becker C, Gomez JE, Damotte D, Dieu-Nosjean MC, Sautes-Fridman C, Fridman WH, Powell CA, Altorki NK, Merad M, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191(4):377–90.
Acknowledgements
We thank Tsui-Ping Tang and I-Chun Lin for the valuable assistance with data collection.
Funding
The authors have no support or funding to report.
Availability of data and materials
The raw of the data would not be shared at this moment because there are several papers under preparation based on this raw data.
Authors’ contributions
Conceived and designed the experiments: YMC CHL HC Chang TYC CCT WFF SFL HC Chen YCC YPC MCL. Analyzed the data: YMC CCW YHC MCL. Contributed materials/analysis tools: YMC KMR CHH YHW MCL MCS KTH. Wrote the paper: YMC MCL. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital. IRB number: 102-4571b. The need for informed consent was waived. (The data were analyzed retrospectively, and all identifying patient data were removed prior to analysis.)
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Lines and regimens of Intercalated chemotherapies. (DOCX 12 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, YM., Lai, CH., Rau, KM. et al. Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 16, 868 (2016). https://doi.org/10.1186/s12885-016-2917-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2917-6