Abstract
Background
The preferred chemotherapy method for gastric cancer continues to be matter of debate. We performed a meta-analysis to comparing prognosis and safety between perioperative chemotherapy and adjuvant chemotherapy to identify the better chemotherapy option for gastric cancer.
Methods
We searched the PubMed, EMBASE, Cochrane Library, and Ovid databases for eligible studies until February 2016. The main endpoints were prognostic value (hazard ratio [HR] for overall survival [OS] and 1-, 2-, 3-, and 5-year survival rate), response rate of chemotherapy, radical resection rate, post-operative complication rate, and adverse effects of chemotherapy.
Results
Five randomized controlled trials and six clinical controlled trials involving 1,240 patients were eligible for analysis. Compared with the adjuvant chemotherapy group, the perioperative chemotherapy group had significantly better prognosis (HR, 0.74; 95 % CI, 0.61 to 0.89; P < 0.01). The difference between the two groups remained significant in the studies that used combination chemotherapy as the neoadjuvant chemotherapy regimen (HR, 0.59; 95 % CI, 0.46 to 0.76; P < 0.01) but were not significant in the studies that used fluoropyrimidine monotherapy (HR, 0.93; 95 % CI, 0.56 to 1.55; P = 0.84). Furthermore, the two groups showed no significant differences in the post-operative complication rates (relative risk, 0.98; 95 % CI, 0.63 to 1.51; P = 0.91) or adverse effects of chemotherapy (P > 0.05 for all adverse effects).
Conclusion
Perioperative chemotherapy showed improved survival compared to adjuvant chemotherapy for gastric cancer. In addition, combination chemotherapy resulted in better survival compared to monotherapy in the neoadjuvant chemotherapy regimens.
Similar content being viewed by others
Background
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide [1, 2]. To date, surgery is the only curative treatment for GC. However, the results are still unsatisfactory, owing to the high rate of metastasis and relapse [1, 3].
Chemotherapy together with surgery has shown promising results. For instance, a randomized controlled trial conducted by Cunningham et al. [4] showed that perioperative chemotherapy (PC) could result in better survival than surgery alone. Similarly, Bang et al. [5] showed that adjuvant chemotherapy (AC) could improve survival over surgery alone. However, the method of delivery of chemotherapy for GC is still a matter of debate. PC consists of preoperative (neoadjuvant) chemotherapy and postoperative chemotherapy, and is provided as standard of care in NCCN guideline for GC. At the same time, the application of AC is limited to situations where neoadjuvant therapy had not been given prior to surgery [6]. However chemotherapy given prior to surgery may reduce tumor burden and eradicate micrometastatic foci outside the surgical field [7, 8]. Several studies have emphasized the survival benefits of PC to the patients [9, 10]. However, chemortherapy given prior to surgery can cause fibrosis and tissue edema, which may cause difficulties during surgery [11], causing adverse effects to the patients [12, 13]. Therefore, we performed a meta-analysis to compare the prognostic value, side effects, and post-operative complications of PC and AC in patients with GC.
Methods
Search strategy
Studies were selected by searching major medical databases (PubMed, EMBASE, Cochrane Library, and Ovid) for all articles published until February 1, 2016. We used the following keywords: “neoadjuvant”, “preoperative”, “perioperative”, “chemotherapy”, “stomach neoplasm”, “gastric cancer”, and “gastrectomy” Then, we narrowed the search by browsing the abstracts, methods, and references of the articles retrieved.
Inclusion and exclusion criteria
The studies that met the following criteria were included: (i) publications that compared PC with AC in patients with GC undergoing surgery; (ii) the full text of the articles was available, with a clear description of the chemotherapy regimens used in the study; (iii) at least one of the outcome measures mentioned below was reported or could be calculated from the data provided. In cases of overlap between authors or institutions, only the higher-quality or more recent study was selected. Studies were excluded for the following reasons: (i) PC and AC were not compared in the patients with GC; (ii) post-operative chemotherapy was not applied in either the PC or AC groups; (iii) radiotherapy was part of treatment.
Outcome measures, data extraction, and assessment of the risk of bias
The primary outcomes were prognostic value (hazard ratio [HR] for overall survival [OS] and 1-, 2-, 3-, and 5-year survival rate), response rate of chemotherapy (response rate: complete response [CR] or partial response [PR] after chemotherapy), radical resection rate; total post-operative complication rate (defined on the basis of the system for reporting complications established by the Memorial Sloan-Kettering Cancer Center [14]), and the adverse effects of chemotherapy. Two authors independently extracted data from full-text articles using a unified datasheet. Randomized controlled trials (RCTs) were evaluated using the Jadad Composite Scale (JCS), wherein high-quality trials should score ≥ 3 of a maximum possible score of 5. Controlled clinical trials (CCTs) were evaluated using the Newcastle–Ottawa Scale [15], wherein high-quality trials should score ≥ 7 of a maximum possible score of 9, and moderate-quality trials should score ≥ 5. Disagreements were presented to a third author and resolved by discussions among the investigators.
Statistical analysis
This meta-analysis was conducted using RevMan software version 5.2 (Cochrane Collaboration). The risk ratio (RR) and HR were used to evaluate the prognostic effect. If the HR and its variance were not reported directly in the original study, these values were calculated using a software designed by Tierney et al. [16]. For HR, we performed subgroup analysis based on available method, such as study design, NAC regimen, et al. In addition, the RR was used to analyze other discontinuous variables. Both ratios were reported with 95 % confidence intervals (CIs). Heterogeneity was determined using the χ2 test or Cochran Q test. I2 was used to quantify heterogeneity. P < 0.10 and I2 > 50 % indicated significant heterogeneity. The inverse variance method with a fixed-effects model was applied when heterogeneity was not found, whereas the random-effects model was used when heterogeneity was found. Publication bias was tested using funnel plots. P < 0.05 was considered significant when measuring the effect sizes. This manuscript reporting adheres to PRISMA guidelines for reporting systematic reviews and meta-analyses.
Results
Eligible studies
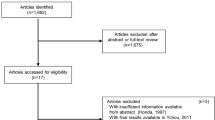
The search for the aforementioned keywords allowed the identification of 4538 articles. Five RCTs [17–21] and six CCTs [9, 11, 22–25] were considered eligible for this meta-analysis (Fig. 1). The analyses included 1240 patients who were in the PC group (n = 557) or in the AC group (n = 683). The detailed characteristics of the patients are listed in Table 1 and Additional file 1. Four RCTs scored 3 in the JCS, indicating that they were high-quality studies (Table 2). Three CCTs scored 6 (moderate-quality study) on the Newcastle–Ottawa scale and 3 CCTs scored 7 (high-quality study) (Table 3).
Hazard ratio for the overall survival
Nine [9, 17–22, 24, 25] out of eleven studies (5 RCTs and 4 CCTs) evaluated provided effective data for the calculation of the HR for OS. Compared with the AC group, the PC group had significantly better prognosis (HR, 0.74; 95 % CI, 0.61 to 0.89; P < 0.01, Fig. 2a-c). The difference between the two groups remained significant subgroup analysis that only consisted of RCTs (HR, 0.74; 95 % CI, 0.60 to 0.93; P = 0.01) (Fig. 2a).
We also performed a subgroup analysis examining the nine studies divided into two subgroups: the fluoropyrimidine monotherapy subgroup and combination chemotherapy subgroup (Fig. 2b). PC did not demonstrate improved prognosis in the fluoropyrimidine monotherapy subgroup (HR, 0.93; 95 % CI, 0.56 to 1.55; P = 0.84) but did show improved prognosis in the combination chemotherapy subgroup (HR, 0.59; 95 % CI, 0.46 to 0.76; P < 0.01).
We also performed a subgroup analysis considering the study locations (Fig. 2c). Five where Chinese studies and four Japanese studies. PC resulted in significantly better prognosis in the Chinese studies (HR, 0.61; 95 % CI, 0.47 to 0.80; P < 0.01) but not in the Japanese studies (HR, 0.88; 95 % CI, 0.68 to 1.13; P = 0.30).
1-, 2-, 3-, 5-year survival rates
Nine [9, 17–22, 24, 25], nine [9, 17–22, 24, 25], seven [9, 17, 18, 20–22, 25], six [9, 18, 20, 21, 23, 25] studies reported 1-, 2-, 3-, 5- year survival rates, respectively. There were no significant differences in the 1- and 2-year survival rates between the two study groups (1-year survival rate: RR, 0.81; 95 % CI, 0.60 to 1.09; P = 0.17, Additional file 2A; 2-year survival rate: RR, 0.90; 95 % CI, 0.77 to 1.04; P = 0.15, Additional file 2B). However, the PC group showed significantly better prognosis for 3- and 5-year survival rates (3-year survival rate: RR, 0.80; 95 % CI, 0.67 to 0.96; P = 0.01, Additional file 2C; 5-year survival rate: RR, 0.77; 95 % CI, 0.64 to 0.92; P < 0.01, Additional file 2D).
Response rate to neoadjuvant chemotherapy
Eight studies [9, 11, 17–20, 22, 24] reported the response rates to NAC in 358 patients. The response rate ranged between 33.3 and 70.0 %. In total, 199 patients achieved CR or PR. The overall response rate was 55.6 %.
Radical resection rate
Seven studies [9, 11, 17–19, 22, 24] reported the radical resection rate. A total of 218 out of 265 patients (82 %) in the PC group and 218 out of 292 (74 %) patients in the AC group received radical resection. Although no significant difference was observed, the PC group showed a trend towards a higher radical resection rate (RR, 1.10; 95 % CI, 0.96 to 1.27; P = 0.17, Fig. 3a).
Total post-operative complication rate
Five studies [9, 11, 19, 22, 24] reported the prevalence of complications. A total of 31 out of the 213 patients in the PC group and 37 out of 243 patients in the AC group suffered postoperative complications. There was no significant difference between two groups (RR, 0.98; 95 % CI, 0.63 to 1.51; P = 0.91, Fig. 3b).
Adverse effects of chemotherapy
Three studies [17, 19, 24] reported adverse effects of chemotherapy in detail. Our meta-analysis indicated that all the adverse effects (including nausea and vomit, gastrointestinal problem, liver toxicity, neurologic effects, leukopenia, thrombocytopenia, and neutropenia) were not significantly different between the two study groups (P > 0.05 for all the comparisons, Additional file 3).
Publication bias
A funnel-plot analysis was performed to determine the publication bias on the basis of the measurement of HR for OS (Fig. 4). The analysis indicated that all the studies were within the funnel plot and were distributed symmetrically.
Discussion
Two methods of chemotherapy delivery, perioperative chemotherapy (PC) and adjuvant chemotherapy (AC) are widely used. However, there is no high level evidence comparing the prognosis and safety between PC and AC. Our meta-analysis that synthesized the results of several smaller studies showed that PC was superior to AC when considering the HR for OS and the 3–, 5-year survival rates. This result indicates that the addition of chemotherapy prior to surgery could provide additional benefits over chemotherapy provided after surgery alone. This may occur because of the effects of NAC in reducing tumor burden and eradicating micrometastatic foci [8]. In this meta-analysis, the response rate to NAC reached 55.6 %. In addition, the radical resection rate was relatively higher in the PC group.
Fluoropyrimidine is the most common and widely accepted chemotherapy drug for GC [26, 27]. However, combination chemotherapy that includes fluoropyrimidine rather than fluoropyrimidine alone is used and recommended by most professionals [4, 28–31]. In our meta-analysis, PC failed to show significant benefits compared with AC in the fluoropyrimidine monotherapy subgroup. However, a significant difference was observed between the two groups in the combination chemotherapy subgroup. This suggests that combination chemotherapy is a better option for NAC in GC and provides significant advantage in relation to fluoropyrimidine monotherapy.
The stage of cancer is another important issue when choosing the treatment method. In the NCCN guideline, for early-stage GC, PC is not routinely recommended [6]. To better understand this issue, we considered the tumor stages reported in the studies included in this meta-analysis. In addition, in almost all the studies included, the majority, if not all, of the patients were at advanced stages. However, the RCT by Nio et al. [20] was an exception. Of the 295 patients evaluated in the RCT, 170 patients were stage 1, and this subgroup failed to show any advantage of PC over AC. On the other hand, when we redid the meta-analysis excluding stage 1 patients and only including stage 2 and 3 patients, the survival benefits of PC became more significant (HR 0.68; 95 % CI, 0.56 to 0.83; P < 0.01). Therefore, the conclusion that “perioperative chemotherapy was superior to adjuvant chemotherapy in the survival benefits” seems to be more suitable for advanced GC.
The results of two large European RCTs [4, 32], which compared PC and surgery alone, established PC as another alternative option for GC. However, no European studies have compared PC and AC. Furthermore, all 11 studies included in this meta-analysis were from Asian countries (China and Japan). We performed a subgroup analysis for these two countries and the results indicated that the Chinese group showed an advantage compared with the Japanese group. This observation may be explained by the fact that Nio et al. [20] included many early-stage patients and most Japanese studies used fluoropyrimidine monotherapy as their NAC method. This suggests that Japanese groups should give more consideration to the use of combination chemotherapy, especially in the pre-operative setting.
Another potential advantage of perioperative chemotherapy is that it increases the likelihood that patients will receive at least part of their planned systemic chemotherapy regimen. For example, Yonemura [17] reported that patients in PC group received relative more courses of regimen in fact. In that study, patients in PC and AC received an average of 2.9 and 2.3 courses of regimen respectively. However, this issue is not reported by the others studies. We believe that studies should pay more attention to this matter in the future.
Safety is always of the utmost concern in clinical practice. Because fibrosis, tissue edema and toxicity may result from chemotherapy [11, 33], there is a concern that the addition of chemotherapy prior to surgery may increase the risk during surgery as well as increase complication rates and adverse effects [34]. In our study, the complication rates and adverse effects during PC were similar to those of AC. Postoperative complication rates were also consistent with the studies that evaluated the effects of NAC (without the restrictions of AC administration) [35, 36]. However, only three studies reported adverse effects, and these studies were not enough to draw a solid conclusion. More investigations are needed to evaluate the side effects of PC.
To the best of our knowledge, this is the first meta-analysis that compares PC with AC in GC. In our meta-analysis, we included 11 studies, five of which were RCTs, and found that PC was superior to AC in the survival effects without compromising safety. In addition, combination chemotherapy was a better option in the pre-operative setting over monotherapy. A subgroup analysis involving three studies [10] in a previous meta-analysis evaluated the effects of NAC in GC and found similar survival benefits. Several limitations of our study should be considered. First, we included some retrospective studies, which may influence the statistical power. Second, all studies were from Asia and limits its generalizability for GC patients worldwide. Third, only a few studies compared the adverse effects of chemotherapy, which did not allow us to draw conclusions about the safety of PC.
Conclusion
Perioperative chemotherapy provides a survival advantage over adjuvant chemotherapy for GC patients, especially for the patients with advanced GC. In addition, combination chemotherapy is a better option for neoadjuvant chemotherapy regimen over monotherapy.
Abbreviations
- AC:
-
Adjuvant Chemotherapy
- CCTs:
-
Clinical Controlled Trials
- CIs:
-
Confidence Intervals
- CR:
-
Complete Response
- GC:
-
Gastric Cancer
- HR:
-
Hazard Ratio
- NAC:
-
Neoadjuvant Chemotherapy
- OS:
-
Overall Survival
- PC:
-
Perioperative Chemotherapy
- PR:
-
Partial Response
- RCTs:
-
Randomized Controlled Trials
- RR:
-
Risk Ratio
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14(4):249–58.
Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, Group EW. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45(6):931–91.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS et al.: NCCN Clinical Practice Guidelines in Oncology: Gastric cancer. Version 1, 2016. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
Frei 3rd E. Clinical cancer research: an embattled species. Cancer. 1982;50(10):1979–92.
Frei 3rd E, Miller D, Clark JR, Fallon BG, Ervin TJ. Clinical and scientific considerations in preoperative (neoadjuvant) chemotherapy. Recent Results Cancer Res. 1986;103:1–5.
Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L, et al. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol. 2012;105(8):793–9.
Yang Y, Yin X, Sheng L, Xu S, Dong L, Liu L. Perioperative chemotherapy more of a benefit for overall survival than adjuvant chemotherapy for operable gastric cancer: an updated Meta-analysis. Sci Reports. 2015;5:12850.
Feng D, Leong M, Li T, Chen L, Li T. Surgical outcomes in patients with locally advanced gastric cancer treated with S-1 and oxaliplatin as neoadjuvant chemotherapy. World J Surg Oncol. 2015;13:11.
Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Okada K, Mori M, Doki Y. Neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal dissemination. Ann Surg Oncol. 2011;18(13):3726–31.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204(3):356–64.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Yonemura Y, Sawa T, Kinoshita K, Matsuki N, Fushida S, Tanaka S, Ohoyama S, Takashima T, Kimura H, Kamata T, et al. Neoadjuvant chemotherapy for high-grade advanced gastric cancer. World J Surg. 1993;17(2):256–61. discussion 261–252.
Sun XC, Lin J, Ju AH. Treatment of Borrmann type IV gastric cancer with a neoadjuvant chemotherapy combination of docetaxel, cisplatin and 5-fluorouracil/leucovorin. J Int Med Res. 2011;39(6):2096–102.
Qu JJ, Shi YR, Liu FR, Ma SQ, Ma FY. A clinical study of paclitaxel combined with FOLFOX4 regimen as neoadjuvant chemotherapy for advanced gastric cancer. Chin J Gastrointestinal Surg. 2010;13(9):664–7.
Nio Y, Koike M, Omori H, Hashimoto K, Itakura M, Yano S, Higami T, Maruyama R. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer--a single institute study. Anticancer Res. 2004;24(3b):1879–87.
Kobayashi T, Kimura T. Long-term outcome of preoperative chemotherapy with 5′-deoxy-5-fluorouridine (5′-DFUR) for gastric cancer. Gan Kagaku ryoho Cancer Chemotherapy. 2000;27(10):1521–6.
Zhang J, Chen RX, Zhang J, Cai J, Meng H, Wu GC, Zhang ZT, Wang Y, Wang KL. Efficacy and safety of neoadjuvant chemotherapy with modified FOLFOX7 regimen on the treatment of advanced gastric cancer. Chin Med J. 2012;125(12):2144–50.
Zhang CW, Zou SC, Shi D, Zhao DJ. Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer. World J Gastroenterol. 2004;10(20):3070–2.
Sun Z, Zhu RJ, Yang GF, Li Y. Neoadjuvant chemotherapy with FOLFOX4 regimen to treat advanced gastric cancer improves survival without increasing adverse events: a retrospective cohort study from a Chinese center. Sci World J. 2014;2014:418694.
Nishioka B, Ouchi T, Watanabe S, Umehara M, Yamane E, Yahata K, Muto F, Kojima O, Nomiyama S, Sakita M, et al. Follow-up study of preoperative oral administration of an antineoplastic agent as an adjuvant chemotherapy in stomach cancer. Gan Kagaku ryoho Cancer Chemotherapy. 1982;9(8):1427–32.
Fukushima M, Satake H, Uchida J, Shimamoto Y, Kato T, Takechi T, Okabe H, Fujioka A, Nakano K, Ohshimo H, et al. Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol. 1998;13(4):693–8.
Schoffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anti-Cancer Drugs. 2004;15(2):85–106.
Mackenzie M, Spithoff K, Jonker D. Systemic therapy for advanced gastric cancer: a clinical practice guideline. Curr Oncol. 2011;18(4):e202–9.
Iacovelli R, Pietrantonio F, Maggi C, de Braud F, Di Bartolomeo M. Combination or single-agent chemotherapy as adjuvant treatment of gastric cancer: A systematic review and meta-analysis of published trials. Crit Rev Oncol/Hematol. 2015;98:24–8.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–21.
Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25(22):3205–9.
Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
Friedman MA, Ogawa M, Carter SK, Sakurai Y, Kimura K, Hannigan J. Chemotherapy of disseminated gastric cancer. A joint effort of the Northern California Oncology Group and the Japanese Gastric Cancer Chemotherapy Group. Cancer. 1983;52(10):1771–7.
Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant chemotherapy followed by surgery versus surgery alone for gastric carcinoma: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(1):e86941.
Xiong BH, Cheng Y, Ma L, Zhang CQ. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Investig. 2014;32(6):272–84.
Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16(44):5621–8.
Acknowledgments
We thank the department of Surgical Oncology of First Hospital of China Medical University and the College of China Medical University for technical assistance.
Funding
This work was supported by Natural Science Foundation of Liaoning Province (No. 2014029201), Program of Education Department of Liaoning Province (L2014307), the Key Laboratory Programme of Education Department of Liaoning Province (LZ2015076).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
JZ and PG contributed equally to this work. ZW participated in the conception and design of the study and coordination; JZ and XC participated in design of the study, data extraction, article selection and manuscript preparation and interpreted the results in collaboration with BM and YS; YY and JS participated in data extraction, article selection and data extraction; PG performed the statistical analysis and participated in the critical revision of the manuscript. All authors drafted and critically revised the manuscript and approved the final version.
Competing interests
All the authors declare that they have no competing interests. And our manuscript has not been published previously and is not under consideration by any other publications.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Concrete chemotherapy regimens used in included studies. (DOCX 22 kb)
Additional file 2:
(A) Meta-analysis of 1 year survival rate; (B) Meta-analysis of 2 year survival rate; (C) Meta-analysis of 3 year survival rate; (D) Meta-analysis of 5 year survival rate. (TIF 457 kb)
Additional file 3:
meta-analysis of chemotherapy adverse effects. (A) Nausea and vomit, (B) gastrointestinal problem, (C) liver toxicity, (D) neurologic effects, (E) leukopenia, (F) thrombocytopenia, (G) neutropenia. (TIF 507 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, Jh., Gao, P., Song, Yx. et al. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: a meta-analysis. BMC Cancer 16, 631 (2016). https://doi.org/10.1186/s12885-016-2667-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2667-5