Abstract
Background
Early pregnancy nutritional status can be associated with adverse birth outcomes such as small-for-gestational age (SGA) and low birth weight (LBW). BMI (Body Mass Index) and MUAC (Mid-upper arm circumference) are easy to use assessments and are indicative of the pre-pregnancy nutritional status if obtained in the first trimester. This study primarily assesses the association of maternal nutritional status using BMI and MUAC with SGA in a community-based cohort of Pakistani women. It also aims to determine the predictive ability of MUAC and BMI in predicting SGA. Secondarily, we assessed the association between maternal nutrition and large for gestational age (LGA) and LBW.
Methods
This study is a secondary analysis of an ongoing pregnancy cohort “Pregnancy Risk Infant Surveillance and Measurement Alliance (PRISMA)“in Ibrahim Hyderi and Rehri Goth, Karachi. PRISMA participants who were enrolled between January 2021 to August 2022 were included given they had a gestational age < 14 weeks confirmed via ultrasound, MUAC and BMI measurements were available and birth weight was captured within 72 hours. Multivariable logistic regression was used to determine an association between maternal nutritional status and SGA. The PRISMA study was approved by the Aga Khan University Ethics Review Committee (2021–5920-15,518).
Results
Of 926 women included in the analysis, 26.6% (n = 247) had a low MUAC (< 23 cm) while 18.4% (n = 171) were underweight (BMI < 18.5 kg/m2). Nearly one third of low MUAC and underweight women delivered SGA infants (34.4 and 35.1% respectively). Underweight women and women with low MUAC had a statistically significant association with SGA (Underweight: OR 1.49, 95% CI 1.1,2.4; Low MUAC-OR 1.64, 95% CI 1.2,2.3) as well as LBW (Underweight: OR-1.63, 95% CI 1.1,2.4; Low MUAC-OR-1.63, 95% CI 1.2,2.3). ROC curves showed that MUAC and BMI had modest predictability for SGA (AUC < 0.7).
Conclusion
Maternal nutritional status as indicated by BMI and MUAC are strongly associated with adverse pregnancy outcomes including SGA, LGA and LBW. Although MUAC and BMI are widely used to determine maternal nutritional status, they have poor predictive ability for newborn size. Further research is needed to identify other tools or a combination of tools to better predict adverse birth outcomes in resource-limited settings and plan interventions.
Similar content being viewed by others
Introduction
Pre-conceptional nutritional status as well as maternal nutrition during pregnancy is pivotal to both mother and child’s health. Evidence suggests that maternal malnutrition can be associated with adverse birth outcomes such as preterm birth (PTB), low birth weight (LBW), small-for-gestational age (SGA) and intrauterine growth restriction (IUGR) [1,2,3]. The global prevalence of maternal underweight is reported to be 9.7% while in South Asia prevalence of maternal underweight status is nearly three times higher (24%) [4]. In Pakistan, a country grappling with poor maternal and neonatal indicators, women of reproductive age (WRA) face a triple burden of malnutrition: undernutrition, overweight/obesity, and micronutrient deficiencies all of which contribute to poor pregnancy outcomes [5]. According to the Pakistan National Nutrition Survey 2018, 14.4% WRA fall under the category of undernutrition defined as BMI < 18.5 kg/m2 [6].
Abnormal birth weight, whether classified small for gestational age (SGA) when birth weight falls below the 10th percentile or large for gestational age (LGA) when birth weight exceeds the 90th percentile for gestational age, are associated with increased infant mortality and morbidity [7, 8]. LGA infants often experience macrosomia, leading to complications during delivery, such as shoulder dystocia, Erb’s palsy, fractures, and neonatal asphyxia [9]. In contrast, SGA infants, particularly when born prematurely, are at a higher risk of various issues, including infections, hypothermia, hypoglycemia, respiratory problems, and feeding difficulties [9]. A recent Lancet Commission on Small Vulnerable Newborns (SVN) includes preterm, LBW and SGA infants as they collectively contribute significantly to poor neonatal outcomes [10, 11]. It is also notable that South Asia has the highest rates of SVN where 52.1% of all newborns are affected [11].
Maternal undernutrition is identified as a strong contributor to the development of SVNs [10]. Establishing nutritional status of the mother early in pregnancy is important so that adequate interventions can be proposed to prevent adverse pregnancy outcomes. Body Mass Index (BMI) and Mid-upper arm circumference (MUAC) are easy to use assessments for maternal nutritional status which are indicative of the pre-pregnancy nutritional status if obtained in the first trimester [12]. Several studies have highlighted MUAC as a viable alternative to BMI for nutritional screening of pregnant women in low-resource settings due to its ability to reduce chances of misclassification in case of late antenatal visits [13,14,15]. This study aims to contribute to the existing knowledge by assessing the association of maternal nutritional status using BMI and MUAC in predicting SGA in a community-based cohort of Pakistani women. It also aims to determine the predictive ability of MUAC and BMI in predicting SGA. The secondary objectives for this study included assessment of association of maternal nutritional status and LGA and LBW.
Methods
Study design and setting
The Pregnancy Risk Infant Surveillance and Measurement Alliance (PRISMA) is an ongoing prospective longitudinal cohort in Ibrahim Hyderi and Rehri Goth located in the peri-urban communities of Karachi, Pakistan. These communities are low socio-demographic communities situated in Bin Qasim town of the coastal region of Karachi. Pregnant women receive midwifery-led antenatal care services during their visit to the primary health care (PHC) facility where relevant laboratory and ultrasound investigations are performed, and women are facilitated for skilled delivery to higher level health care facilities. Further details regarding the PRISMA cohort have been described elsewhere [16].
Study period and population
For this study, we included PRISMA participants who were enrolled between January 2021 to August 2022. Pregnant women with a viable intrauterine pregnancy < 14 weeks of gestation as confirmed by ultrasound, BMI and MUAC measurements in the first trimester and birth weights of their infant captured within 72 hours were included for this analysis. There was no predefined exclusion criteria for this analysis. All participants provided individual written informed consent before undergoing study procedures. The PRISMA study was approved by the Aga Khan University Ethics Review Committee (2021–5920-15,518).
Study procedures
All women underwent ultrasound scans by certified sonologists at the time of enrolment to establish viable pregnancy and gestational age (GA). Trained community health workers (CHWs) conducted anthropometric measurements for women, including height (using SECA scale, model no. 213), weight (using SECA scale, model no. 876), and MUAC (using standardized UNICEF tapes), at each ANC visit. BMI was calculated using the WHO international BMI cut-offs and were categorized into underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2) [17]. MUAC values were categorized as low MUAC for those with MUAC < 23 cm and normal if MUAC ≥23 cm [18]. These measurements were performed using SECA 876 weight machine, with a graduation of 100 g < 150 kg > 200 g and a maximum capacity of 250 kg. This underwent weekly calibration using 10 kg and 5 kg weights to ensure accurate and reliable measurements [19]. Additionally, height was measured using SECA 217 which covers a measuring range of 20–205 cm, while UNICEF MUAC tape were used (range from 1 to 59 cm) [20].
The pregnant mother was followed during pregnancy and for labor and delivery to capture relevant outcomes including baby’s birth weight within 72 hours of life. The primary outcome of this analysis was SGA while secondary outcomes included LGA and LBW. SGA and LGA were calculated based on INTERGROWTH 21 guidelines, utilizing the web-based INTERGROWTH 21 newborn size calculator [21, 22] while LBW was defined as birthweight less than 2500 g.
Data analysis
The baseline characteristics of the study cohort such as current age, age at the time of marriage, were reported through mean and standard deviation using t-test and categorical variables such as education, wealth index, and other socio-demographic indicators as well as frequencies of outcome variables including SGA, LGA, LBW were reported as frequency and percentages using chi2 (Tables 1 and 2). Simple and multivariable logistic regression models were run for SGA, LGA and LBW separately to determine the association of these outcomes with BMI and MUAC while adjusting for age, age at marriage, smoking status, gravid status, comorbidities, severity of anemia and number of ANC visits in the current pregnancy. At the stage of crude logistic regression, a p-value of < 0.25 was taken for determining the level of significance and at multivariable level, p-value of < 0.05 was used for determining the level of significance. Stepwise model building approach was used for multivariable regression, however, the final adjusted models used were those that were not developed as stepwise rather those that adjusted all clinically important variables. Overall significance of the models were assessed to check fit of the models (p < 0.05) To assess the predictive capability of BMI and MUAC for SGA and LBW, we employed receiver operating curves (ROC) to calculate the area under curve (AUC). All statistical analyses were performed using STATA version 17.0.
Results
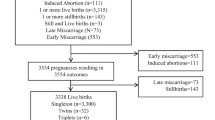
The PRISMA Cohort enrolled 3775 participants from January 2021 to August 2022, of which 926 participants were included in this analysis (Fig. 1).
The study participants’ sociodemographic and clinical characteristics are presented in Table 1.
Among women with low MUAC, 66% (n = 163) women had no primary education, and 78.1% (n = 193) women were multigravida. Approximately 40% (n = 95) of women were smokers and 17.8% (n = 44) had moderate to severe anemia. Similarly, 48% (n = 82) of underweight women were smokers and 18.1% (n = 31) had moderate to severe anemia.
Among women with low MUAC, 34.4% (n = 85) women had neonates who were SGA and 21.2% (n = 144) were LBW. When stratified by BMI status, a similar burden of SGA neonates was reported (35.1%, n = 60) while the burden of LBW was higher (32.2%, n = 55) (Refer to Table 2).
When adjusted for woman’s current age, age at marriage, gravida, smoking status, comorbidities, severity of anemia and number of ANC visits, women with low MUAC had higher likelihood of SGA (OR = 1.64 95% CI 1.2, 2.3) compared to women with normal MUAC. Similarly, women who were underweight had higher likelihood of SGA (OR = 1.49,95% CI 1.1, 2.2) as compared to women with normal BMI (Table 3). Women with low MUAC and those who were underweight also had higher odds of LBW neonates (OR = 1.63, 95% CI 1.2, 2.3 and OR = 1.63, 95%CI 1.1, 2.4 respectively) (Table 3).
Discussion
This study aimed to assess the association between maternal nutritional status in early pregnancy with adverse outcomes such as SGA, LGA and LBW in a community-based cohort in a low resource setting like Pakistan. Being underweight or having a low MUAC were significantly associated with SGA and LBW whereas the overweight and obese category of BMI were significantly associated with LGA, thus highlighting the impact of maternal nutritional status on neonatal size at birth.
Maternal nutrition and its impact on low-resource settings is multifaceted. Our study substantiates the exiting findings demonstrating a strong association between low MUAC and BMI as a risk factor for the likelihood of having SGA. This aligns with the global trends where underweight women face increased risks of fetal growth restriction (FGR), LBW and SGA [23,24,25]. A systematic review including LMICs reported that underweight women had 1.13 [95%CI, 1.01–1.27], 1.66 [95% CI, 1.50–1.84] and 1.85 [95%CI, 1.69–2.02] times higher odds of having a PTB, LBW and SGA, respectively. However, the absence of method used for specifying GA (LMP or Ultrasound) in this review may cause misclassification [26]. Additionally, a population-based prospective pregnancy registry from Pakistan and India linked underweight women to LBW, yet missed to include other outcomes like SGA or PTB [27]. Maternal pre-pregnancy overweight and obesity along with multiparity are strong determinants for delivering LGA infants [28]. A Swedish study reported that maternal overweight and obesity exhibited a significant association with LGA [28]. The current study enhances methodological rigor by employing first trimester ultrasounds for determining accurate GA, contributing evidence on association between maternal nutritional status and poor pregnancy outcomes like SGA, LGA and LBW.
BMI has conventionally served as a gauge for maternal nutritional status and fetal growth prediction [29]. However, growing evidence is suggesting that MUAC could be used as an alternative in assessing pregnancy outcomes [15, 30]. A study in India found a significant association (aOR = 7.91, p-value < 0.001) and also a moderate positive correlation between MUAC and BMI (r = 0.57, p < 0.001), suggesting MUAC to be a simple yet effective tool for assessing maternal nutritional status in pregnancy especially in low resource settings [30]. Results from a Zambian study assessing BMI and MUAC for predicting SGA revealed an inadequate overall predictive model (AUCBMI = 0.66, AUCMUAC = 0.68). However, it showed high discriminatory power for severe SGA in HIV-positive women (AUCBMI = 0.8 and AUCMUAC = 0.83) [13]. Our study also showed a slightly higher odds of SGA (OR = 1.64) with MUAC as compared with BMI (OR = 1.49); however, the predictive ability of both BMI and MUAC was poor on ROC curves.
This study had a few strengths and limitations. We confirmed the gestational age of each pregnant woman through ultrasound in early pregnancy which gave us accurate dating of the pregnancies. This is a major strength of this study as gestational age based on ultrasound is more accurate than LMP in determining gestational age [31, 32]. BMI and MUAC were measured with standard instruments and trained staff in a prospective manner. However, there are certain limitations to this study. Birthweight was not available for 21% of the women enrolled in the PRISMA study which could have led to selection bias. Also, the sample for this study was obtained from communities in the coastal regions of Karachi where the majority have a lower socioeconomic background thus may not be a representative sample for the country. Furthermore, we were unable to analyze gestational weight gain over the course of pregnancy due to insufficient data.
Conclusion
This study concludes that early maternal nutritional status determined by BMI and MUAC is associated with adverse pregnancy outcomes including SGA,LGA, and LBW in low resource settings. BMI and MUAC are the most common and easily available tools to assess the mother’s nutritional status in early pregnancy despite its limited performance. Considering the importance of nutritional assessment of the mother in early pregnancy, there is a need to explore other proxy measures such as dietary diversity score, mid-arm muscle area or use combination of existing tools to enhance our understanding of this important association. These measures can then be effectively utilized in resource limited settings to assess the effect of nutritional interventions in pregnancy and their impact on neonatal outcomes.
Availability of data and materials
The dataset and material related to this study can be available from the corresponding author on reasonable request.
Abbreviations
- GA:
-
Gestational age
- BMI:
-
Body Mass Index
- MUAC:
-
Mid upper arm circumference
- SGA:
-
Small-for-gestational age
- LGA:
-
Large-for-gestational age
- LBW:
-
Low birthweight
- SVN:
-
Small vulnerable newborns
- FGR:
-
Fetal growth restriction
- IUGR:
-
Intra-uterine growth restriction
- WHO:
-
World Health Organization
- PRISMA:
-
Pregnancy Risk Infant Surveillance and Measurement Alliance
- ROC:
-
Receiver operating curve
- AUC:
-
Area under curve
- LMICs:
-
Low- and middle-income countries
References
Han Z, Mulla S, Beyene J, Liao G, SD MD, Group obotKS. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2010;40(1):65–101.
Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32(1):5–25.
Imdad A, Lassi Z, Salaam R, Bhutta ZA. Chapter 1 - prenatal nutrition and nutrition in pregnancy: effects on long-term growth and development. In: Saavedra JM, Dattilo AM, editors. Early Nutrition and Long-Term Health. Woodhead Publishing; 2017. p. 3–24.
NRFCJT. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 192 million participants. Lancet. 2016;387(10026):1377–96.
Maternal Nutrition Strategy 2022–2027 Pakistan UNICEF Pakistan https://www.unicef.org/pakistan/media/4356/file/Pakistan%20Maternal%20Nutrition%20Strategy%202022-27.pdf. Accessed 30 Sept 2023.
Nutrition Wing MoNHS, Regulations and Coordinations, Government of Pakistan. National Nutrition Survey 2018-key findings report. Pakistan; 2018.
Kerényi Z, Tamás G, Kivimäki M, Péterfalvi A, Madarász E, Bosnyák Z, et al. Maternal Glycemia and risk of large-for-gestational-age babies in a population-based screening. Diabetes Care. 2009;32(12):2200–5.
Lawn JEPEOVLSF, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting - the lancet. Lancet. 2023;401(10389)
Vargas-Terrones M, Nagpal TS, RJBjopt B. Impact of exercise during pregnancy on gestational weight gain and birth weight: an overview. Braz J Phys Ther. 2019;23(2):164–9.
Ashorn P, Ashorn U, Muthiani Y, Aboubaker S, Askari S, Bahl R, et al. Small vulnerable newborns—big potential for impact. Lancet. 2023;401(10389):1692–706.
Lawn JE, Ohuma EO, Bradley E, Idueta LS, Hazel E, Okwaraji YB, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–19.
Natamba BK, Sanchez SE, Gelaye B, Williams MA. Concordance between self-reported pre-pregnancy body mass index (BMI) and BMI measured at the first prenatal study contact. BMC Pregnancy Childbirth. 2016;16(1):187.
Appiagyei A, Vwalika B, Spelke MB, Conner MG, Mabula-Bwalya CM, Kasaro MP, et al. Maternal mid-upper arm circumference to predict small for gestational age: findings in a Zambian cohort. Int J Gynecol Obstet. 2023;161(2):462–9.
Fakier A, Petro G, Fawcus S. Mid-upper arm circumference: a surrogate for body mass index in pregnant women. S Afr Med J. 2017;107(7):606–10.
Salih Y, Omar SM, AlHabardi N, Adam I. The mid-upper arm circumference as a substitute for body mass index in the assessment of nutritional status among pregnant women: a cross-sectional study. Medicina. 2023;59(6):1001.
Sabahat N, Ali J, Nida Y, Muhammad K, Zaid H, Uzma K, et al. Cohort profile: the pregnancy risk infant surveillance and measurement Alliance (PRISMA) – Pakistan. BMJ Open. 2023;13(12):e078222.
Weir CB, Jan A. BMI classification percentile and cut off points. StatPearls; 2019.
Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013;5
SHOP S. SECA 876 Weight Machine Germany: All Rights Reserved Seca 2024; 2024 [Available from: https://www.seca.com/en_us/products/all-products/product-details/seca876.html. Accessed 6 Mar 2024.
SHOP S. SECA 217 Height Scale [Online]. Germany: All Rights Reserved Seca2024; 2024 [updated 2024. Available from: https://us.secashop.com/products/height-measuring-instruments/seca-217/2171821009. Accessed 6 Mar 2024.
Papageorghiou AT, Kennedy SH, Salomon LJ, Altman DG, Ohuma EO, Stones W, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218(2):S630-SS40.
Papageorghiou AT, et al. International standards for fetal growth based on serial ultrasound measurements: the fetal growth longitudinal study of the INTERGROWTH-21st project. Lancet. 2014;384(9946)
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51.
Young Melissa F, Ramakrishnan U. Maternal undernutrition before and during pregnancy and offspring health and development. Ann Nutr Metab. 2021;76(Suppl. 3):41–53.
Kirchengast S, Hartmann BJAMS. Maternal prepregnancy nutritional status influences newborn size and mode of delivery. AIMS Med Sci. 2018;5(1):53–66.
Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, Bilano V, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. 2015;16(9):758–70.
Short VL, Geller SE, Moore JL, McClure EM, Goudar SS, Dhaded SM, et al. The relationship between body mass index in pregnancy and adverse maternal, perinatal, and neonatal outcomes in rural India and Pakistan. Am J Perinatol. 2018;35(09):844–51.
Lwin MW, Timby E, Ivarsson A, Eurenius E, Vaezghasemi M, Silfverdal S-A, et al. Abnormal birth weights for gestational age in relation to maternal characteristics in Sweden: a five year cross-sectional study. BMC Public Health. 2023;23(1):976.
Liu Y, Dai W, Dai X, Li Z. Prepregnancy body mass index and gestational weight gain with the outcome of pregnancy: a 13-year study of 292,568 cases in China. Arch Gynecol Obstet. 2012;286(4):905–11.
Mishra KG, Bhatia V, Nayak R. Association between mid-upper arm circumference and body mass index in pregnant women to assess their nutritional status. J Family Med Prim Care. 2020;9(7):3321–7.
Macaulay S, Buchmann EJ, Dunger DB, Norris SA. Reliability and validity of last menstrual period for gestational age estimation in a low-to-middle-income setting. J Obstet Gynaecol Res. 2019;45(1):217–25.
ISUOG Practice Guidelines. Performance of first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2013;41(1):102–13.
Acknowledgements
The authors are grateful to the participants who voluntarily took part in this study and the data collectors for their help in data collection.
Funding
There was no funding for this study as it is a secondary analysis. The PRISMA study was funded by the Bill &Melinda Gates Foundation (BMGF) (INV-005776).
Author information
Authors and Affiliations
Contributions
ZH and SA contributed equally to this paper. ZH conceptualized this paper. SA and MFQ performed the analysis under supervision of ZH. ZH, NY, SA and ASA drafted the manuscript. All authors reviewed and approved the final version of the manuscript. ZH is the guarantor of this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The PRISMA study was approved by the Aga Khan University Ethics Review Committee (2021–5920-15518). All participants provided individual written informed consent before undergoing study procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ambreen, S., Yazdani, N., Alvi, A.S. et al. Association of maternal nutritional status and small for gestational age neonates in peri-urban communities of Karachi, Pakistan: findings from the PRISMA study. BMC Pregnancy Childbirth 24, 214 (2024). https://doi.org/10.1186/s12884-024-06420-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06420-3