Abstract
Background
Postoperative delirium (POD) is a frequent neurologic dysfunction that often leads to more negative outcomes. Early identification of patients who are vulnerable to POD and early implementation of appropriate management strategies could decrease its occurrence and improve patient prognosis. Therefore, this meta-analysis comprehensively and quantitatively summarized the prevalence and related predictive factors of POD in head and neck cancer surgical patients.
Methods
PubMed, Embase, and Cochrane Library were searched for observational studies that reported the prevalence and risk factors for POD after head and neck cancer surgery and were published from their inception until December 31, 2022. Two reviewers independently selected qualified articles and extracted data. The qualities of related papers were assessed using the Newcastle-Ottawa scale (NOS). RevMan 5.3 and Stata 15.0 were applied to analysis the data and conduct the meta-analysis.
Results
Sixteen observational studies with 3289 inpatients who underwent head and neck cancer surgery were included in this review. The occurrence of POD ranged from 4.2 to 36.9%, with a pooled incidence of 20% (95% CI 15–24%, I2 = 93.2%). The results of this pooled analysis demonstrated that the statistically significant risk factors for POD were increased age (OR: 1.05, 95% CI: 1.03–1.07, P < 0.001), age > 75 years (OR: 6.52, 95% CI: 3.07–13.87, P < 0.001), male sex (OR: 2.29, 95% CI: 1.06–4.97, P = 0.04), higher American Society of Anesthesiologists grade (OR: 2.19, 95% CI: 1.44–3.33, P < 0.001), diabetes mellitus (OR: 2.73, 95% CI: 1.24–6.01, P = 0.01), and history of smoking (OR: 2.74, 95% CI: 1.13–6.65, P = 0.03).
Conclusions
POD frequently occurs after head and neck cancer surgery. Several independent predictors for POD were identified, which might contribute to identifying patients at high risk for POD and play a prominent role in preventing POD in patients following head and neck cancer surgery.
Similar content being viewed by others
Introduction
Head and neck cancer is one of the most frequent malignancies, including cancers of the oral cavity, pharynx, larynx, paranasal sinuses, and nasal cavity [1]. There were more than 878,000 new cases of head and neck cancers in 2020 and approximately 445,000 deaths each year worldwide [2]. Over the past few decades, researchers have made great efforts to explore therapeutic strategies for head and neck cancer, such as radiotherapy, chemotherapy, and immunotherapy; however, surgical resection is still the main treatment method [3]. Unfortunately, owing to the complex nature, multiple comorbidities, highly invasive and extensive surgical procedures, and longer operation time, surgery may inevitably lead to postoperative complications, which not only prolong the hospital stay and decrease the quality of life but also increase the total hospital cost and the risk of mortality [4]. Postoperative delirium (POD), a relatively frequent neuropsychiatric disorder after anesthesia and surgery, is an acute and transient cerebral disorder characterized by disturbance of attention, perception, and consciousness [5]. It was reported that approximately 11.50 to 36.11% of inpatients experienced POD after head and neck cancer surgery, depending on the frequency of assessment, age of the patient, and different types of surgical interventions [6]. POD can lead to unfavourable events such as prolonged hospital stay, increased risk of dementia, mortality, high medical expenses, functional impairment, and other clinical complications [7, 8]. Fortunately, 30–40% of POD can be prevented by early identification and treatment of its related risk factors, although the present pathophysiology of POD remains obscure [9]. Therefore, it is reasonable to believe that early identification of patients at risk for POD and timely implementation of targeted intervention strategies might play critical roles in reducing POD incidence and its related detrimental effects.
Based on different clinical psychomotor behaviours, POD could be further categorized into three subtypes: hyperactive, hypoactive, or mixed. The subtype of POD may be influenced by factors related to specific surgical patient populations. For example, hypoactive-type POD, characterized by lethargy, apathy, and reduced motor activity, occurs more frequently after cardiac and hip fracture surgery [10, 11], while hyperactive-type POD, characterized by agitation, restlessness, and insomnia, is common after head and neck cancer surgery [12]. Recently, several meta-analyses demonstrated that some predisposing risk factors (ageing, low albumin, diabetes, history of delirium, preoperative depression, preoperative functional dependence, mild cognitive impairment, and carotid artery stenosis) and precipitating risk factors (time of mechanical ventilation, surgery delay > 48 h, and intensive care unit stay time) could increase the incidence of POD in patients after cardiac and orthopedic surgery [13, 14]. In regard to patients undergoing head and neck cancer surgery, there may be different risk factors for POD. Risk factors for POD after head and neck cancer surgery were reported in the individual studies, but the results were inconsistent or even conflicting [12, 15]. Additionally, in 2017, Zhu et al. identified several potential risk factors for POD after head and neck cancer surgery using univariate analysis [6]. However, this meta-analysis only included 8 articles, which contributed to the unreliability of results. In addition, the majority of included studies in this review were from Japan in this review, which might reduce the generalizability of the conclusions. Furthermore, this review used univariable analysis to summarize the risk factors for POD rather than multivariate analysis, which led to the results being less mathematically robust. Over the past five years, several studies reporting the risk factors for POD after head and neck cancer surgery have been published, which may offer some new evidence. Consequently, this study was conducted to comprehensively and quantitatively analyze the prevalence and related risk factors for POD in patients who underwent head and neck cancer surgery, and thus providing guidance for clinical prevention decision-making.
Methods
Our meta-analysis strictly complied with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16].
Literature search
PubMed, Cochrane Library, and Embase were comprehensively searched for articles published from their inception until December 31, 2022. Based on the combination of medical subject heading terms and text words, a basic search strategy was conducted using the following terms: “delirium”, “postoperative delirium”, “mixed origin delirium”, “head and neck”, “neoplasms”, “cancer”, and “risk factors”, etc. See Additional File 1 for the detailed search strategies.
Study selection
Inclusion criteria included the following: (1) studies designed as cohort, case-control, or cross-sectional studies; (2) studies including patients undergoing surgery for head and neck cancer; (3) studies reporting the prevalence and risk factors for POD in patients undergoing surgery for head and neck cancer; (4) studies in which POD was diagnosed by some validated methods, such as the Nursing Delirium Screening Scale (Nu-DESC), Intensive Care Delirium Screening Checklist (ICDSC), Confusion Assessment Method (CAM) or Diagnostic and Statistical Manual of Mental Disorders (DSM); (5) studies written in English; and (6) studies with complete data that could be extracted, including ORs of multivariable risk factors with 95%CIs. Exclusion criteria included the following: (1) reviews, letters, abstract-only publications, animal experiments, and case reports; (2) studies with overlapping populations or duplicate publications; (3) studies that did not investigate the predictors for POD by multivariate logistic regression analysis; and (4) articles with insufficient data for statistics.
Data extraction and quality assessment
Two authors screened the full text of the articles, extracted the data and assessed the quality of the papers separately. The extracted data comprised authors, year of publication, country, study design, sample size, mean age of the patients, diagnostic methods for POD and its incidence, risk factors for POD, and study quality score. Since all the selected studies were observational studies, the quality of the eligible papers was rated using the Newcastle-Ottawa Scale (NOS), which is recognized as a standardized method for the quality assessment of non-randomized studies [17]. The maximum total score for the included studies was 9 points using the NOS which contains eight items. Papers with NOS scores ≥ 7.0 were considered high quality, and NOS scores < 7.0 were regarded as low quality. Any disagreements were eventually resolved through discussion or negotiation.
Statistical analysis
Stata 15.0 and RevMan 5.3 were used to analyze all data. If multivariable risk factors were reported in more than two studies, we performed a meta-analysis. Pooled ORs with corresponding 95% CIs were applied to assess the relationship between the predictors and POD, and P < 0.05 was regarded as statistically significant. I2 values and Q-test statistics were applied to detect heterogeneity among articles, where P < 0.1 and I2 > 50% were deemed to indicate significant heterogeneity. If the articles showed high heterogeneity, a random-effect model analysis was utilized; otherwise, a fixed-effect model was chosen. The final result for each relevant variable was presented as forest plots. When the heterogeneity of the pooled effect was significant (I2 > 50%), we further explored the source of heterogeneity using the sensitivity or subgroup analysis. Publication bias with a funnel plot was also conducted.
Results
Literature search
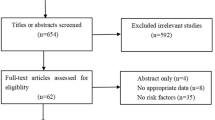
The initial literature search retrieved 542 citations from the PubMed (n = 122), Embase (n = 354), and Cochrane Library (n = 66). After the removal of duplicate articles (n = 113) by EndNote X9, 429 articles were retained. After preliminary headline and abstract screening, 394 studies were eliminated. The 35 remaining studies consequently underwent full-text review. Subsequently, 19 papers were eliminated for the following reasons: conference abstract (6 studies); not conducting multivariate analysis (3 studies); systematic review or letter (3 studies); without validated POD tools (3 studies); incomplete data (2 studies); randomized controlled trial (1 study);and duplicated population (1 study). Ultimately, 16 articles were eligible for this meta-analysis. The detailed process of the database search is shown in Fig. 1.
Characteristics of identified studies
In total, 16 observational studies with 3289 patients were published from 2009 to 2022, and the sample size ranged from 69 to 515. Of these 16 observational studies included, 1 was a case-control study, 14 were retrospective cohort studies, and 1 was a prospective cohort study. Of the 16 articles, 12 studies were conducted in Asia (7 in Japan [12, 18,19,20,21,22,23], 3 in China [24,25,26], and 2 in South Korea [27, 28]), while 3 studies were performed in the Germany [5, 15, 29] and the remaining 1 study was conducted in the United States [30]. The most common diagnostic method was the DSM-IV in 8 of 16 included studies [12, 18, 19, 21, 22, 24, 27, 30], the DSM-V in 4 studies [5, 15, 20, 29], the CAM in 2 studies [25, 26], and the ICDSC [23] and Nu-DESC [29] in the remaining 2 studies. Table 1 presents the basic information of the selected literature.
Methodological quality evaluation
Data on the quality of the eligible articles based on the NOS is presented in the Table 2. The NOS score of all included studies was no less than 7 points, suggesting that all of these were high-quality.
Incidence of POD
All eligible articles provided the incidence of POD, varying from 4.2 to 36.9% with a pooled incidence of 20% (95% CI 15–24%, I2 = 93.2%) (Fig. 2). In addition, subgroup analyses were performed on region, criteria for POD, number of samples, quality of included studies, and study design (Table 3). Furthermore, we performed a sensitivity analysis and the results suggested that none of the included articles had a great influence on the pooled estimates (Fig. 3). Begg’s funnel plot provided significant evidence of publication bias (Fig. 4).
Risk factors of POD
Originally, we identified 53 risk factors from included studies based on multivariate analysis. Of these, 8 risk factors were reported in two or more studies, and finally 6 risk factors were considered statistically significant, which are displayed in Table 4. All of these identified factors were divided into 2 categories, including predisposing and precipitating risk factors.
Predisposing risk factors
Age
A total of 5 articles showed that older age was a potential risk factor for POD. The meta-analysis of these articles suggested that older age was a significant predictor for POD (OR: 1.05, 95% CI: 1.03–1.07, P < 0.001, I2 = 18%, Table 4; Fig. 5). Moreover, age > 75 years was deemed as a risk factor for POD in 2 studies, and the results of this meta-analysis suggested that patients older than 75 years were more prone to experience POD (OR: 6.52, 95% CI: 3.07–13.87, P < 0.001, I2 = 0%, Table 4; Fig. 6).
Male sex
Five papers reported that male sex was a potential risk factor for POD. The meta-analysis results indicated that male gender was an independent risk factor for POD (OR: 2.29, 95% CI: 1.06-4.97, P = 0.04), with mild heterogeneity (I2= 55%, P = 0.06, Table 4, Fig. 7). Furthermore, the potential sources of heterogeneity were sought through sensitivity analysis, and the results indicated that no single study significantly changed the pooled result.
American society of anesthesiologists (ASA) physical states
Three studies reported the association between ASA physical status and POD, and the meta-analysis indicated that a 2.19-fold increased risk of POD in patients with higher ASA grades (OR: 2.19, 95% CI: 1.44–3.33, P < 0.001, I2 = 31%, Table 4; Fig. 8).
Diabetes mellitus
Diabetes mellitus was recognized as a risk factor for POD in 3 papers. In our meta-analysis, patients with diabetes mellitus had a 2.73-fold increased risk of POD compared to patients without diabetes mellitus (OR: 2.73, 95% CI: 1.24–6.01, P = 0.01, I2 = 0%, Table 4; Fig. 9).
History of smoking
Two articles reported that the impact of the smoking history on risk of POD. Our results indicated that the pooled OR was 2.74 (95% CI: 1.13–6.65, P = 0.03) with no heterogeneity (I2 = 0%, P = 0.86, Table 4; Fig. 10).
Precipitating risk factors
In this category, only 2 risk factors were analyzed by meta-analysis, including operative time and ICU stay time. The meta-analysis of 2 risk factors showed that surgery time (OR: 1.00, 95% CI: 1.00-1.01, P = 0.003, I2 = 0%, Table 4; Fig. 11) and ICU stay time (OR: 2.08, 95% CI: 0.46–9.46, P = 0.34, I2 = 93%, Table 4; Fig. 12) were not significant risk factors for POD.
Discussion
POD is a common postoperative complication among head and neck cancer surgery patients and is related to notable morbidity and mortality. In light of these adverse prognoses, it is imperative to identify related predictive factors of POD and take appropriate preventive measures to prevent POD in patients undergoing surgery for head and neck cancer. Thus, we conducted this meta-analysis and found several significant predictive factors of POD after head and neck cancer surgery including increased age, age > 75 years, male sex, higher ASA level, diabetes mellitus and history of smoking.
Our study was not the first systematic review and meta-analysis exploring the risk factors for POD after head and neck cancer surgery. Compared with a previous meta-analysis [6], our systematic review and meta-analysis included articles that were published within the latest 5 years and that summarized the incidence of POD after head and neck cancer surgery. The prevalence of POD varies significantly among surgical populations; for instance, the incidence of POD is reportedly 5.45 to 28.57% after urological surgery, 10.09 to 51.28% after hip fracture surgery, and 4.1 to 54.9% after cardiac surgery [13, 14, 32]. In our study, the incidence of POD ranged from 4.2 to 36.9% with a pooled incidence of 20% and high heterogeneity (I2 = 93.2%, P < 0.001), which was consistent with a previous meta-analysis [6]. The heterogeneity of POD incidence may be a consequence of the sample size, the region of surgery, or the POD diagnostic criteria [33]. In addition, the incidence of POD in Western countries was significantly higher than that in Asia, possibly due to ethnic differences.
It is widely accepted that advanced age is an important predisposing predictor for POD [34]. In this meta-analysis, older age was associated with a relatively low risk of POD after head and neck cancer surgery. However, for patients over 75 years old, the risk of POD was 6.52 times higher than that in patients younger than 75 years old, which was in line with a previous meta-analysis [35]. The results may be explained by the presence of more comorbidities such as depression, preexisting cerebrovascular disease, insomnia, and frailty in elderly patients [36]. An alternative explanation might be age-related inflammatory response changes, which might play a role in the pathophysiology of POD [37]. In addition, the effect of sex differences on POD risk remains controversial. A previous meta-analysis suggested that female sex was a predictor for POD in patients undergoing spinal surgery [38]. Conversely, our results indicated that male sex was significantly associated with POD development in head and neck cancer surgery patients. A possible explanation was that males were more likely to develop obstructive sleep apnoea and alcohol dependence, which have been confirmed as significant risk factors for POD [39].
ASA classification is a well-known grading system for evaluating patients’ tolerance to anesthesia and their physical status before surgery. In line with our recent study [40], our results indicated that an ASA level increase was a strong predictor for POD. Therefore, those who have a higher ASA level should be of great concern to clinicians. In our study, we also investigated whether diabetes mellitus was associated with POD after head and neck cancer surgery. Diabetes mellitus is a well-established risk factor for the development of dementia [41]. Additionally, the study by Liu and colleagues demonstrated that the increased risk of POD after hip fracture surgery was explained by diabetes mellitus [42]. Our results also revealed that head and neck cancer surgical patients with diabetes mellitus were more susceptible to POD. Diabetes mellitus could weaken insulin signaling pathways in the regulation of the functions of neurons and glial cells [43]. In addition, diabetes mellitus is characterized by hyperglycemia, oxidative stress, and chronic inflammation that can lead to blood-brain barrier impairment [44]. Thus, it is not surprising that patients with diabetes mellitus had a higher risk for POD after head and neck cancer surgery.
Several studies have demonstrated that a history of smoking was associated with surgical complications, including postoperative pneumonia and wound infection [45]. However, the correlation between a history of smoking and perioperative neurocognitive disorders remains obscure to date. A previous study showed that a preoperative smoking history could decrease the incidence of early postoperative cognitive dysfunction by stimulating the cholinergic anti-inflammatory pathway [31]. Interestingly, our results suggested that a history of smoking was considered as a significant predictor for POD after head and neck cancer surgery. Similar to our current study, Zhou et al. also found that smoking was positively related to POD in patients undergoing non-cardiac and non-obstetric surgery [46], which was associated with the impaired cholinergic function due to nicotine withdrawal resulting from sudden cessation of smoking [47].
This meta-analysis did not conclude that operative time and intensive care unit duration were predictive factors of POD after head and neck cancer surgery, which might be related to the small number of studies included. However, according to the Consensus-based Guideline on POD of the European Society of Anesthesiology, operative time and ICU stay time should be considered as risk factors for POD after surgery [48]. Thus, the connection between the 2 risk factors and POD needs to be validated in future large-sample prospective multicentre cohort studies.
Limitations
There were some limitations in our study. First, only articles published in English were included in the current study, resulting in unavoidable selection bias. Second, we identified several risk factors for POD after head and neck cancer surgery, however, the association between these risk factors and POD in other surgical populations still need to be explored further. Third, some significant risk factors were identified in a few studies with small sample sizes, which should be interpreted with caution.
Conclusions
To sum up, our meta-analysis indicated that POD was common after head and neck cancer surgery. Based on the multivariate analysis, some significant predictors were identified, including increased age, age > 75 years, male sex, higher ASA grade, diabetes mellitus, and history of smoking, which might play a critical role in optimizing clinical management of POD.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- POD:
-
Postoperative delirium
- NOS:
-
Newcastle-Ottawa scale
- OR:
-
Odds ratio
- CIs:
-
Confidence intervals
- Nu-DESC:
-
Nursing Delirium Screening Scale
- ICDSC:
-
Intensive Care Delirium Screening Checklist
- CAM:
-
Confusion Assessment Method
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- ASA:
-
American Society of Anesthesiologists
References
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60–72. https://doi.org/10.1056/NEJMra1715715.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Mody MD, Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398(10318):2289–99. https://doi.org/10.1016/S0140-6736(21)01550-6.
Li D, Wang C, Wei W, Li B, Liu H, Cheng A, Niu Q, Han Z, Feng Z. Postoperative complications of free flap reconstruction in moderate-advanced head and neck squamous cell carcinoma: a prospective cohort study based on real-world data. Front Oncol. 2022;12:792462. https://doi.org/10.3389/fonc.2022.792462
Kolk A, Kolk A, Schwarzer C, Wolff K-D, Grill F, Weingart J. Factors associated with postoperative delirium in patients undergoing complex head and neck flap surgery. J Oral Maxillofac Surg. 2022;80(2):372-379.e5. https://doi.org/10.1016/j.joms.2021.08.153.
Zhu Y, Wang G, Liu S, Zhou S, Lian Y, Zhang C, Yang W. Risk factors for postoperative delirium in patients undergoing major head and neck cancer surgery: a meta-analysis. Jpn J Clin Oncol. 2017;47(6):505–11. https://doi.org/10.1093/jjco/hyx029.
Guenther U, et al. Preoperative cognitive impairment and postoperative delirium predict decline in activities of daily living after cardiac surgery-a prospective, observational cohort study. Geriatrics (Basel). 2020;5(4):69.
Mohanty S, Gillio A, Lindroth H, Ortiz D, Holler E, Azar J, Boustani M, Zarzaur B. Major surgery and long term cognitive outcomes: the effect of postoperative delirium on dementia in the year following discharge. J Surg Res. 2022;270:327–34. https://doi.org/10.1016/j.jss.2021.08.043.
Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin. 2015;33(3):505–16. https://doi.org/10.1016/j.anclin.2015.05.007.
Colwill JP, Bena JF, Morrison SL, Bakaeen F, Albert NM. Postoperative cardiovascular surgery delirium: interrater agreement between nurses and clinical nurse specialist and factors associated with prevalence. Clin Nurse Spec. 2021;35(5):238–45. https://doi.org/10.1097/NUR.0000000000000619.
Albrecht JS, Marcantonio ER, Roffey DM, Orwig D, Magaziner J, Terrin M, Carson JL, Barr E, Brown JP, Gentry EG, Gruber‐Baldini AL. Stability of postoperative delirium psychomotor subtypes in individuals with hip fracture. J Am Geriatr Soc. 2015;63(5):970–6. https://doi.org/10.1111/jgs.13334.
Ishibashi-Kanno N, Takaoka S, Nagai H, Okubo-Sato M, Fukuzawa S, Uchida F, Yamagata K, Yanagawa T, Bukawa H. Postoperative delirium after reconstructive surgery for oral tumor: a retrospective clinical study. Int J Oral Maxillofac Surg. 2020;49(9):1143–8. https://doi.org/10.1016/j.ijom.2020.01.018.
Chen H, Mo L, Hu H, Ou Y, Luo J. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. J Cardiothorac Surg. 2021;16(1):113. https://doi.org/10.1186/s13019-021-01496-w.
Qi Y-M, Li Y-J, Zou J-H, Qiu X-D, Sun J, Rui Y-F. Risk factors for postoperative delirium in geriatric patients with hip fracture: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:960364.
Taxis J, Spoerl S, Broszio A, Eichberger J, Grau E, Schuderer J, Ludwig N, Gottsauner M, Spanier G, Bundscherer A, Reichert TE, Ettl T. Postoperative delirium after reconstructive surgery in the head and neck region. J Clin Med. 2022;11(22):6630.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Shiiba M, Takei M, Nakatsuru M, Bukawa H, Yokoe H, Uzawa K, Tanzawa H. Clinical observations of postoperative delirium after surgery for oral carcinoma. Int J Oral Maxillofac Surg. 2009;38(6):661–5. https://doi.org/10.1016/j.ijom.2009.01.011.
Hasegawa T, Saito I, Takeda D, Iwata E, Yonezawa N, Kakei Y, Sakakibara A, Akashi M, Minamikawa T, Komori T. Risk factors associated with postoperative delirium after surgery for oral cancer. J Craniomaxillofac Surg. 2015;43(7):1094–8. https://doi.org/10.1016/j.jcms.2015.06.011.
Booka E, Kamijo T, Matsumoto T, Takeuchi M, Kitani T, Nagaoka M, Imai A, Iida Y, Shimada A, Takebayashi K, Niihara M, Mori K, Onitsuka T, Tsubosa Y, Takeuchi H, Kitagawa Y. Incidence and risk factors for postoperative delirium after major head and neck cancer surgery. J Craniomaxillofac Surg. 2016;44(7):890–4. https://doi.org/10.1016/j.jcms.2016.04.032.
Makiguchi T, Yamaguchi T, Nakamura H, Ogawa M, Harimoto N, Shirabe K, Yokoo S. Impact of skeletal muscle mass on postoperative delirium in patients undergoing free flap repair after oral cancer resection. J Plast Surg Hand Surg. 2020;54(3):161–6. https://doi.org/10.1080/2000656X.2020.1724545.
Takahashi N, Hiraki A, Kawahara K, Nagata M, Yoshida R, Matsuoka Y, Tanaka T, Obayashi Y, Sakata J, Nakashima H, Arita H, Shinohara M, Nakayama H. Postoperative delirium in patients undergoing tumor resection with reconstructive surgery for oral cancer. Mol Clin Oncol. 2021;14(3):60. https://doi.org/10.3892/mco.2021.2222.
Kinoshita H, Saito J, Takekawa D, Ohyama T, Kushikata T, Hirota K. Availability of preoperative neutrophil-lymphocyte ratio to predict postoperative delirium after head and neck free-flap reconstruction: a retrospective study. PLoS One. 2021;16(7):e0254654.
Zhang C, Xi MY, Zeng J, Li Y, Yu C. Prognostic impact of postoperative complications on overall survival in 287 patients with oral cancer: a retrospective single-institution study. J Oral Maxillofac Surg. 2019;77(7):1471–9. https://doi.org/10.1016/j.joms.2019.01.020.
Wang Y, Yu H, Qiao H, Li C, Chen K, Shen X. Risk factors and incidence of postoperative delirium in patients undergoing laryngectomy. Otolaryngol Head Neck Surg. 2019;161(5):807–13. https://doi.org/10.1177/0194599819864304.
Kong S, Wang J, Xu H, Wang K. Effect of hypertension and medication use regularity on postoperative delirium after maxillofacial tumors radical surgery. Oncotarget. 2021;12(18):1811–20. https://doi.org/10.18632/oncotarget.28048.
Choi NY, Kim EH, Baek CH, Sohn I, Yeon S, Chung MK. Development of a nomogram for predicting the probability of postoperative delirium in patients undergoing free flap reconstruction for head and neck cancer. Eur J Surg Oncol. 2017;43(4):683–8. https://doi.org/10.1016/j.ejso.2016.09.018.
Kim JH, Lee YS, Kim YH, Cho KJ, Jung YH, Choi S-H, Nam SY, Kim SY. Early ambulation to prevent delirium after long-time head and neck cancer surgery. Front Surg. 2022;9:880092.
Obermeier KT, et al. Postoperative delirium in patients with oral cancer: is intraoperative fluid administration a neglected risk factor? Cancers (Basel). 2022;14(13):3176.
Densky J, Eskander A, Kang S, Chan J, Tweel B, Sitapara J, Ozer E, Agrawal A, Carrau R, Rocco J, Teknos TN, Old M. Risk factors associated with postoperative delirium in patients undergoing head and neck free flap reconstruction. JAMA Otolaryngol Head Neck Surg. 2019;145(3):216–21. https://doi.org/10.1001/jamaoto.2018.3820.
Wang R, Wang G, Liu Y, Zhang M. Preoperative smoking history is associated with decreased risk of early postoperative cognitive dysfunction in patients of advanced age after noncardiac surgery: a prospective observational cohort study. J Int Med Res. 2019;47(2):689–701. https://doi.org/10.1177/0300060518808162.
Hua Y, et al. Risk factors for postoperative delirium in elderly urological patients: a meta-analysis. Med (Baltim). 2022;101(38):e30696.
Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572–90. https://doi.org/10.1213/ANE.0000000000004641.
Bramley P, McArthur K, Blayney A, McCullagh I. Risk factors for postoperative delirium: an umbrella review of systematic reviews. Int J Surg. 2021;93:106063. https://doi.org/10.1016/j.ijsu.2021.106063.
Scholz AF, et al. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21–28.
Ramos MD, et al. Risk for post-operative delirium related to comorbidities in older adult cardiac patients: an integrative review. J Clin Nurs. 2022;32(9–10):2128–39. https://doi.org/10.1111/jocn.16389.
Cortese GP, Burger C. Neuroinflammatory challenges compromise neuronal function in the aging brain: postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res. 2017;322(Pt B):269–79.
Zhang HJ, Ma XH, Ye JB, Liu CZ, Zhou ZY. Systematic review and meta-analysis of risk factor for postoperative delirium following spinal surgery. J Orthop Surg Res. 2020;15(1):509. https://doi.org/10.1186/s13018-020-02035-4.
Koo DL, et al. White matter tract-specific alterations in male patients with untreated obstructive sleep apnea are associated with worse cognitive function. Sleep. 2020;43(3):zsz247.
Liu J, et al. High ASA physical status and low serum uric acid to creatinine ratio are independent risk factors for postoperative delirium among older adults undergoing urinary calculi surgery. Clin Interv Aging. 2023;18:81–92.
Savelieff MG, Chen KS, Elzinga SE, Feldman EL. Diabetes and dementia: clinical perspective, innovation, knowledge gaps. J Diabetes Complications. 2022;36(11):108333. https://doi.org/10.1016/j.jdiacomp.2022.108333.
Liu K, Song Y, Yuan Yi, Li Z, Wang X, Zhang W, Li Y, Mi X, Han D, Rong Y, Guo X, Wang G. Type 2 diabetes mellitus with tight glucose control and poor pre-injury stair climbing capacity may predict postoperative delirium: a secondary analysis. Brain Sci. 2022;12(7):951. https://doi.org/10.3390/brainsci12070951.
Shpakov AO, Zorina II, Derkach KV. Hot spots for the use of intranasal insulin: cerebral ischemia, brain injury, diabetes mellitus, endocrine disorders and postoperative delirium. Int J Mol Sci. 2023;24(4):3278. https://doi.org/10.3390/ijms24043278.
Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol. 2017;12(4):593–601. https://doi.org/10.1007/s11481-017-9752-7.
Zheng L-M, Zhang Z-W, Wang W, Li Y, Wen F. Relationship between smoking and postoperative complications of cervical spine surgery: a systematic review and meta-analysis. Sci Rep. 2022;12(1):9172. https://doi.org/10.1038/s41598-022-13198-x.
Zhou S, Shi S, Xie C, Chen G. Association between smoking and postoperative delirium in surgical patients with pulmonary hypertension: a secondary analysis of a cohort study. BMC Psychiatry. 2022;22(1):371. https://doi.org/10.1186/s12888-022-03981-5.
Nagoshi N, et al. Impact of tobacco smoking on outcomes after posterior decompression surgery in patients with cervical spondylotic myelopathy: a retrospective multicenter study. Clin Spine Surg. 2020;33(10):E493-e498.
Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, Cherubini A, Jones C, Kehlet H, MacLullich A, Radtke F, Riese F, Slooter AJC, Veyckemans F, Kramer S, Neuner B, Weiss B, Spies CD. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. https://doi.org/10.1097/EJA.0000000000000594.
Acknowledgements
Not applicable.
Funding
The study was supported by the Key Research and Development Program of Hebei Province (Grant No. 19277714D).
Author information
Authors and Affiliations
Contributions
BD and JLL conceived the study and drafted the manuscript. DDY applied the search strategy. LJ and MNL extracted the data. BD made the figures and tables. BD and JLL drafted this manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, B., Yu, D., Jiang, L. et al. Incidence and risk factors for postoperative delirium after head and neck cancer surgery: an updated meta-analysis. BMC Neurol 23, 371 (2023). https://doi.org/10.1186/s12883-023-03418-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03418-w