Abstract
Background
Retinoblastoma is a rare intraocular malignancy and typically initiated by inactivating biallelic mutations of RB1 gene. Each year, ~ 8000 children worldwide are diagnosed for retinoblastoma. In high-income countries, patient survival is over 95% while low-income countries is ~ 30%.If disease is diagnosed early and treated in centers specializing in retinoblastoma, the survival might exceed 95% and many eyes could be safely treated and support a lifetime of good vision. In China, approximate 1100 newly diagnosed cases are expected annually and 28 hospitals covering 25 provinces established centers classified by expertise and resources for better treatment options and follow-up. Comparing with other province of eastern China, Yunnan province is remote geographically. This might result that healthcare staff have low awareness of the role of genetic testing in management and screening in families.
Methods
The patients with retinoblastoma were selected in Yunnan. DNA from blood was used for targeted gene sequencing. Then, an in-house bioinformatics pipeline was done to detect both single nucleotide variants and small insertions/deletions. The pathogenic mutations were identified and further confirmed by conventional methods and cosegregation in families.
Results
Using our approach, targeted next generation sequencing was used to detect the mutation of these 12 probands. Bioinformatic predictions showed that nine mutations were found in our study and four were novel pathogenic variants in these nine mutations.
Conclusions
It’s the first report to describe RB1 mutations in Yunnan children with retinoblastoma. This study would improve role of genetic testing for management and family screening.
Similar content being viewed by others
Background
Retinoblastoma (RB, OMIM#180200) is a rare malignant tumor which rapidly develops from the immature cells of a retina and occurs in infancy or in children, usually before the age of 5 years (about 2/3 children below the age of 2 years, about 95% children below the age of 5 years) [1, 2].
To explain mechanisms of RB, Knudson proposed the famous “two-hit hypothesis”. A germline mutation (‘first hit’, M1) and an acquired somatic mutation (‘second hit’, M2) arise heritable retinoblastomas; two somatic mutations presented in the same transformation suppressor gene in a susceptible cell, result from non-heritable retinoblastomas [3]. In some patients, Chromosomal deletions pointed to a chromosome (chr.) 13q14 locus. In 70% of retinoblastoma tumors, the heterozygosity of chr. 13q14 polymorphic loss suggested the second hit involved the same locus [4].
The first cloned gene of tumor suppressor is retinoblastoma 1 (Rb1) gene. The protein which is encoded by this gene is a cell cycle negative regulator. To maintain the overall chromatin structure, this encoded protein stabilizes constitutive heterochromatin. Defect in Rb1 is a cause of childhood cancer Rb, osteogenic sarcoma, and bladder cancer [5].
The gene of RB1 displays a wide spectrum of mutations, including small insertions/deletions (indels), large deletions/duplications, structural variations (SVs), and single nucleotide variations (SNVs) [6,7,8]. These mutations which are spanning 27 exons are distributed throughout the entire length of the RB1gene. No hotspots have been reported in this gene [9].
The incidence of retinoblastoma is approximately 1 in 16,000–18,000 live births, regardless of sex, race, or geography [10, 11]. In the United States and Europe, incidence rate of RB is 2–5 cases per million children according to WHO [12]. In India, rates of RB incidence are 1.9–12.3 and 1.3–6.7per million in boys and girls, respectively [13]. Dimaras Helen et al. have estimated that approximate 8000 new cases are predicted each year in worldwide [4]. In China, there are about 1100–1500 new cases each year, but only 50% those children survive [14]. The disease-free survival rate of children with RB in developed countries is over 95% [15] while this is substantially lower, at 10–30% in developing countries [16, 17].
Six countries of Asia-Pacific region bear 42.6% global burden of RB (3452 of 8099 children):1486 children in India (18.3%), 1103 children in China (13.6%), 277 children in Indonesia (3.4%), 260 children in Pakistan (3.2%), 184 (2.3%) children in Bangladesh, 142 (1.8%) children in Philippines [18].
A heritable form and non-heritable form are two forms of the RB disease. Heritable RB accounts for 45% of all cases which 80% bilateral, 15% unilateral and 5% trilateral. Approximately 55% of cases are non-heritable RB that is always unilateral [19]. The mode of RB inheritance is autosomal dominant (AD) found in 10% of cases.
[20]. The majority of RB patients are sporadic and the only affected members of otherwise unaffected families. The disease is labeled “sporadic” if the family has no history of RB [21].
From presenting signs and clinical examination, diagnosis of RB is usually clear [22]. Leukocoria (white pupil) is the most common sign; Strabismus is the second most common sign when central vision is lost. Due to increased pressure, or non-infective orbital inflammation, advanced disease might present with enlarged cornea and iris colour change [4, 23].
The primary goal of management is to save the child’s life, followed by salvage of the eye and optimization of visual function. Many treatment options of RB are available and depend on the laterality and extent of RB disease [24]. The various treatment modalities for retinoblastoma includes enucleation of the eye, external beam radiotherapy, brachytherapy, thermotherapy, laser photocoagulation, cryotherapy,systemic chemotherapy, intra-arterial chemotherapy, nanoparticulate chemotherapy and chemoreduction [4]. When children with RB have symptoms, such as leukokoria and strabismus, it is late for these children to have best time for cure. Delayed diagnosis results in incurable invasion of the optic nerve and brain and metastases elsewhere in the body. However, if noticed early, prompt treatment can cure the cancer and save the eye(s) [4]. According to Intraocular International Retinoblastoma Classify (IIRC) which divides retinoblastomas into 5 groups (labeled A through E; Group A means small tumors while group E means large tumor and the eye have no chance to be saved),Children’s Hospital of Fudan University reported that about 75% children with RB were diagnosed at group D and E of IIRC when these children saw doctor for the first time [25, 26].
In the management of RB, genetic testing and counseling are extremely important [27]. Heritability form of RB can be identified by genetic testing of the proband [28]. But, genetic testing has not been carried out in Yunnan whose permanent population was 47,368,000, including 51.48% male population and 48.52% female population, 17.79% children and 62.21% adults, 66.43%Han population and 33.57% minority population according to the 1% population sample survey by Yunnan Bureau of Statistics (http://stats.yn.gov.cn/).
Therefore, how to carry out genetic testing is an urgent problem in the RB clinical in developing countries. In this paper, the genes of RB family in Yunnan were studied in order to obtain genetic forms of RB by genetic testing of the probands and then give counseling to these families. We use targeted next generation sequencing (NGS) to screen probands and then Sanger sequencing was used to identify variants in other members of pedigrees. In our study, nine mutations were found and four were novel mutations.
Methods
Subjects
The non-consanguineous families from the Yun-Gui Plateau was recruited by the Children’s Hospital of Kunming Medical University for genetic diagnosis. In these families, the children suffered from RB, but the parents and other members were normal. This study was approved by the Ethics Committee of the Children’s Hospital of Kunming Medical University; in accordance with the principles of the Declaration of Helsinki written informed consent was obtained from the participants or their guardians. The age range of the patients, in which we obtained parental consent, is from 6 to 117 months (36.50 ± 38.61). In this study, the normal control includes 100 individuals (55 males and 45 females) aged between 5 and 35 years old without associated hereditary diseases.
Clinical evaluations
All clinical and physical examinations were conducted in the Children’s Hospital of Kunming Medical University in Kunming. CT and ultrasound imaging were performed in these probands.
Targeted NGS and variant analysis
Two-millilitre peripheral blood samples were collected from the probands, siblings and their parents in tubes containing 0.2 M EDTA. Using the QIAamp DNA blood extraction kit (TIANGEN, Beijing, China), DNA was extracted from the venous blood of each subject. According to the manufacturer’s protocol (MyGenostics, Inc., Beijing, China), 3 micrograms of genomic DNA was fragmented by Covaris 32, and the 3′ end of each DNA fragment was A-tailed. Then, Illumina adapters were ligated to these fragments. We aimed for a 350–400 base-pair product, and all samples were checked with Nanodrop 2000 or Qubit systems to determine if they represented a captured library.
Each resulting captured library was loaded on an Illumina MiSeq 2000 sequencing platform, and the sequences were determined to ensure that each sample met the desired average sequencing coverage.
Mutation analysis
Using Bcl2Fastq software (Bcl2Fastq 2.18.0.12, Illumina, Inc.), raw image files were processed for base calling and raw data generation. In addition, to get a quality score ≥ 20, low-quality variations were filtered out. Then, Short Oligonucleotide Analysis Package (SOAP) aligner software (SOAP2.21, soap.genomics.org.cn/soapsnp.html) was used to align the clean reads to the reference human genome (UCSC hg19, http://genome.ucsc.edu/). Polymerase chain reaction (PCR) duplicates were removed by the Picard programme [29, 30] .The single nucleotide polymorphisms (SNPs) were determined by the SOAPsnp programme [31]. The reads were realigned by Burrows-Wheeler Aligner (BWA) software 0.7.15, and the deletions and insertions (InDels) were detected by Genome Analysis Toolkit software 3.7. In addition, the identified indel SNPs were annotated using the Exome-assistant programme (http://122.228.158.106/exomeassistant). To determine their pathogenicity, non-synonymous variants were evaluated by four algorithms, namely, PolyPhen (http://genetics.bwh.harvard.edu/pph2/), Protein Analysis Through Evolutionary Relationships (PANTHER, www.pantherdb.org), Sorting Intolerant from Tolerant [SIFT, (http://sift.jcvi.org/)] and Pathogenic Mutation Prediction (Pmut; http://mmb.pcb.ub.es/PMut/).
Mutation validation
PCR and Sanger sequencing with an ABI3500 sequencer were used to confirm potential causative variants in this family. The sites of variation were identified to compare the DNA sequences with the corresponding GenBank (www.ncbi.nlm.nih.gov) reference sequences. The sequences of forward and reverse primers are presented in supplementary Table 1 and were used to confirm potential causative variants in this family.
Thermocycling conditions: An initial denaturation of 95 °C for 10 min, 35 cycles of denaturation at 94 °C for 30 s., annealing at 64 °C for 30 s, extension at 72 °C for 45 s and a final extension of 72 °C for 5 min. The sequences of forward and reverse primers are in supplementary Table 1.
Results
Clinical findings
Twelve patients with RB were selected for this study of targeted RB1 sequencing from non-consanguineous Yunnan families. There are no pathogenic or likely pathogenic variants were detected in three probands with our approach while nine variants in nine families.
The clinical findings of probands
In these nine families,there are seven boys and two girls (See Fig. 1). Minority nationalities have Dai, Hani, Bai, Hui, Yi and Han. Mean age at diagnosis is 23 ± 9.9 months for unilateral and 11 ± 8.5 months for bilateral retinoblastoma in our study, respectively. Six probands were diagnosed with bilateral RB while three with the unilateral form. There are common sign leukocoria in probands of families 1, 3, 4, 6, 7; in family 2, left eye of the proband had been removed; the proband of 5 had strange reflection; the proband had blurred vision and shed tears in 8 and 9 (Table 1).
Pedigree of families with RB. Unaffected subjects are denoted as blank while affected subjects are represented with darkened symbols. The arrow indicates the proband. a .Pedigree of the family 1; b. Pedigree of the family 2; c. Pedigree of the family 3; d. Pedigree of the family 4;. e. Pedigree of the family 5; f. Pedigree of the family 6; g. Pedigree of the family 7; h. Pedigree of the family 8; i. Pedigree of the family 9
The clinical findings of parents
There was no congestion in the conjunctiva, no secretions in the conjunctival sac., and no tumor in both eyes. In both eyes (OU), the microscopy of slit lamp showed that the cornea is smooth and transparent, the thickness of corneal is normal, the depth of anterior chamber is normal, cornea is clear, iris texture is clear, pupil is isometric, lens is transparent, interstitial is clear and light emission exists.
Targeted NGS
The mean read depth of coverage for each proband sample ranged from 52 to 66X, and the average throughput depth of the target region in each sample ranged from 90 to 99X. NGS produced reads from 32.8 to 56.12 million reads and the read length from 148 to 149 bp. The reads aligned to the human genome and mapped to the target region with a mean coverage from 99.8 to 99.9%. The SNPs and Indels are reported in supplementary Table 2.
Identification of SNVs and InDels in children with RB patients
To detect SNVs and InDels, we analyzed blood samples of 6 bilateral patients and 3 familial unilateral patient and identified pathogenic variants in 8 patients and likely pathogenic variants in 1 patient. Four were novel and five were previously reported (Table 2). The spectrum of identified mutations includes 5 SNVs (1 nonsynonymous and 4 stop gain), one deletion (1 frameshift), and 3 splice site variants (splice).
Eight of them were shown to be de novo, and remaining one was inherited from one of their mothers.
Identification of pathogenic mutation
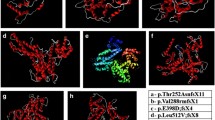
Sanger sequencing and cosegregation further confirmed all the pathogenic variants (See Figs. 2 and 3). We detected 9 mutations which 4 were novel and 5 were known (Table 2). Additionally, these four novel mutations were absent in 100 normal control individuals.
Discussion
RB is the most common intraocular malignancy in childhood and presents in one or both eyes. Through presenting signs and clinical examination, diagnosis of RB is usually clear [22]. In our study, five probands had leukocoria; one proband had strange reflection; one proband’s left eye was enucleated; two had blurred vision. Mean age at diagnosis was 23 ± 9.9 months for unilateral and 11 ± 8.5 months for bilateral. According to Gene Reviews and AlAli et al. (Mean ageat diagnosis is 24 months for unilateral and 15 months for bilateral; mean age at diagnosis to be 27 months for unilateral and 15 months for bilateral, respectively), our mean age at diagnosis is not later than elsewhere in the world [24]. However, there no tumor in parents of these families.. And especially in family 3 and 7, the visions of the parents were normal.
Genetic testing of RB1 is beneficial to provide counselling for families. In patients with RB, identification of gene alterations improves clinical management of patient and relatives at risk [32]. Here, we have used targeted NGS approach for the molecular analysis of Yunnan Children with RB, based on targeted gene enrichment and bioinformatics pipeline. We used in-house pipeline to successfully detect both pathogenic variants in RB patients. The average throughput depth of the target region in each sample ranged from 90-99X. These met the desired average sequencing coverage which was ensured to provide high quality bases for sensitive and efficient variant detection. To detect SNVs and InDels for all the samples, we developed an automatic in-house variant calling pipeline as freely available tools. The pathogenic SNVs and InDels were identified by stringent criteria, and 8 the pathogenic and 1 likely pathogenic variants were further confirmed by conventional methods and cosegregation with phenotype. Targeted mutation analysis is useful to study mutations in blood and can detect DNA variations. The RB1 gene of seven families’ member are normal and only in family 3and 7, the unaffected mother of this family are heterozygous and the same to the proband. Seven proband’s genes are spontaneous mutation. Four mutations were newly discovered mutations and never reported before. With genetic testing, mutation profiles might be created to precisely screen mutations of relatives or subsequent generations in these families. Four families’ other children had no mutations by gene testing while other five families are one-child family.
However, no RB1 mutations were found in the remaining 25% (3/12) of cases. This could be due to the fact that there are the weakness of study, like not doing cytogenetic studies such as chromosomal microarray (Identification of chromosome translocations or large gross deletions) and multiplex ligation-dependent probe amplification assays (MLPA identifies deletions or rearrangements of 1 or several exons, which account for 16% of all aberrations in RB1). This possibly results in 3 unsolved cases. The combined and cost-effective approach, such as method using a combination of direct sequencing and MLPA, were needed to accurately detect this 3 “negative cases” in next study.
Clearly, study of social determinants of health, such as health seeking behavior, perceptions of medical care, and sociocultural issues related to cancer inheritance would inform counselling approaches that meet the needs of families [33]. This study is helpful for the molecular diagnosis of RB in a comprehensive in Yunnan. These might provide molecular diagnosis to doctor for RB management because lack of genetic testing counseling, poor access to multidisciplinary retinoblastoma-specific health care and socioeconomic factors are one of the factors of higher mortality globally. For the probands with germlinemutation, the molecular diagnosis also provides counseling because the later life of patients with heritable RB displayed that these patients have a high life¬time risk of developing second primary malignancies, such as osteosarcomas, melanomas and soft-tissue sarcomas [34,35,36].
However, in this cohort, the small number of patients is not enough to establish a significant frequency reference and functional studies are necessary for assigning pathogenicity to these novel variants.
Conclusion
Here, we reported that this approach with bioinformatics pipeline could detect variants including novel pathogenic variants. This comprehensive approach reduces the time and number of assays required for the detection of pathogenic variants by conventional methods. To the best of our knowledge, this is the first such study using targeted NGS approach to detect pathogenic variants in Yunnan children with RB. Overall, targeted NGS approach is becoming more feasible in clinical settings.
Availability of data and materials
The raw sequencing data of NGS are available in the NCBI’s Sequence Read Archive (SRA) with accession number PRJNA666751 (SRR12762344, SRR12762345, SRR12762346, SRR12762347) https://dataview.ncbi.nlm.nih.gov/object/PRJNA666751?reviewer=mlmsrbpj2u60r1ss0ijmkgmole”. The other data and materials are available from the corresponding author (JM) or first author (ZZ) upon reasonable request.
Abbreviations
- RB:
-
Retinoblastoma
- IIRC:
-
Intraocular International Retinoblastoma Classify
- NGS:
-
Next generation sequencing
- SOAP:
-
Short Oligonucleotide Analysis Package
- PCR:
-
Polymerase chain reaction
- SNPs:
-
Single nucleotide polymorphisms
- BWA:
-
Burrows-Wheeler Aligner
- InDels:
-
Deletions and insertions
- SIFT:
-
Sorting Intolerant from Tolerant
- SNVs:
-
Single nucleotide variants
References
Bornfeld N, Biewald E, Bauer S, Temming P, Lohmann D, Zeschnigk M. The interdisciplinary diagnosis and treatment of intraocular tumors. Dtsch Arztebl Int. 2018;115(7):106–11.
Dimaras H, Khetan V, Halliday W, Orlic M, Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RJ, Squire JA, et al. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet. 2008;17(10):1363–72.
Comings DE. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973;70(12):3324–8.
Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, Abramson DH, Shields CL, Chantada GL, Njuguna F, et al. Retinoblastoma. Nat Rev Dis Primers. 2015;1(1):15021..
Lohmann D. Retinoblastoma. Adv Exp Med Biol. 2010;685:220–7.
Valverde JR, Alonso J, Palacios I, Pestana A. RB1 gene mutation up-date, a meta-analysis based on 932 reported mutations available in a searchable database. BMC Genet. 2005;6:53.
Devarajan B, Prakash L, Kannan TR, Abraham AA, Kim U, Muthukkaruppan V, Vanniarajan A. Targeted next generation sequencing of RB1 gene for the molecular diagnosis of retinoblastoma. BMC Cancer. 2015;15:320.
Lohmann DR. RB1 gene mutations in retinoblastoma. Hum Mutat. 1999;14(4):283–8.
Jaya Singh AMAJ. Next-generation sequencing-based method shows increased mutation detection sensitivity in an Indian retinoblastoma cohort. Mol Vis. 2016;22:1036–47.
Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–31..
Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93(1):21–3.
Retinoblastoma union for International Cancer Control WHO.2014.
Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: a review of population-based cancer registries. Indian Pediatr. 2014;51(3):218–20.
Song Z. Effect and mechanism of silencing SHH gene on the proliferation and apoptosis of retinoblastoma cell: Jilin University; 2016..
Canadian Retinoblastoma Society. National Retinoblastoma Strategy Canadian Guidelines for Care: Strategie therapeutique du retinoblastome guide clinique canadien. Can J Ophthalmol. 2009;44(Suppl 2):S1–S88.
Canturk S, Qaddoumi I, Khetan V, Ma Z, Furmanchuk A, Antoneli CB, Sultan I, Kebudi R, Sharma T, Rodriguez-Galindo C, et al. Survival of retinoblastoma in less-developed countries impact of socioeconomic and health-related indicators. Br J Ophthalmol. 2010;94(11):1432–6.
Dean M, Bendfeldt G, Lou H, Giron V, Garrido C, Valverde P, Barnoya M, Castellanos M, Jimenez-Morales S, Luna-Fineman S. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guatemala. Cancer Lett. 2014;351(1):59–63.
Jain M, Rojanaporn D, Chawla B, Sundar G, Gopal L, Khetan V. Retinoblastoma in Asia. Eye (Lond). 2019;33(1):87–96.
Soliman SE, Racher H, Zhang C, MacDonald H, Gallie BL. Genetics and molecular diagnostics in retinoblastoma--an update. Asia Pac J Ophthalmol (Phila). 2017;6(2):197–207.
Vogel F. Genetics of retinoblastoma. Hum Genet. 1979;52(1):1–54.
Yun J, Li Y, Xu CT, Pan BR. Epidemiology and Rb1 gene of retinoblastoma. Int J Ophthalmol. 2011;4(1):103–9.
Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, Gallie BL. Retinoblastoma. Lancet. 2012;379(9824):1436–46.
Dimaras H. Retinoblastoma genetics in India: from research to implementation. Indian J Ophthalmol. 2015;63(3):219–26.
AlAli A, Kletke S, Gallie B, Lam WC. Retinoblastoma for pediatric ophthalmologists. Asia Pac J Ophthalmol (Phila). 2018;7(3):160–8..
Linn MA. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin N Am. 2005;18(1):41–53.
Gao YJ, Qian J, Yue H, Yuan YF, Xue K, Yao YQ. Clinical characteristics and treatment outcome of children with intraocular retinoblastoma: a report from a Chinese cooperative group. Pediatr Blood Cancer. 2011;57(7):1113–6.
Skalet AH, Gombos DS, Gallie BL, Kim JW, Shields CL, Marr BP, Plon SE, Chevez-Barrios P. Screening children at risk for retinoblastoma: consensus report from the American Association of Ophthalmic Oncologists and Pathologists. Ophthalmology. 2018;125(3):453–8.
Roelofs K, Shaikh F, Astle W, Gallie BL, Soliman SE. Incidental neuroblastoma with bilateral retinoblastoma: what are the chances? Ophthalmic Genet. 2018;39(3):410–3.
Etherington GJ, Ramirez-Gonzalez RH, MacLean D. bio-samtools 2: a package for analysis and visualization of sequence and alignment data with SAMtools in ruby. Bioinformatics. 2015;31(15):2565–7.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–9.
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25(15):1966–7.
Richter S, Vandezande K, Chen N, Zhang K, Sutherland J, Anderson J, Han L, Panton R, Branco P, Gallie B. Sensitive and efficient detection of RB1 gene mutations enhances Care for Families with retinoblastoma. Am J Hum Genet. 2003;72(2):253–69.
He LQ, Njambi L, Nyamori JM, Nyenze EM, Kimani K, Matende I, Rono H, Njom V, Bett J, Mukuria M, et al. Developing clinical cancer genetics services in resource-limited countries: the case of retinoblastoma in Kenya. Public Health Genom. 2014;17(4):221–7.
Kleinerman RA, Tucker MA, Abramson DH, Seddon JM, Tarone RE, Fraumeni JJ. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99(1):24–31.
Kleinerman RA, Yu CL, Little MP, Li Y, Abramson D, Seddon J, Tucker MA. Variation of second cancer risk by family history of retinoblastoma among long-term survivors. J Clin Oncol. 2012;30(9):950–7.
MacCarthy A, Bayne AM, Brownbill PA, Bunch KJ, Diggens NL, Draper GJ, Hawkins MM, Jenkinson HC, Kingston JE, Stiller CA, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br J Cancer. 2013;108(12):2455–63.
Acknowledgements
We thank all subjects who participated in this study.
Funding
This work was supported by a grant from the Joint Fund of the Yunnan Science and Technology Department and Kunming Medical-University [grants nos. 2019FE001(− 274)], the Health and Family Planning Commission of Kunming and Yunnan [grants nos. 2017-SW (Yang)-02 and H-2018012], Key R & D plan of Yunnan Provincial Department of science and technology----International cooperation in science and technology (grants nos. 2018IA047), Cultivation of Reserve Talents for Young and Middle-aged Academic and Technical Leaders of Yunnan Province (2019HB102), and Innovation team of Yunnan Province for comprehensive Prevention and Treatment of Children’s hearing impairment and language diseases (2019HC026), respectively. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Z Z, Y S X, W J H and R S conceived and designed the experiments; Z Z, R S, W J H, H C J, L T, R Q Land J M performed the experiments; Z Z, Y S X, J M, H C J, X H Y, W J H and L T analyzed the data; Y S X, R Q Land W J H recruited patients and collected clinical information.. YS X, R Q L,R S, H C J, and H Y G contributed to accumulation and interpretation of clinical data. W J H, J M H Y G and Z Z coordinated the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Kunming Children’s Hospital, Kunming Medical University. Written informed consents were obtained from all participants in this study. Moreover, written informed consents were obtained from the parents of all participants under the age of 16. The ethics committee of Kunming Children’s Hospital, Kunming Medical University, were acquired by our team to access the data we used in our research.
Consent for publication
All participants have provided written informed consents to publish all identifying images, personal details and clinical details anonymously. Moreover, written informed consent for publication of identifying images or other personal or clinical details was obtained from the parents of any participant under the age of 18.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1.PCR primers for amplification. Supplementary Table 2. Coverage level through the target region for patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Z., Xiao, Ys., Shen, R. et al. Next generation sequencing of RB1gene for the molecular diagnosis of ethnic minority with retinoblastoma in Yunnan. BMC Med Genet 21, 230 (2020). https://doi.org/10.1186/s12881-020-01150-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-020-01150-7