Abstract
Background
Acute pancreatitis is one of the most common diseases requiring emergency surgery. Rapid and accurate recognition of acute pancreatitis can help improve clinical outcomes. This study aimed to develop a deep learning-powered diagnostic model for acute pancreatitis.
Materials and methods
In this investigation, we enrolled a cohort of 190 patients with acute pancreatitis who were admitted to Sichuan Provincial People’s Hospital between January 2020 and December 2021. Abdominal computed tomography (CT) scans were obtained from both patients with acute pancreatitis and healthy individuals. Our model was constructed using two modules: (1) the acute pancreatitis classifier module; (2) the pancreatitis lesion segmentation module. Each model’s performance was assessed based on precision, recall rate, F1-score, Area Under the Curve (AUC), loss rate, frequency-weighted accuracy (fwavacc), and Mean Intersection over Union (MIOU).
Results
Upon admission, significant variations were observed between patients with mild and severe acute pancreatitis in inflammatory indexes, liver, and kidney function indicators, as well as coagulation parameters. The acute pancreatitis classifier module exhibited commendable diagnostic efficacy, showing an impressive AUC of 0.993 (95%CI: 0.978–0.999) in the test set (comprising healthy examination patients vs. those with acute pancreatitis, P < 0.001) and an AUC of 0.850 (95%CI: 0.790–0.898) in the external validation set (healthy examination patients vs. patients with acute pancreatitis, P < 0.001). Furthermore, the acute pancreatitis lesion segmentation module demonstrated exceptional performance in the validation set. For pancreas segmentation, peripancreatic inflammatory exudation, peripancreatic effusion, and peripancreatic abscess necrosis, the MIOU values were 86.02 (84.52, 87.20), 61.81 (56.25, 64.83), 57.73 (49.90, 68.23), and 66.36 (55.08, 72.12), respectively. These findings underscore the robustness and reliability of the developed models in accurately characterizing and assessing acute pancreatitis.
Conclusion
The diagnostic model for acute pancreatitis, driven by deep learning, exhibits excellent efficacy in accurately evaluating the severity of the condition.
Trial Registration
This is a retrospective study.
Similar content being viewed by others
Background
Acute pancreatitis is one the most common diseases in emergency departments and is characterized by local and systemic inflammation with different clinical courses [1, 2]. The symptoms of acute pancreatitis are non-specific and may include abdominal pain, nausea, vomiting and fever. These symptoms can be difficult to distinguish from those of other gastrointestinal diseases, such as cholecystitis, acute gastroenteritis and acute appendicitis. Furthermore, acute pancreatitis may present with atypical symptoms, such as back pain, which can result in incorrect or delayed diagnosis. Although elevated serum amylase and lipase levels are characteristic of acute pancreatitis, these enzymes can also be elevated under other conditions, which can lead to false-positive results. In 2017, there were about 1.6 million new cases of acute pancreatitis worldwide, of which about 100,000 resulted in death [3]. Acute pancreatitis is mostly self-limited. However, around 20% of the patients develop acute severe pancreatitis and the death rate is about 30% [4]. Although several models have been developed to predict pancreatitis-related outcomes, their accuracy is unsatisfactory [5, 6]. At the present, there are many clinical scoring systems for the early classification of acute pancreatitis severity, among which Acute Physiological and Chronic Health Score (APACHE) II and Acute Pancreatitis Severity Bed Side Index (BISAP) are widely used in clinical practice [7]. The BISAP score can be evaluated on the first day of admission, but the accuracy and sensitivity of its prediction are not high [8]. In imaging, the assessment of acute pancreatitis relies on the Balthazar CT [9] rating and the Modified Computed Tomography Severity Index Score (MCTSI) [10]. However, in early stages of acute pancreatitis, morphological changes of the pancreas may not be apparent on CT or MRI images in some patients, especially pancreatic necrosis, which may lead to underestimation of the severity of the disease [11, 12]. The severity of symptoms and manifestations of acute pancreatitis varies from person to person, and it can result in complications, including the formation of pseudocysts and organ failure. The early recognition of these complications is of the utmost importance for the appropriate management of the condition and the improvement of patient outcome.
Recently, artificial intelligence (AI) is poised to revolutionize the future development of medicine [13]. Through AI models, an accurate prediction of results can be achieved by learning complex relationships among the data presented [14]. With the advancement of computing technologies and the development of medical databases, machine learning has become an active area of medical research. Machine learning in medicine can generate more accurate diagnostic algorithms and individualized patient treatment plans [15, 16]. In recent years, AI has also been widely applied to acute pancreatitis diagnosis and treatment, especially in severity evaluation [17,18,19], complications [20,21,22], mortality [23, 24], recurrence [25, 26], and surgery time prediction [27, 28] with various degrees of breakthroughs.
Traditional machine learning (ML) constructs models for diagnosis and predictions based on clinical and laboratory data of patients with acute pancreatitis. Deep learning (DL), as a primary research direction in the field of ML, has its unique advantages. DL can learn patterns and features within data, and the information obtained in the process of learning can be made highly interpretable for numerical, image and other data. The Convolutional Neural Networks (CNN) is a class of feedforward neural networks that uses convolutional computation with a deep neural network structure and is one of the representative algorithms of DL [29]. Among the different variants of CNN-based networks, U-Net has become one of the main choices, a network model proposed by Ronneberger et al. [30] in 2015, which consists of a symmetric encoder-decoder network with hopping connections for enhanced detail retention. The U-Net network was used for semantic segmentation of medical imaging data when it was proposed and was extended to semantic segmentation of 3D video data [31] and generation of super-resolution images [32] in subsequent applied research. Numerous studies [33,34,35,36,37,38,39,40] have proposed novel model architectures based on the U-net model architecture, which have demonstrated significant advancements in image segmentation performance and model parameter requirements. EasyDL is a DL platform developed by Baidu company that allows researchers to create and train models easily [41]. EasyDL’s underlying layers combine AutoDL and AutoML technologies to automatically obtain optimal networks, which avoids tedious network selection and hyperparameter tuning for non-specialists in building DL models. Therefore, this study merges two autonomous modules to develop an ensemble learning model, which not only improves the performance of the model, but also enhances its interpretability. Previous research in the diagnosis and prediction of acute pancreatitis has only been conducted at the level of traditional ML [17,18,19,20,21,22,23,24,25,26,27,28]. The advantages of DL have not been effectively utilized, and this study proposes to construct a unique diagnostic prediction model for acute pancreatitis through DL methods.
To that end, we broke into new territories in the following areas: (1) This is the first instance in which DL has been employed to construct a diagnostic model for acute pancreatitis. (2) We innovatively combined the acute pancreatitis classifier and lesion segmentation modules to construct a diagnostic model, which can not only quickly identify pancreatitis, but also identify pancreatitis-associated foci and directly assess the severity of the disease.
This paper is structured as follows: Sect. 2 outlines the methodology employed in this study. This includes an overview of the inclusion and exclusion criteria, the data collection and processing process, and the model construction. The model was made up of two modules: (1) the acute pancreatitis classifier module; (2) the pancreatitis lesion segmentation module. The statistical methods employed, and the evaluation methods used to assess the final model. Section 3 presents the results section of this study, including the statistical results of the patients’ baseline data and the results of the modelling. Section 4 is devoted to the strengths of the model and some of the current limitations. It concludes with a discussion of future directions for improving the study.
Materials and methods
Patients
The protocol of this retrospective study was approved by the Medical Ethics Committee of Sichuan Provincial People’s Hospital, with consent information waived (approval number: Ethical Review (Research) No. 99 of 2022). The investigation involved patients admitted to the emergency medicine center of Sichuan Provincial People’s Hospital for acute pancreatitis from January 2020 to December 2021. Additionally, healthy control individuals from the Physical Examination Center of Sichuan Provincial People’s Hospital during the same period were included in the study. Inclusion and exclusion criteria are shown in Table 1.
Data collection
Clinical and laboratory data collection
Upon admission to the emergency department, comprehensive clinical and laboratory data were meticulously gathered. This encompassed demographic information such as gender and age, along with pertinent medical histories including hypertension, diabetes, smoking habits, and alcohol consumption. The laboratory dataset included a range of parameters: blood routine, inflammatory marker, liver and kidney function indicators, serum amylase, lipase, blood lipids, and coagulation function.
Imaging data collection
For the purposes of DL in this study, abdominal CT images served as the primary dataset. A meticulous process was followed, where two independent radiologists screened the abdominal CT images of both patients and healthy control individuals. Subsequently, images depicting noticeable pancreatic swelling indicative of acute pancreatitis or those exhibiting a normal pancreas in physical examinations were selected. The final step involved a thorough review of the screened images by a senior radiologist, ensuring the precision and reliability of the dataset for subsequent analyses.
Development of classifier module for acute pancreatitis
In our research, we employed Baidu’s EasyDL platform (https://ai.baidu.com/easydl/) as the foundation for constructing a classifier module dedicated to acute pancreatitis. Adhering to EasyDL’s operational protocol, we uploaded CT images representing both acute pancreatitis and healthy pancreas, and these images were subsequently trained using EasyDL’s optimal network. We observed and recorded the performance metrics of the module during training. To comprehensively assess the module’s robustness, an untrained dataset was used for external validation, providing valuable insights into its generalization capabilities beyond the training dataset.
Development of lesion segmentation module for acute pancreatitis
In this study, the delineation of pancreatic conditions, including normal pancreas, swollen pancreas, peripancreatic inflammatory exudate, peripancreatic effusion, and peripancreatic abscess necrosis, was anchored on the Balthazar CT rating. To execute this segmentation task, we used the open-source software Genie Annotation Assistant. Two radiologists performed pixel-level segmentation of the lesions, and subsequently, a senior radiologist reviewed the segmented content.
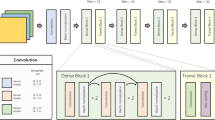
The U-Net network [30], illustrated in Fig. 1, was chosen as the foundational architecture for the segmentation module. This module exhibits a straightforward structure, with the left section devoted to feature extraction and the right part to up-sampling. Termed the Encoder-Decoder structure in the realm of research, the U-Net network maximizes the effectiveness of segmentation data utilization by employing a data enhancement method, particularly advantageous when dealing with a limited number of segmented images.
For optimal module training, the dataset was intelligently partitioned into a training set and a validation set through a computer-generated random division, maintaining an 8:2 ratio. This strategic division ensures robust training and reliable validation, contributing to the overall efficacy of the segmentation module. The workflow of the methodology is shown in Fig. 2.
Statistical methods
For the analysis of small sample continuous data, the normality of the data distribution was assessed using the Shapiro-Wilk test. In instances where the data exhibited normal distribution, they were presented as mean ± standard deviation (± S), and inter-group comparisons were conducted using the independent sample t-test. Alternatively, for skewed data, representation was made using the median and quartile [M (P25, P75)], and inter-group comparisons were performed using the Mann-Whitney U test.
Dichotomous data underwent inter-group comparisons through Chi-square tests. The statistical analysis was executed using IBM SPSS Statistics 26.0 software (IBM, America).
Model evaluation
In the acute pancreatitis classifier module, the assessment of module efficacy relied on the area under the Receiver Operating Characteristic (ROC) curve. The acute pancreatitis lesion segmentation module’s performance was gauged by analyzing the accuracy, loss rate, frequency-weighted accuracy (fwavacc), and Mean Intersection over Union (MIOU) across both the training and validation sets.
Result
Demographic characteristics
A total of 190 patients with acute pancreatitis were included in this study, of which 121 (63.68%) were males and 69 (36.32%) were females. According to the severity of the disease, 100 cases (52.63%) were classified as mild acute pancreatitis and 90 cases (47.37%) as severe acute pancreatitis (moderate and severe acute pancreatitis are defined as severe acute pancreatitis in this study). The clinical and laboratory data of patients with pancreatitis were collected in accordance with the methodology detailed in Table 2. The differences between the two groups in smoking, leukocytes, neutrophils, hematocrit (HCT), platelets (PLT), C-reactive protein (CRP), procalcitonin (PCT), urea, creatinine (Cr), glucose (Glu), calcium (Ca), albumin (ALB), aspartate transaminase (AST), lactate dehydrogenase (LDH), total bilirubin (TB), amylase (AMY), lipase (LPS), total cholesterol (TC), LDL, PT, APTT, DD, and FDP were statistically significant (P < 0.05).
Imaging data
Image study was performed by two radiologists outlining the target area and one senior radiologist reviewing the result. A total of 945 segmented images of swollen pancreas, 592 segmented images of normal pancreas, 475 segmented images of peripancreatic inflammatory exudate, 153 segmented images of peripancreatic effusion, and 42 segmented images of peripancreatic abscess necrosis were obtained. Figure 3 shows the lesion segmentation diagram.
Classifier module for acute pancreatitis
The classifier module obtained by EasyDL was highly effective, with 99.1% precision rate, 100% recall rate and 100% f1-score for predicting acute pancreatitis. Among 352 random samples, 350 were correctly predicted by the module and 2 samples were incorrectly predicted (Table 3). The heat map is shown in Fig. 4. To verify the performance of the module, 186 untrained images were selected and inputted to EasyDL for validation. The ROC curves of the classifier module in the test set and external validation set are shown in Fig. 5 [AUC 0.993 (95%CI: 0.978–0.999) in the test set for healthy patients vs. patients with acute pancreatitis, P < 0.001]; [AUC 0.850 (95%CI: 0.790–0.898) in the external validation set for healthy patients vs. patients with acute pancreatitis), P < 0.001]. The AUC of the classifier module in the test set and external validation set are detailed in Table 4.
Lesion segmentation module for identifying acute pancreatitis
Pancreas segmentation module
The module was constructed to distinguish swollen and normal pancreas, using 675 segmentation images of swollen pancreas and 500 segmentation images of normal pancreas. The training parameters of this module were EPOCH-NUM = 300, BATCH-SIAZE = 16, train-num = 10. The module performed very well in pancreatic segmentation, and in the validation set, the median and quartiles of accuracy, loss rate, fwavacc, and mean cross-ratio were [99.54 (99.48, 99.59), 1.74 (1.36, 2.19), 99.14 (99.02, 99.23), 86.02 (84.52, 87.20)]. The accuracy, loss rate, fwavacc, and mean crossover sum of the training and validation sets of the module are shown in Fig. 6. The segmentation effects are shown in Fig. 7. The results of each parameter of the segmentation module in the validation set are shown in Table 5.
Peripancreatic inflammatory exudate segmentation module
This module was constructed using 457 segmentation images of peripancreatic inflammatory exudate. The training parameters of this module were EPOCH-NUM = 300, BATCH-SIAZE = 16, train-num = 10. The module performed well in segmentation of peripancreatic inflammatory exudate, and in the validation set, the median and quartiles of accuracy, loss rate, fwavacc, and mean cross-comparison ratio were [99.06 (98.89, 99.20), 3.64 (2.85, 4.98), 98.29 (97.97, 98.57), and 61.81 (56.25, 64.83)]. The accuracy, loss rate, fwavacc, and mean crossover sum of the training and validation sets of the module are shown in Fig. 8. The splitting effect is shown in Fig. 9.
Peripancreatic effusion segmentation module
The peripancreatic effusion module was constructed using 153 segmentation images of peripancreatic effusion. The training parameters of this module were EPOCH-NUM = 400, BATCH-SIAZE = 16, train-num = 10. The module performed well in segmentation of peripancreatic inflammatory exudate, and in the validation set, the median and quartiles of accuracy, loss rate, fwavacc, and mean cross-comparison ratio were [98.86 (98.40, 99.05), 4.07 (3.12, 5.50), 97.79 (96.91, 98.16), 57.73 (49.90, 68.23)]. The accuracy, loss rate, fwavacc, and mean crossover sum of the training and validation sets of the module are shown in Fig. 10. The splitting effect is shown in Fig. 11.
Peripancreatic abscess necrosis segmentation module
The peripancreatic abscess necrosis module was constructed using 42 segmentation maps of peripancreatic abscess necrosis. The training parameters of this module were EPOCH-NUM = 400, BATCH-SIAZE = 16, train-num = 10. The module performed well in segmentation of peripancreatic abscess necrosis, and in the validation set, the median and quartiles of accuracy, loss rate, fwavacc, and mean cross-comparison ratio were [97.94 (97.64, 98.26), 4.80 (4.29, 6.15), 96.36 (95.51, 96.98), 66.36 (55.08, 72.12)]. The accuracy, loss rate, fwavacc, and mean crossover sum of the training and validation sets of the module are shown in Fig. 12. The splitting effect is shown in Fig. 13.
Discussion
In this study, we constructed a deep learning-powered diagnostic model for acute pancreatitis, which was able to effectively recognize acute pancreatitis and assess its severity by segmenting out the relevant lesions. The acute pancreatitis classifier module of this model showed high accuracy for the diagnosis of acute pancreatitis. In the test set [AUC of 0.993 (95% CI: 0.978–0.999), sensitivity of 100.00% and specificity of 98.59% for healthy patients vs. patients with acute pancreatitis]. In the external validation set [AUC of 0.850 (95% CI: 0.790–0.898), sensitivity of 80.85% and specificity of 89.13% for healthy patients vs. patients with acute pancreatitis]. In the pancreatitis segmentation module of this model, its segmenting ability of acute pancreatitis related lesions was also good, MIOU on the validation set was as high as 86.02%. This indicates that our model can diagnose acute pancreatitis quickly and accurately. As a result, it can have a positive impact on clinical practice. For example, the model can be deployed in more primary hospitals, which can assist emergency physicians to diagnose acute pancreatitis quickly and accurately, reducing the misdiagnosis rate while increasing the success rate of patient treatment.
The acute pancreatitis classifier module of this model achieved satisfactory results in terms of AUC-ROC, sensitivity, and specificity in both the test set and the external validation set. The credibility of this module is increased by the heatmap. Traditional DL models are deficient in interpretability and many studies [42,43,44] treated DL models as black boxes. In our study we have applied heatmap based on Shapley value [45] to improve the interpretability. In the pancreatitis classifier module of, we know exactly which regions the model is transforming with high weights to obtain the final discriminative results by the heatmap. From the correct classification Fig. 4(1a-1d) and Fig. 4(1e-1h) we can see that the module classifies the peripancreatic area as a high weight region. In addition, Fig. 4(1i-1l) shows the incorrectly classified images, and the module classified these two normal pancreatic images as pancreatitis images based on the peripancreatic region. It is not difficult to see from the figure that the module incorrectly considers the residual stomach as a high-weighted region. It is known that this is difficult even for a well-trained imaging physician.
EasyDL is a DL platform developed by Baidu that facilitates the entire process of model creation, data uploading, training the model, and model release. The underlying layer of EasyDL integrates AutoDL and AutoML technologies to automatically identify the optimal network. The platform eliminates the need for non-professionals to engage in tedious network selection and hyper-parameter tuning when constructing DL networks. Furthermore, EasyDL provides a heatmap, constructed using the Pixel-wise Shapley Value technique, which enables the user to identify the focus area. This is the primary reason this platform was selected for the construction of the pancreatitis classifier module. However, as this platform is a commercial platform, its most significant drawback is that it is not possible to ascertain which network it employs for training purposes or the training process itself.
In terms of segmentation method for acute pancreatitis lesions, we established our model by distinguishing four pathological types such as swell pancreases, peripancreatic inflammatory exudate, peripancreatic effusion, and peripancreatic abscess necrosis. In general, the model runs well (Table 5), but the performance on peripancreatic inflammatory exudate, peripancreatic effusion, and peripancreatic abscess necrosis are not satisfactory. The reasons for this were analyzed in conjunction with the results of the module segmentation. Peripancreatic inflammatory exudate and peripancreatic effusion are randomly distributed around the pancreas. From the segmentation results of the two modules in the validation set, the segmentation module identifies not only our pre-segmented lesions, but also some small and scattered lesions, which is equivalent to increasing the denominator of the MIOU. On the other hand, although the manual segmentation of the lesion is done by an imaging physician, selection bias is inevitable. However, by looking at the segmentation results, we found that the constructed module can in fact correct the selection bias, which explains the low MIOU and good segmentation results. Therefore, for these two modules, we cannot evaluate the performance of the module simply based on the magnitude of the MIOU and should instead combine the segmentation effects to make a comprehensive analysis. This also confirms that the DL model, as mentioned in study by Meglič J et al. [46], is actually learning and not simply mimicking the training dataset. This is a significant breakthrough in the field of medical image segmentation. As for the segmentation module of pancreatic abscess necrosis, the lack of sample size is the main reason for the low MIOU of the module, but the present results achieved by 42 segmented images have already shown that the module itself is highly successful.
To our best knowledge, our model is the first one that can distinguish acute pancreatitis in CT images. In addition, our model provides a segmentation function that can distinguish acute pancreatitis lesions, which is also unprecedented. This intelligent diagnostic model can assist clinicians to quickly recognize and assess the severity of acute pancreatitis through the segmentation of related lesions in a clinical setting. In terms of processing image data, this research ensured the quality of the dataset through manual segmentation by well-trained imaging physicians.
Currently, DL techniques have been applied to medical image segmentation and have demonstrated expert performance. In Li’s study [47], a meta-learning approach based on frequency domain feature mixing was proposed, which achieved a new level of generalization in MRI segmentation of nasopharyngeal carcinoma, with MIOU of 75.74%. The Swin MoCo network, a momentum contrast learning network with a Swin Transformer backbone, proposed by Xu et al. [48], has been shown to improve parotid segmentation to 85.18% MIOU. In Wang’s study [49], the deep learning model based on the U-net network demonstrated efficacy in fully automated image segmentation of adenoid and airway of nasopharynx in children, with MIOU values of 86.28% and 86.32%, respectively. Similar findings [50, 51] have been reported in other medical image segmentation models, more details can be found in Table 6. From the results presented in the table, it can be concluded that optimizing the model architecture is an urgent problem to be solved if the objective is to further improve the segmentation performance of the model.
Although the segmentation function of U-Net is powerful, the acceptance domain of convolution operation in CNN is limited by the size of convolution kernel, resulting in a lack of long-distance dependence [52]. Therefore, CNN-based methods often have obvious limitations when it comes to displaying remote relationships in modeling. This is also the reason U-Net cannot make further breakthroughs. Transformer [53] is a popular approach in natural language processing that has been shown to be effective in learning global contextual features in computer vision and has demonstrated superior portability to downstream tasks under large-scale pre-training. It has been successful in the field of machine translation and natural language processing [54]. Therefore, it is proposed that TransUNet uses a CNN encoder to obtain local features, and then merges the Transformer into a hybrid encoder in the U-Net down-sampling path to obtain global contextual features [55]. The use of U-net alone to segment the pancreas is problematic when the basic textural features of the pancreas are not obvious compared to the surrounding peripheral organs. By combining CNN, which is good at capturing local features, and Transformer, which is good at capturing surrounding features, we can obtain more accurate segmentation than any traditional methods. Although our method achieves some good results in the segmentation of acute pancreatitis lesions, the module still has much room for improvement, potentially by capturing the surrounding features through Transformer.
Among the models for DL, the application of appropriate preprocessing approaches to the data or model can frequently enhance the learning results. Such examples include noise reduction, data balancing, data enhancement, and model architecture optimization, among others. In medical imaging, noise may have multiple sources and may affect the ability of the model to learn meaningful features. The importance of noise reduction to improve segmentation accuracy was highlighted in a study [56]. We can explore similar techniques such as denoising self-encoders or wavelet-based methods to mitigate noise in CT images. Category imbalance is a prevalent issue in DL models, particularly in classification models. When the number of samples of different categories in a dataset varies greatly, the model may exhibit a tendency to predict most of the categories while ignoring a few, which may subsequently affect the overall performance of the model. Although our dataset does not exhibit significant imbalances, the techniques discussed in Singh’s Study [57] can be borrowed, albeit with different application scenarios. For instance, oversampling a limited number of classes or the generation of synthetic data may be employed to achieve a more balanced distribution of acute pancreatitis and healthy cases. The study by Vaisali Chandrasekar [58] emphasizes the significance of data enhancement in improving the generalization capacity of models. It is possible to apply a number of enhancement techniques to CT images, including rotation, flipping and cropping, in order to artificially expand the dataset and expose the model to a greater variety of situations. Finally, optimization of the model architecture is often important as well. A study [36] demonstrated the effectiveness of an improved U-Net architecture for a segmentation task. Although our study employed the U-Net architecture, we may wish to consider integrating selected elements, such as dense connections or pyramid pooling modules, with the aim of enhancing our lesion segmentation model.
Although our model performs well on both the training and validation sets, it is prudent to exercise caution when employing AI to diagnose and treat diseases in clinical practice. One study [59] has demonstrated the potential risks associated with the use of Computer-aided Detection or Diagnostic (CAD) in clinical settings. However, we still encourage clinicians to consider it as a tool to assist in diagnosis.
This study has some limitations. Firstly, it is a single-center study, which increases the risk of bias. Secondly, the data set included in this study met the model requirements, but not every patient with pancreatitis had local complications such as effusion or necrosis, so there was still a lack of sufficient data to construct a better segmentation module for effusion and necrosis in acute pancreatitis.
Conclusion
This study presents an innovative approach to the construction of an intelligent diagnostic model for acute pancreatitis, employing a DL algorithm. The model is designed to assist clinicians in rapidly and accurately identifying the presence of pancreatitis and segmenting lesions associated with acute pancreatitis. The model assists clinicians in assessing the severity of the disease in a more intuitive manner and in developing appropriate treatment plans for patients. Furthermore, in future work, we will continue to optimize the network and incorporate patients’ laboratory data into the model based on the existing model to construct a more comprehensive diagnostic model.
Data availability
The source code and relevant data for this study can be accessed at https://github.com/dcpengjin/PancreaseCT. The code is protected under the MIT license. EasyDL is a commercial deep learning platform developed by Baidu. The underlying layer of EasyDL combines AutoDL and AutoML technologies to automatically obtain the optimal network. However, it is important to note that the platform has some limitations, including the inability to provide algorithms in certain cases.
Abbreviations
- AI:
-
Artificial intelligence
- ALB:
-
Albumin
- AMY:
-
Amylase
- APACHE II:
-
Acute Physiological and Chronic Health Score II
- AST:
-
Aspartate transaminase
- AUC:
-
Area Under the Curve
- BISAP:
-
Acute Pancreatitis Severity Bed Side Index
- Ca:
-
Calcium
- CNN:
-
Convolutional Neural Networks
- Cr:
-
Creatinine
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DL:
-
Deep learning
- fwavacc:
-
frequency-weighted accuracy
- Glu:
-
Glucose
- HCT:
-
Hematocrit
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipase
- MCTSI:
-
Modified Computed Tomography Severity Index Score
- MIOU:
-
Mean Intersection over Union
- ML:
-
Machine learning
- MR:
-
Magnetic Resonance
- PCT:
-
Procalcitonin
- ROC:
-
Receiver Operating Characteristic
- TB:
-
Total bilirubin
- TC:
-
Total cholesterol
References
Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55.
Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396:726–34.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392:1736–88.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Acute Pancreatitis classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11.
Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, et al. Computed Tomography Severity Index vs. other indices in the prediction of severity and mortality in Acute Pancreatitis: a predictive accuracy Meta-analysis. Front Physiol. 2019;10:1002.
Di MY, Liu H, Yang ZY, Bonis PA, Tang JL, Lau J. Prediction models of mortality in Acute Pancreatitis in adults: a systematic review. Ann Intern Med. 2016;165:482–90.
Simoes M, Alves P, Esperto H, Canha C, Meira E, Ferreira E, et al. Predicting Acute Pancreatitis Severity: comparison of prognostic scores. Gastroenterol Res. 2011;4:216–22.
Gao W, Yang HX, Ma CE. The value of BISAP score for Predicting Mortality and Severity in Acute Pancreatitis: a systematic review and Meta-analysis. PLoS ONE. 2015;10:e0130412.
Cheng T, Han TY, Liu BF, Pan P, Lai Q, Yu H, et al. Use of Modified Balthazar Grades for the early prediction of Acute Pancreatitis Severity in the Emergency Department. Int J Gen Med. 2022;15:1111–9.
Liao Q, He WH, Li TM, Lai C, Yu L, Xia LY, et al. [Evaluation of severity and prognosis of acute pancreatitis by CT severity index and modified CT severity index]. Zhonghua Yi Xue Za Zhi. 2022;102:2011–7.
Shinagare AB, Ip IK, Raja AS, Sahni VA, Banks P, Khorasani R. Use of CT and MRI in emergency department patients with acute pancreatitis. Abdom Imaging. 2015;40:272–7.
Spanier BW, Nio Y, van der Hulst RW, Tuynman HA, Dijkgraaf MG, Bruno MJ. Practice and yield of early CT scan in acute pancreatitis: a Dutch Observational Multicenter Study. Pancreatology. 2010;10:222–8.
Dias R, Torkamani A. Artificial intelligence in clinical and genomic diagnostics. Genome Med. 2019;11(1):70.
Li J, Zhou Z, Dong J, Fu Y, Li Y, Luan Z, et al. Predicting breast cancer 5-year survival using machine learning: a systematic review. PLoS ONE. 2021;16:e0250370.
Weiss J, Kuusisto F, Boyd K, Liu J, Page D. Machine learning for treatment assignment: improving individualized risk attribution. AMIA Annu Symp Proc. 2015;2015:1306–15.
Weiss JC, Natarajan S, Peissig PL, McCarty CA, Page D. Machine learning for personalized medicine: predicting primary myocardial infarction from electronic health records. AI Magazine. 2012;33:33.
Choi HW, Park HJ, Choi SY, Do JH, Yoon NY, Ko A, et al. Early Prediction of the severity of Acute Pancreatitis using Radiologic and Clinical Scoring systems with classification Tree Analysis. AJR Am J Roentgenol. 2018;211:1035–43.
Yang Z, Dong L, Zhang Y, Yang C, Gou S, Li Y, et al. Prediction of severe Acute Pancreatitis using a decision Tree Model based on the revised Atlanta classification of Acute Pancreatitis. PLoS ONE. 2015;10:e0143486.
Lin Q, Ji YF, Chen Y, Sun H, Yang DD, Chen AL, et al. Radiomics model of contrast-enhanced MRI for early prediction of acute pancreatitis severity. J Magn Reson Imaging. 2020;51:397–406.
Qiu Q, Nian YJ, Tang L, Guo Y, Wen LZ, Wang B, et al. Artificial neural networks accurately predict intra-abdominal infection in moderately severe and severe acute pancreatitis. J Dig Dis. 2019;20:486–94.
Xu F, Chen X, Li C, Liu J, Qiu Q, He M, et al. Prediction of multiple organ failure complicated by moderately severe or severe Acute Pancreatitis based on machine learning: a Multicenter Cohort Study. Mediators Inflamm. 2021;2021:5525118.
Fei Y, Hu J, Gao K, Tu J, Li WQ, Wang W. Predicting risk for portal vein thrombosis in acute pancreatitis patients: a comparison of radical basis function artificial neural network and logistic regression models. J Crit Care. 2017;39:115–23.
Ding N, Guo C, Li C, Zhou Y, Chai X. An Artificial neural networks Model for Early Predicting In-Hospital mortality in Acute Pancreatitis in MIMIC-III. Biomed Res Int. 2021;2021:6638919.
Mofidi R, Duff MD, Madhavan KK, Garden OJ, Parks RW. Identification of severe acute pancreatitis using an artificial neural network. Surgery. 2007;141:59–66.
Chen Y, Chen TW, Wu CQ, Lin Q, Hu R, Xie CL, et al. Radiomics model of contrast-enhanced computed tomography for predicting the recurrence of acute pancreatitis. Eur Radiol. 2019;29:4408–17.
Mashayekhi R, Parekh VS, Faghih M, Singh VK, Jacobs MA, Zaheer A. Radiomic features of the pancreas on CT imaging accurately differentiate functional abdominal pain, recurrent acute pancreatitis, and chronic pancreatitis. Eur J Radiol. 2020;123:108778.
Lan L, Guo Q, Zhang Z, Zhao W, Yang X, Lu H, et al. Classification of infected necrotizing pancreatitis for surgery within or beyond 4 weeks using machine learning. Front Bioeng Biotechnol. 2020;8:541.
Luo J, Lan L, Peng L, Li M, Zhou X. Predicting timing of Surgical intervention using recurrent neural network for Necrotizing Pancreatitis. IEEE Access. 2020;8:207905–13.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. Medical Image Computing and Computer-assisted Intervention–MICCAI 2015. Springer Int Publishing. 2015;2015:234–41.
Roth HR, Shen C, Oda H, Oda M, Hayashi Y, Misawa K, et al. Deep learning and its application to medical image segmentation. Med Imaging Technol. 2018;36:63–71.
Wu S, Xu J, Tai YW, Tang CK. Deep high dynamic range imaging with large foreground motions. Proceedings of the European Conference on Computer Vision (ECCV). 2017;2018:117–132.
Ansari MY, Yang Y, Balakrishnan S, Abinahed J, Al-Ansari A, Warfa M, Almokdad O, et al. A lightweight neural network with multiscale feature enhancement for liver CT segmentation. Sci Rep. 2022;12(1):14153.
Han Z, Jian M, Wang GG, ConvUNeXt. An efficient convolution neural network for medical image segmentation. Knowledge-based systems. 2022.
Xie Y, Zhang J, Shen C, Xia Y. Cotr: efficiently bridging cnn and transformer for 3d medical image segmentation. 2021.
Ansari MY, Yang Y, Meher PK, Dakua SP. Dense-PSP-UNet: a neural network for fast inference liver ultrasound segmentation. Comput Biol Med. 2023;153:106478.
Jafari M, Auer D, Francis S, Garibaldi J, Chen X. DRU-net: an efficient deep convolutional neural network for Medical Image Segmentation. IEEE. 2020.
Ansari MY, Abdalla A, Ansari MY, Ansari MI, Malluhi B, Mohanty S, et al. Practical utility of liver segmentation methods in clinical surgeries and interventions. BMC Med Imaging. 2022;22(1):97.
Ansari MY, Qaraqe M, Righetti R, Serpedin E, Qaraqe K. Unveiling the future of breast cancer assessment: a critical review on generative adversarial networks in elastography ultrasound. Front Oncol. 2023;13:1282536.
Ansari MY, Mangalote IAC, Meher PK, Meher PK, Aboumarzouk O, Al-Ansari A et al. Advancements in Deep Learning for B-Mode Ultrasound Segmentation: a Comprehensive Review. IEEE Transactions on Emerging Topics in Computational Intelligence 8.
Du Y, Yang R, Chen Z, Wang L, Weng X, Liu X. A deep learning network-assisted bladder tumour recognition under cystoscopy based on Caffe deep learning framework and EasyDL platform. Int J Med Robot. 2021;17:1–8.
Haight TJ, Eshaghi A. Deep Learning algorithms for Brain Imaging: from Black Box to Clinical Toolbox. Neurology. 2023;100:549–50.
Khan AA, Ibad H, Ahmed KS, Hoodbhoy Z, Shamim SM. Deep learning applications in neuro-oncology. Surg Neurol Int. 2021;12:435.
Sarker IH. Deep learning: a comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci. 2021;2:420.
Lundberg SM, Lee SI. A unified approach to interpreting model predictions. 31st Conference on Neural Information Processing Systems. 2017.
Meglič J, Sunoqrot MRS, Bathen TF, Elschot M. Label-set impact on deep learning-based prostate segmentation on MRI. Insights Imaging. 2023;14:157.
Li Y, Chen Q, Li H, Wang S, Chen N, Han T, et al. MFNet: Meta-learning based on frequency-space mix for MRI segmentation in nasopharyngeal carcinoma. J Cell Mol Med. 2024;28(9):e18355.
Xu Z, Dai Y, Liu F, Wu B, Chen W, Shi L. Swin MoCo: improving parotid gland MRI segmentation using contrastive learning. Med Phys. 2024 May 15.
Wang L, Luo Z, Ni J, Li Y, Chen L, Guan S, et al. Application of U-Net network in automatic image segmentation of adenoid and airway of nasopharynx. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023;37(8):632–636641.
Dzieniszewska A, Garbat P, Piramidowicz R. Improving skin lesion segmentation with self-training. Cancers (Basel). 2024;16(6):1120.
Zhu S, Fang X, Qian Y, He K, Wu M, Zheng B, et al. Pterygium Screening and Lesion Area Segmentation based on deep learning. J Healthc Eng. 2022;2022:3942110.
Wang X, Girshick R, Gupta A, He K. Non-local neural networks. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2018: 7794–7803.
Raghu M, Unterthiner T, Kornblith S, Zhang C. Do vision transformers seem like convolutional neural networks. Adv Neural Inf Process Syst. 2021;34:12116–28.
Li Z, Zhang Z, Zhao H, Wang R, Chen K, Utiyama M, et al. Text Compression-aided transformer encoding. IEEE Trans Pattern Anal Mach Intell. 2022;44:3840–57.
Poudel S, Lee SW. Deep multi-scale attentional features for medical image segmentation. Appl Soft Comput. 2021;109:107445.
Dakua PS. Towards left ventricle segmentation from magnetic resonance images. IEEE Sens J, 2017:1–1.
Singh AV, Chandrasekar V, Laux P, Luch A, Dakua SP, Zamboni P, et al. Micropatterned neurovascular interface to mimic the blood-brain barrier’s neurophysiology and micromechanical function: a BBB-on-CHIP model. Cells. 2022;11(18):2801.
Chandrasekar V, Singh AV, Maharjan RS, Dakua SP, Balakrishnan S, Dash S, et al. Perspectives on the Technological aspects and Biomedical Applications of Virus-Like Particles/Nanoparticles in Reproductive Biology: insights on the Medicinal and Toxicological Outlook. Adv NanoBiomed Res. 2022;2(8):19.
Akhtar Y, Dakua SP, Abdalla A, Aboumarzouk OM, Ansari MY, Abinahed J, et al. Risk assessment of computer-aided diagnostic software for hepatic resection. IEEE Trans Radiation Plasma Med Sci. 2021;PP(99):1–1.
Acknowledgements
We would like to thank Dr. Charles Damien Lu of Los Angeles for English language editing.
Funding
This work was supported by funding from the Sichuan Science and Technology Program (Grant ID: 2021YFS0378) to Hua Jiang.
Author information
Authors and Affiliations
Contributions
CZ, JP and HJ designed the study. CZ collected the date. CZ and YW performed the statistical analysis. CZ, JP, and LW were involved in model training and data analysis. CZ, HJ, M-WS and WC were involved in drafting or revising the intellectual content of the manuscript. Grant proposal and funding acquisition was HJ. CZ and HJ contributed to Supervision and mentoring. All authors read and approved the final manuscript and take responsibility for its publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this retrospective study was approved by the Medical Ethics Committee of Sichuan Provincial People’s Hospital, with consent information waived (approval number: Ethical Review (Research) No. 99 of 2022)Consent for publication.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Peng, J., Wang, L. et al. A deep learning-powered diagnostic model for acute pancreatitis. BMC Med Imaging 24, 154 (2024). https://doi.org/10.1186/s12880-024-01339-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-024-01339-9