Abstract
Background
Preoperative identification of rectal cancer lymph node status is crucial for patient prognosis and treatment decisions. Rectal magnetic resonance imaging (MRI) plays an essential role in the preoperative staging of rectal cancer, but its ability to predict lymph node metastasis (LNM) is insufficient. This study explored the value of histogram features of primary lesions on multi-parametric MRI for predicting LNM of stage T3 rectal carcinoma.
Methods
We retrospectively analyzed 175 patients with stage T3 rectal cancer who underwent preoperative MRI, including diffusion-weighted imaging (DWI) before surgery. 62 patients were included in the LNM group, and 113 patients were included in the non-LNM group. Texture features were calculated from histograms derived from T2 weighted imaging (T2WI), DWI, ADC, and T2 maps. Stepwise logistic regression analysis was used to screen independent predictors of LNM from clinical features, imaging features, and histogram features. Predictive performance was evaluated by receiver operating characteristic (ROC) curve analysis. Finally, a nomogram was established for predicting the risk of LNM.
Results
The clinical, imaging and histogram features were analyzed by stepwise logistic regression. Preoperative carbohydrate antigen 199 level (p = 0.009), MRN stage (p < 0.001), T2WIKurtosis (p = 0.010), DWIMode (p = 0.038), DWICV (p = 0.038), and T2-mapP5 (p = 0.007) were independent predictors of LNM. These factors were combined to form the best predictive model. The model reached an area under the ROC curve (AUC) of 0.860, with a sensitivity of 72.8% and a specificity of 85.5%.
Conclusion
The histogram features on multi-parametric MRI of the primary tumor in rectal cancer were related to LN status, which is helpful for improving the ability to predict LNM of stage T3 rectal cancer.
Similar content being viewed by others
Background
The treatment of locally advanced rectal cancer includes neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME). LNM is an important prognostic factor for local recurrence and distant metastasis [1]. The European Society for Medical Oncology (ESMO) guidelines recommend that low-risk T3N0 patients do not need preoperative nCRT and only need TME. For most patients with T3N1-2 cancer, preoperative nCRT is necessary, achieving tumor degradation and reducing the risk of postoperative recurrence [2]. Therefore, the preoperative identification of lymph node status in patients with T3 rectal cancer plays an important role in guiding treatment decision-making. At present, high-resolution MRI is the standard method for evaluating LNM of rectal cancer. However, the accuracy of using MRI morphological criteria such as diameter, shape, boundary, and signal heterogeneity to assess the status of lymph nodes is unsatisfactory [3,4,5].
Texture analysis can reflect tumor heterogeneity and assist doctors in diagnosis by extracting many quantitative features from the region of interest of medical images [6]. Features extracted from medical images can be defined as first-order, second-order, or higher-order features [7]. Texture analysis based on first-order statistics of tumor images is called histogram analysis, which only reflects the distribution of voxel gray intensity and has no spatial information or relationship between voxels [8, 9]. Histogram analysis based on medical images has been proved to be helpful for early non-invasive identification of tumor heterogeneity, prediction of LNM, evaluation of curative effect, and prognosis of the patients [10,11,12,13].
However, the few articles about lymph node prediction in rectal cancer mainly focus on single sequence image analysis but do not give full play to the characteristics of multi-parametric MRI, and the diagnostic ability needs to be improved. Therefore, this study takes stage T3 rectal cancer as an example to explore the predictive ability of multi-parametric MRI features in lymph node diagnosis to better guide the choice of treatment for patients with stage T3 rectal cancer.
Methods
Patients
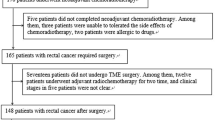
This retrospective study was approved by the Ethics Review Committee of Harbin Medical University Cancer Hospital and the need for signed informed consent was waived. We included 710 patients with rectal cancer who underwent total mesorectal resection from January 2015 to May 2017 and obtained preoperative MRI images and postoperative pathological reports. Case inclusion criteria were as follows: (1) Patients with rectal cancer confirmed by histopathology as stage T3Nx; and (2) Patients who underwent multi-parametric MRI within two weeks before the operation. Case exclusion criteria were as follows: (1) Patients with particular histological types confirmed by a histopathologist, such as mucinous adenocarcinoma or neuroendocrine tumor (n = 41); (2) Patients who received treatment such as nCRT before MRI or surgery (n = 414); (3) Patients who underwent MRI without DWI (n = 50); and (4) Patients with poor image quality (n = 30) (Fig. 1). Finally, we conducted a retrospective study of 175 patients. Clinical data, including patients’ age, height, weight, history of smoking and alcohol consumption were obtained by reviewing the medical records. Tumor marker information was obtained from laboratory-based tests in each patient. We assessed the expression status of carcinoma embryonic antigen (CEA), carbohydrate antigen (CA) 199, CA724, and α-fetoprotein (AFP), according to practical guidelines for the use of tumor markers [14].
High resolution rectal MRI parameters
All patients underwent rectal MRI with a 3.0 T MR scanner (Achieva; Philips, Eindhoven, Netherlands), with a 16-channel torso array coil. The MRI scan sequence included T2-weighted imaging (T2WI) sequence (sagittal, short axial, and coronal position), and diffusion weighted imaging sequence (DWI) (b = 0/1000). The MRI scanning parameters were: TR/TE = 3000 ms/100 ms; number of signal frequency (NSA) = 2; layer thickness = 3 mm; layer spacing = 0.4 mm; field of view (FOV) = 240 × 240 mm. Sagittal T2WI images determined the location of the lesion, and the short axis T2WI of the lesion was located perpendicular to the long axis of the intestinal canal (TR = 3000–4000 ms; TE = 110 ms; NSA = 3; layer thickness = 3.5 mm; layer spacing = 0.2 mm). According to the location of the sagittal lesion, the coronal T2WI was located on the long axis of the parallel lesion (TR = 3824 ms; TE = 110 ms; NSA = 3; layer thickness = 3.0 mm; layer spacing = 0.2 mm).
Analysis of radiological features
MR images of all patients were collected by a RIS/PACS (GE Healthcare centrality, USA) system. Two abdominal radiologists (R1 and R2, with 5 and 8 years of experience in MRI diagnosis of rectal cancer) determined the stage of the patient's rectal cancer. The subjective evaluation information of patients with rectal lesions was recorded on T2WI images, including the following: (1) Lesion thickness and length: measured at the largest T2WI level. (2) Lesion location: categorized as low (0–5 cm from the anal verge), middle (5.1–10 cm from the anal verge), and high (10.1–15 cm from the anal verge); (3) mrT3 stage: classified into four categories depending on the distance between the outermost edge of the muscularis propria and the maximum extramural spread of the tumor (T3a, < 1 mm; T3b, 1–5 mm; T3c, 5–15 mm; and T4d, > 15 mm) [15]; (4) mrN stage: doctors observed and classified the size and morphological characteristics of visible lymph nodes on T2WI; the criteria for a malignant node: (1) Short axis diameter ≥ 9 mm; (2) Short axis diameter 5–8 mm and ≥ 2 morphologically suspicious characteristics; (3) Short axis diameter < 5 mm and 3 morphologically suspicious characteristics. Morphologically suspicious criteria were: round shape, irregular border, and heterogeneous signal [4]. The number of suspected lymph nodes in the region was evaluated according to the above criteria; no suspicious lymph nodes was mrN0; 1–3 suspected lymph nodes was mrN1; 4 or more suspected lymph nodes was mrN2 [16]. (5) Extramural vascular invasion (EMVI): EMVI is an extension of the tumor to the vessels in the mesorectum, resulting in wall irregularity, focal enlargement, and signal intensity (SI) of the tumor within the vessel [15]. (6) Mesorectal fascia (MRF): the shortest distance between the primary tumor of rectal cancer, suspected lymph nodes or tumor deposit, and the MRF was measured. If the measured value was < 1 mm, it was evaluated as MRF positive [15]. If there was a difference in the radiologist's opinion in the above characteristic assessment, a solution was determined through consultation.
Histogram features extraction
All images were stored in DICOM format and input into Matlab software. Another radiologist (R3, with 3 years of experience in MRI diagnosis of rectal cancer) used Matlab software (MathWorks, Natick, MA, USA) to identify the tumor and select the region of interest (ROI), and a senior doctor (R1) supervised this. The radiologist (R3) only knew that the focus was rectal cancer and ignored the clinical and pathological features. The T2WI-ROI and DWI-ROI were obtained by delineating ROI directly along the margin of the whole tumor on the maximum cross-sections of T2W images and DW images (high b value) on the axial plane, excluding the intraluminal gas, and the obvious necrotic and cystic areas. On the basis of T2WI-ROIs we obtained T2WI texture features. Then we mapped the DWI-ROIs to T2WI and the ADC map, respectively, obtained the T2-map-ROI and ADC-ROI, and extracted texture features from these ROIs. Finally, four groups of histogram features of the T2WI-ROI, DWI-ROI, T2-map-ROI and ADC-ROI were obtained from each patient.
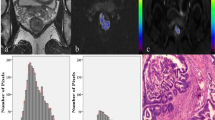
The histogram of signal strength distribution was then generated in the ROI, including mean, median, maximum, minimum, standard deviation, coefficient of variation (CV), kurtosis, skewness, and mode (Fig. 2).
An example of manual segmentation of MRI in the primary tumor of rectal cancer. a T2W maximum cross-sectional view, manually sketched T2WI-ROI (green intra-line region) and its histogram of primary tumors in patients with stage T3 rectal cancer. b The same slice DWI image from the same patient, the manually sketched DWI-ROI (red intra-line area), and its histogram. c The T2-map-ROI (red mosaic region) and its histogram of T2-map were obtained by mapping DWI ROI to T2WI. d The ADC-ROI (magenta mosaic region) and its histogram of the ADC diagram were obtained by mapping DWI ROI to the ADC graph
Histopathological evaluation
Histopathological assessment is the gold standard for LN malignancy. The pathological report of surgically resected specimens contained a standard data set based on the 8th edition of the American Joint Commission on Cancer (AJCC) TNM staging system. Tumor differentiation, depth of invasion, and pathological N stage (pN) were obtained retrospectively. According to the pathological data, the patients were divided into the positive and negative lymph node groups.
Statistical analysis
Medcalc (version 11.4, Medcalc, Belgium), SPSS (version 24.0 IBM, NY, USA), and R software (version 4.0.3, The R Project) were used for statistical analysis. Medcalc was used to draw the receiver operating characteristic (ROC) curve of all histogram features, and the value corresponding to the maximum Youden index was the best diagnostic cutoff value. These values were used to transform histogram features into classification variables. Statistical analysis of the clinical, doctor's diagnosis and histogram characteristics was carried out using SPSS software. The continuous variables were compared by the independent sample t-test or Mann Whitney U test, and the classified variables were compared by the chi-square test. The statistically significant characteristics were analyzed by univariate and multivariate logistic regression analysis to determine the independent predictors of LNM. ROC curve analysis was used to determine the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of the combined model and the independent predictive factor model. p < 0.05 was considered statistically significant. The nomogram was drawn by R software. In addition, the calibration plots were obtained by internal verification of the model by the bootstrap method. Finally, the clinical decision curve was drawn.
Results
Patient characteristics
One hundred and seventy-five patients with stage T3 rectal cancer confirmed by postoperative pathology were enrolled in the study. The clinical and pathological data on lymph nodes in the non-LNM group (n = 113) and LNM group (n = 62) are shown in Table 1. Except for the level of CA199 (p = 0.007), there was no significant difference in clinical characteristics between the two groups.
Radiological findings
The imaging features in the lymph node positive and lymph node negative groups are shown in Table 2. There were significant differences in mrN stage (p < 0.001) and MRF (p = 0.008) between the two groups, but there was no significant difference in tumor thickness (p = 0.255), length (p = 0.306), tumor location (p = 0.325), extent of invasion (p = 0.997), mrT stage (p = 0.212) and EMVI (p = 0.089) between the two groups.
Risk factors for LNM
All the quantitative histogram features were used to draw a ROC curve to obtain cutoff values (see Additional files 1–4) on the basis of LN status. According to the cutoff values, we divided each group into the higher than cutoff value cohort and lower than cutoff value cohort. In these cohorts, DWISkewness, DWIMedian, DWICV, DWIP95, DWIMode, ADCKurtosis, ADCCV, ADCP5, ADCMode, T2WIKurtosis, T2WICV, and T2-mapP5 had statistical significance in the evaluation of LNM in rectal cancer. Multivariate logistic regression analysis showed that CA199 level (odds ratio (OR) = 0.104; p = 0.009), mrN (OR = 0.124; p < 0.001), T2WIKurtosis (OR = 4.101; p = 0.010), T2mapP5 (OR = 0.267; p = 0.007), DWICV (OR = 10.135; p = 0.038), and DWIMode (OR = 7.744; p = 0.038) were independent predictors of LNM, while other covariates were not independent predictors (Table 3, Fig. 3).
Comparison of diagnostic efficiency of different models
The diagnostic performance of independent predictors and the combined model were analyzed, respectively, and the results showed that the combined model had the highest AUC value (0.860) (Table 4, Fig. 4). The clinical-imaging-histogram nomogram has good discrimination (Fig. 5). The calibration plots also showed good accordance between the nomogram prediction and the actual outcome for non-LNM and LNM in this data set (Fig. 6a). The decision analysis curve showed satisfactory positive net benefits of the nomogram on most of the threshold probabilities, indicating a favorable potential clinical effect of the model (Fig. 6b).
a The calibration curves for predicting LNM. The Y axis represents the actual rate of LNM. The X axis represents the predicted probability of LNM. The ideal line represents a perfect prediction by an ideal model. The apparent line represents the performance of the nomogram model, of which a closer fit to the ideal line represents a better prediction. b The decision curve analysis for the morphological-histogram nomogram. The red line represents the net benefit of the morphological-histogram model. Across the various threshold probabilities, the morphological-histogram curve showed great net benefit
Discussion
In this study, we analyzed the histogram features of primary rectal cancer on multi-parametric magnetic resonance images, combined with clinical information and imaging features, and compared the accuracy of doctor diagnosis and the combined model to predict lymph node status; then, based on the combined model, a clinical-imaging-histogram nomogram was constructed. The results showed that imaging features combined with clinical risk factors and medical imaging histogram features have a high value in predicting and diagnosing LMN.
In most cases, the probability of LNM in stage T3 rectal cancer was higher than that in stage T1-2, accounting for 46% [17, 18]. However, there may be differences in prognosis due to different treatment strategies [19]. Therefore, the 2020 NCCN guidelines recommend that the choice of treatment for stage T3 rectal cancer should refer to N stage, and preoperative nCRT should be performed according to the presence or absence of LNM [16]. MRI is the first choice for TNM staging of rectal cancer. Although the diagnostic accuracy of T staging of rectal cancer has reached 88–99% [20], the on-going LNM evaluation accuracy is still less than 80% [21]. 25% of lymph nodes are likely to be over-staged, which will lead to unnecessary preoperative overtreatment, and the possible short-term (such as proctitis) and long-term (such as fecal incontinence, intestinal and genitourinary dysfunction) complications will aggravate the damage caused by the tumor in these patients [22]. Therefore, a definitive diagnosis of lymph node status is a key problem for clinicians in making an appropriate treatment plan for stage T3 patients. In this study, the diagnostic efficiency of radiology features was the same as previous studies, with an accuracy of 71%. The main reason for the deviation in diagnosis may be the diameter of lymph nodes. When the lymph node is smaller than 3 mm, it is difficult to be detected by MRI, which leads to the specificity being 28%, but when it is larger than 9 mm, the sensitivity is 41% [23]. To avoid the above factors, this study evaluated primary tumor lesions to predict the status of lymph nodes and acquired satisfactory results.
In recent years, the application of histograms in the prediction of LNM of primary focus is mainly based on T2WI images. Some studies have shown that histological grade and LNM are positively correlated with kurtosis [24,25,26]. However, in this study, unlike previous studies, the higher the T2WI kurtosis, the less metastatic, in accordance with the results of Yang et al [8], which demonstrated that the low skewness, kurtosis, energy, and high entropy of T2WI were more meaningful for LNM. We speculated that the results might relate to stage T3 cancer, of which 95.5% were moderately differentiated (Table 1), without differences in differentiation degree. Besides kurtosis, T2-map P5 was associated with LNM in this study. Kurtosis and T2-map P5 were probably related to the fibrous components which contained less water, leading to low T2WI SI and heterogeneity.
Based on previous research on T2WI images, we further incorporated DWI and ADC histogram features into the analysis of primary rectal cancer to predict LNM, which obtained better predictive ability than simple T2WI diagnosis. Each sequence had some features associated with LNM (additional files). In multivariate logistic analysis, there were still four features from three sequences which were independent predictors. They all indicated that primary tumor histogram features were related to LNM. The results showed that the lower the DWI, the higher the ADC SI and the larger the CV, and the less probability of LNM. Thus, our fibrosis hypothesis was further confirmed. At the same histopathological grades, a larger CV and lower tumor cellular density may indicate more fibrosis surrounding the lesion. The existence of fibrosis may contract the blood vessels and lymphatic vessels, restrict cancer cells escaping from the primary focus, and avoid further spread [27].
Finally in association with the doctor's diagnosis, we integrated the laboratory results and the histogram features of the above-mentioned multi-parameter images to verify the influence of the quantitative features of the histogram on the clinical diagnosis. The AUC was 0.860, the sensitivity was 72.8%, and the specificity was 85.5%, which was better than those obtained in previous research [8, 28,29,30,31,32,33]. We suggest that clinicians should perform TME as soon as possible in patients with stage T3 rectal cancer with normal CA199, negative mrN diagnosis, DWICV >0.306, DWIMode >100 s/mm2, T2WIKurtosis >5.224, and T2-mapP5 ≤59s/mm2; to reduce treatment cost and to obtain a more significant survival benefit. On this basis, we propose a clinical-imaging-histogram nomogram, which can be easily used for individual prediction of preoperative LNM in patients with rectal cancer.
Our research has some limitations. First, the study was based on a retrospective analysis carried out in a single center, and there may be biases in patient selection. Secondly, our research only analyzed the histogram features on MRI, and other texture features can also be studied, such as the gray-level co-occurrence matrix. Finally, due to the small sample size, the universal applicability of the nomogram we developed may be limited. Studies with larger samples and multi-agency involvement are required to verify our research results.
Conclusions
This study shows that the histogram characteristics of primary rectal cancer on multi-parametric MRI sequences are significantly correlated with lymph node status. The histogram quantitative parameters of MRI can increase the accuracy of lymph node evaluation. The clinical-imaging-histogram nomogram can be used as a novel simple scoring system to predict the risk of LNM.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
01 March 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12880-022-00758-w
Abbreviations
- MRI:
-
Magnetic resonance imaging
- LNM:
-
Lymph node metastasis
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- nCRT:
-
Neoadjuvant chemoradiotherapy
- TME:
-
Total mesorectal excision
- ESMO:
-
European Society for Medical Oncology
- T2WI:
-
T2 weighted imaging
- DWI:
-
Diffusion-weighted imaging
- NSA:
-
Number of signal frequency
- FOV:
-
The field of view
- EMVI:
-
Extramural vascular invasion
- MRF:
-
Mesorectal fascia
- AJCC:
-
American Joint Commission on Cancer
- SI:
-
Signal intensity
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- CV:
-
Coefficient of variation
- MRF:
-
Mesorectal fascia
- EMVI:
-
Extramural vascular invasion
- CEA:
-
Carcinoma embryonic antigen
References
Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29(23):3163–72.
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, Arnold D, Committee EG. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–40.
Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(3):355–64.
Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75.
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging–a meta-analysis. Radiology. 2004;232(3):773–83.
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics. 2017;37(5):1483–503.
Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–9.
Yang L, Liu D, Fang X, Wang Z, Xing Y, Ma L, Wu B. Rectal cancer: can T2WI histogram of the primary tumor help predict the existence of LNM? Eur Radiol. 2019;29(12):6469–76.
Wang F, Wang Y, Zhou Y, Liu C, Liang D, Xie L, Yao Z, Liu J. Apparent diffusion coefficient histogram analysis for assessing tumor staging and detection of LNM in epithelial ovarian cancer: correlation with p53 and Ki-67 expression. Mol Imaging Biol. 2019;21(4):731–9.
Giesel FL, Schneider F, Kratochwil C, Rath D, Moltz J, Holland-Letz T, Kauczor HU, Schwartz LH, Haberkorn U, Flechsig P. Correlation between suvmax and CT radiomic analysis using lymph node density in PET/CT-based lymph node staging. J Nucl Med. 2017;58(2):282–7.
Eriguchi D, Shimada Y, Imai K, Furumoto H, Okano T, Masuno R, Matsubayashi J, Kajiwara N, Ohira T, Ikeda N. Predictive accuracy of lepidic growth subtypes in early-stage adenocarcinoma of the lung by quantitative CT histogram and FDG-PET. Lung Cancer. 2018;125:14–21.
Ceriani L, Milan L, Martelli M, Ferreri AJM, Cascione L, Zinzani PL, Di Rocco A, Conconi A, Stathis A, Cavalli F, et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood. 2018;132(2):179–86.
Farjam R, Tsien CI, Feng FY, Gomez-Hassan D, Hayman JA, Lawrence TS, Cao Y. Investigation of the diffusion abnormality index as a new imaging biomarker for early assessment of brain tumor response to radiation therapy. Neuro Oncol. 2014;16(1):131–9.
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11-79.
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019;39(2):367–87.
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D. Garrido-Laguna I et al NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw. 2020;18(7):806–15.
Landmann RG, Wong WD, Hoepfl J, Shia J, Guillem JG, Temple LK, Paty PB, Weiser MR. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50(10):1520–5.
Guillem JG, Diaz-Gonzalez JA, Minsky BD, Valentini V, Jeong SY, Rodriguez-Bigas MA, Coco C, Leon R, Hernandez-Lizoain JL, Aristu JJ, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26(3):368–73.
Zhao F, Wang J, Yu H, Cheng X, Li X, Zhu X, Xu X, Lin J, Chen X, Yan S. Neoadjuvant radiotherapy improves overall survival for T3/4N+M0 rectal cancer patients: a population-based study of 20300 patients. Radiat Oncol. 2020;15(1):49.
Akasu T, Iinuma G, Takawa M, Yamamoto S, Muramatsu Y, Moriyama N. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol. 2009;16(10):2787–94.
Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(7):2212–23.
Al-Sukhni E, Messenger DE, Charles Victor J, McLeod RS, Kennedy ED. Do MRI reports contain adequate preoperative staging information for end users to make appropriate treatment decisions for rectal cancer? Ann Surg Oncol. 2013;20(4):1148–55.
Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52(1):78–83.
Cui Y, Yang X, Du X, Zhuo Z, Xin L, Cheng X. Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: correlation with clinical pathologic prognostic factors. Eur Radiol. 2018;28(4):1485–94.
Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, Zhan Y, Chen K, Shen K, Yan F. Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology. 2015;277(1):46–55.
Wang Q, Li H, Yan X, Wu CJ, Liu XS, Shi HB, Zhang YD. Histogram analysis of diffusion kurtosis magnetic resonance imaging in differentiation of pathologic Gleason grade of prostate cancer. Urol Oncol. 2015;33(8):337 e315-324.
Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29(6):1797–810.
Zhao L, Liang M, Shi Z, Xie L, Zhang H, Zhao X. Preoperative volumetric synthetic magnetic resonance imaging of the primary tumor for a more accurate prediction of LNM in rectal cancer. Quant Imaging Med Surg. 2021;11(5):1805–16.
Yu J, Dai X, Zou HH, Song JC, Li Y, Shi HB, Xu Q, Shen H. Diffusion kurtosis imaging in identifying the malignancy of lymph nodes during the primary staging of rectal cancer. Colorectal Dis. 2018;20(2):116–25.
Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, Beets-Tan RG. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23(12):3354–60.
Zhang H, Zhang C, Zheng Z, Ye F, Liu Y, Zou S, Zhou C. Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol. 2017;27(9):3845–55.
Li J, Zhou Y, Wang X, Yu Y, Zhou X, Luan K. Histogram analysis of diffusion-weighted magnetic resonance imaging as a biomarker to predict lymph node metastasis in T3 stage rectal carcinoma. Cancer Manag Res. 2021;13:2983–93.
Song L, Yin J. Application of texture analysis based on sagittal fat-suppression and oblique axial T2-weighted magnetic resonance imaging to identify lymph node invasion status of rectal cancer. Front Oncol. 2020;10:1364.
Acknowledgements
We are thankful to the data providers for sharing their precious resources.
Funding
2020 SKY Imaging Research Fund of the Chinese International Medical Foundation (Project No. Z-2014-07-2003-07); Funding for postdoctoral research of Heilongjiang Provincial Government (LBH-Z18140); Harbin Medical University Cancer Hospital Haiyan Funds (No. JJZD2020-17); Harbin Medical University Cancer Hospital Haiyan Funds (No. JJQN2021-07).
Author information
Authors and Affiliations
Contributions
Study concepts: CHZ, YZ, RY. Study design: XXW, CHZ, YZ, RY. Data acquisition: YZ, RY, YW. Quality control of data: MZ, YW, ZY, XYZ, JQX. Data analysis and interpretation: CHZ, XXW, YZ, RY, YW. Statistical analysis: ZY, RY, YW, MZ, XYZ, JQX. Manuscript preparation: CHZ, YZ, XXW, RY. Manuscript editing and reviewing: CHZ, XXW, YZ, RY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Board of Harbin Medical University. The requirement of informed consent from the patients was waived because of the retrospective design of this study, and patients’ information was protected. And the study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised to modify the article title.
Supplementary Information
Additional file 1.
Comparison of T2WI histogram parameters between the LNM and non-LNM groups.
Additional file 2.
Comparison of DWI histogram parameters between the LNM and non-LNM groups.
Additional file 3.
Comparison of ADC histogram parameters between the LNM and non-LNM groups.
Additional file 4.
Comparison of T2-map histogram parameters between the LNM and non-LNM groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Yang, R., Wang, Y. et al. Histogram analysis based on multi-parameter MR imaging as a biomarker to predict lymph node metastasis in T3 stage rectal cancer. BMC Med Imaging 21, 176 (2021). https://doi.org/10.1186/s12880-021-00706-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-021-00706-0