Abstract
Background
Risk scores facilitate the assessment of mortality risk in patients with community-acquired pneumonia (CAP). Despite their utilities, there is a scarcity of evidence comparing the various RS simultaneously. This study aims to evaluate and compare multiple risk scores reported in the literature for predicting 30-day mortality in adult patients with CAP.
Methods
A retrospective cohort study on patients diagnosed with CAP was conducted across two hospitals in Colombia. The areas under receiver operating characteristic curves (ROC-curves) were calculated for the outcome of survival or death at 30 days using the scores obtained for each of the analyzed questionnaires.
Results
A total of 7454 potentially eligible patients were included, with 4350 in the final analysis, of whom 15.2% (662/4350) died within 30 days. The average age was 65.4 years (SD: 21.31), and 59.5% (2563/4350) were male. Chronic kidney disease was 3.7% (9.2% vs. 5.5%; p < 0.001) (OR: 1.85) higher in subjects who died compared to those who survived. Among the patients who died, 33.2% (220/662) presented septic shock compared to 7.3% (271/3688) of the patients who survived (p < 0.001). The best performances at 30 days were shown by the following scores: PSI, SMART-COP and CURB 65 scores with the areas under ROC-curves of 0.83 (95% CI: 0.8–0.85), 0.75 (95% CI: 0.66–0.83), and 0.73 (95% CI: 0.71–0.76), respectively. The RS with the lowest performance was SIRS with the area under ROC-curve of 0.53 (95% CI: 0.51–0.56).
Conclusion
The PSI, SMART-COP and CURB 65, demonstrated the best diagnostic performances for predicting 30-day mortality in patients diagnosed with CAP. The burden of comorbidities and complications associated with CAP was higher in patients who died.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Community-acquired pneumonia (CAP) is the leading infectious cause of death worldwide, with a global incidence ranging from 1 to 14 cases per 1,000 individuals annually, resulting in approximately 2.5 million deaths each year [1, 2]. Hospitalization is required for 22–42% of patients, with 10–14% admitted to the Intensive Care Unit (ICU). Mortality rates range from 10 to 12% in hospitalized patients and can reach up to 35% for those requiring ICU care within 30 days [1, 3]. Early recognition of patients at high risk for complications and mortality, using clinical judgment and various risk scoring systems, is recommended by numerous scientific associations and experts [3, 4]. The initial clinical assessment of CAP is crucial for determining the extent of disease involvement, establishing the appropriate site for in-hospital treatment, and estimating the short- and long-term mortality risk [5,6,7].
The risk scores (RS) most used to predict 30-day mortality in CAP are the Pulmonary Severity Index (PSI) and the CURB-65 (confusion, blood urea nitrogen > 7 mmol/L, respiratory rate ≥ 30/min, systolic blood pression < 90 mmHg or Diastolic Blood Pression ≤ 60 mmHg, age ≥ 65 years) [7,8,9]. Kaal et al. [10] described that the use of the CURB-65 as a severity assessment tool in patients with CAP in the emergency department was associated with a lower risk of 30-day mortality compared to the PSI (OR: 0.89; 95% CI: 0.83–0.96, p = 0.003). Mortality risk in CAP patients has been validated and compared with various other scores [11]. For instance, the Pneumonia Shock Score demonstrated the area under receiver operating characteristic curve (ROC-curve) for 30-day mortality of 0.739 (95% CI: 0.709–0.769, p < 0.001) [12]. Similarly, the ADROP score achieved the area under ROC-curve of 0.846 (95% CI: 0.790–0.903) [11, 12]. However, none of these scores exhibited excellent performances.
Numerous studies have compared the diagnostic performances of up to four or five questionnaires in predicting 30-day mortality in patients with CAP. However, more than ten different scales are currently available for this purpose. This gap in evidence has led to inconsistencies between clinical practice guidelines and expert consensus regarding the best tool for estimating short-term mortality risk [3, 13, 14]. Therefore, the aim of this study was to compare 16 different severity questionnaires, including various clinical variables and radiological findings, to predict 30-day mortality in adult patients with CAP.
Methods
Study design
A retrospective cohort study on patients diagnosed with CAP was conducted across two hospitals in Colombia. Patients were assessed and admitted to emergency room and ICU from January 2010 to December 2019.
Inclusion criteria
Subjects aged 18 years or older, regardless of gender, who had received a diagnosis of CAP in accordance with the guidelines of the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) [2, 14, 15], were included in the study. Patients were required to present the following: respiratory symptoms (including cough, purulent sputum, and dyspnea), systemic involvement (fever and altered consciousness), radiographic findings suggestive of pneumonia, observed in either chest X-rays or chest computed tomography scan (alveolar and/or interstitial opacities, the presence of pulmonary consolidations, and unilateral or bilateral pleural effusion).
Furthermore, the medical records were required to contain sufficient information to calculate the following scores: CURB-65, CRB-65, SCAP, SOAR, CORB, ADROP, NEWS, Pneumonia Shock, REA-ICU, PSI, SMART-COP, SMRT-CO, SRIS, qSOFA, CAPSI and Charlson Comorbidity Index. The variables for each questionnaire are described in Supplementary File 1. Patients with incomplete medical records, those who developed nosocomial pneumonia, individuals with decompensated heart failure, pulmonary fibrosis, and pulmonary embolism were excluded from this study.
Variables
The study included the following variables: sociodemographic variables (age and sex), vital signs, level of consciousness, comorbidities, radiographic finding, arterial blood gas analysis, pulse oximetry, white blood cell count, blood glucose level, blood urea nitrogen, serum albumin, serum sodium, need for ICU admission, invasive mechanical ventilation (IMV) and/or vasopressor support. The dependent variable was survival at 30 days following the diagnosis of CAP. To minimize potential errors in classifying the studied outcomes, the research team obtaining information from clinical records had medical experience for diagnosing CAP. To reduce the risk of data entry errors, at least two team members reviewed the information during the transcription process into the database.
Sample size
To calculate the sample size, data from the study by Lim et al. [16] which reported a sensitivity of 75% and specificity of 69% for CURB-65, and data from España et al. [17] which reported a sensitivity of 92.1% and specificity of 73.8% for SCAP, were used. Using the formula for sample size for paired diagnostic tests, with an expected mortality rate of 14%, a power of 90%, and a statistical significance level of 0.05, a minimum of 2168 subjects were required.
Statistical analysis
The data was obtained directly from medical records and subsequently transcribed into the Research Electronic Data Capture (REDCap) software [18, 19]. Then it was analyzed using licensed SPSS 25 software (IBM Corp. IBM SPSS Statistics for Windows. Version 25.0). Qualitative variables were reported in percentages and frequencies, while quantitative variables were summarized as mean and standard deviation (SD) for those with a normal distribution and median and interquartile range for those with a non-normal distribution. Bivariate analysis was conducted using the chi-square test for qualitative variables and the T-student test or Mann-Whitney U test for quantitative variables, depending on their distribution. Sensitivity, specificity, the areas under ROC-curves, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), and negative likelihood ratio (LR-) were calculated for the outcome of survival or death at 30 days using the scores obtained from all the analyzed questionnaires.
The established cutoff point for each questionnaire, as detailed in Supplementary Table 2, was used. The calculated the areas under ROC-curves were compared using the DeLong test. The interpretation of the areas under ROC-curves were as follows: 0.50 indicated an absence of discriminatory capacity; 0.51 to 0.60 indicated nearly no discriminatory capacity; 0.61 to 0.69 indicated poor discriminatory capacity; >0.7 to 0.8 indicated acceptable discriminatory capacity; >0.80 to 0.90 indicated excellent discriminatory capacity, and > 0.90 indicated outstanding discriminatory capacity [20, 21]. Statistical significance was considered when the p-value was < 0.05.
Missing data
An imputation analysis addressed missing data, employing weighted mean imputation for quantitative variables and logistic regression for qualitative variables with a loss of less than 10% [20]. Variables with more than 10% data loss were excluded. A comparison between non-imputed and imputed results ensured that imputation did not introduce bias or significantly alter the original data.
Results
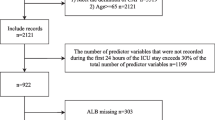
In total, there were 7454 potentially eligible patients, and 4350 entered the final analysis, of which 15.2% (662/4350) died within 30 days Fig. 1. The average age was 65.4 years (SD: 21.31), and 59.5% (2563/4350) were male Table 1. Altered consciousness was present in 33.1% of patients who died compared to 10.1% (371/3688) of patients who survived. The prevalence of systemic hypertension and chronic obstructive pulmonary disease was 12.4% (46.1% vs. 58.5%; p < 0.001) and 3.7% (18.1% vs. 21.8%; p < 0.001) lower in the group of living patients compared to those who died. respectively. Chronic kidney disease was 3.7% (9.2% vs. 5.5%; p < 0.001) higher in subjects who died compared to those who survived.
Laboratory tests
The oxygen arterial pressure/fraction of inspired oxygen in the patients who survived was 233 (SD: 69.7), compared to 203.8 (SD: 88.5) in the patients who died (p < 0.001) Table 2. The pH was 7.38 in the deceased patient’s group versus 7.42 in the surviving group (OR: 4.02 CI: 95%. p < 0.001). Likewise, lactate was 1 unit higher (3 vs. 2; p < 0.001) in the deceased subjects compared to the survivors. Creatinine and blood urea nitrogen were 0.2 (1.3 vs. 1.5; p = 0.041) and 13.2 (23.3 vs. 36.5; OR: 3.92 CI: 95%; p < 0.001) lower in the subjects who survived compared to the deceased group.
In-hospital treatment and complications
Among the patients who died, 33.2% (220/662) presented septic shock compared to 7.3% (271/3688) of the patients who survived (p < 0.001) Table 3. The use of systemic corticosteroids and vasopressor support was 32.8% (217/662) and 33.2% (159/662), respectively, in the mortality group. The ICU requirement was 25.4% (168/662) in deceased patients versus 11.2% (414/3688) in living patients (p < 0.001). Of the patients who deceased, 24% (159/662) required invasive mechanical ventilation, whereas 7.2% (266/3688) of the subjects who survived needed (p < 0.001).
Performances of RS for 30-day mortality prediction
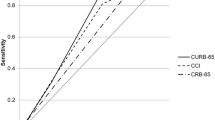
The best performances at 30 days were observed in the PSI, SMART-COP, and CURB 65 scores with the areas under ROC-curves of 0.83 (95% CI: 0.8–0.85), 0.75 (95% CI: 0.66–0.83), and 0.73 (95% CI: 0.71–0.76), respectively, Table 4. Scores with lower performances were SIRS and SOAR with the areas under ROC-curves of 0.53 (95% CI: 0.51–0.56) and 0.65 (95% CI: 0.62–0.68), respectively. The DeLong’s test was significant with a result minor than 0.05.
Discussion
The analysis revealed that PSI, SMART-COP, and CURB 65 emerged as the top-performing predictors, while SIRS and SOAR demonstrated comparatively lower efficacy. PSI exhibited excellent performance in forecasting 30-day mortality, and SMART-COP and CURB 65 demonstrated acceptable accuracy. Noteworthy factors associated with fatal outcomes included advanced age, the presence of comorbidities (specifically cardiovascular, respiratory, and tobacco smoking-related), altered consciousness, acidosis, kidney dysfunction, reliance on vasopressor support, and admission to the ICU.
The PSI risk score has demonstrated greater discriminatory capacity due to variables such as age and comorbidities, which have a significant correlation with increased mortality in individuals over 75 years old with chronic kidney disease, lung neoplasms, and cerebrovascular disease [7, 22,23,24,25]. Zaki et al. [22] mention that the PSI identifies patients who can be treated on an outpatient basis, but it may overestimate severity, especially in young patients with severe respiratory failure and no comorbidities. Our data show that the PSI has greater discriminatory capacity for predicting short-term mortality due to the variables that structure it, which may support the published medical evidence.
Marti et al. [23] performed a meta-analysis to determine the performances of RS in pneumonia prognosis. They concluded new severity scores such as SMART-COP had better discriminative performance in predicting intensive respiratory or vasopressor support, as previously mentioned fatal outcomes, compared to PSI and CURB-65. Also, Saldías et al. [24] determined the performances of RS in predicting 30-day mortality with an area under ROC-curve of 0.77 (95% CI: 0.74–0.81) for SMART-COP. Consistent with our data, the performance of SMART-COP was also acceptable for predicting mortality compared to questionnaires such as PSI, CURB-65, SCAP, among others.
Despite its recognized role as a predictor of mortality [26,27,28,29], none of the examined RS in our study incorporated mechanical ventilation as a variable. Our findings underscored that the need for mechanical ventilation emerged as a significant risk factor for mortality, mirroring the observations made in a cohort of 3719 patients studied by Cilloniz et al. [30]. Mermiri et al. [31] highlighted the association of vasopressor use with increased 30-day mortality (RR: 2.97; 95% CI: 1.72–5.14; p < 0.001) and a heightened risk of acute kidney injury (RR: 3.17: 95% CI: 2.21–4.54; p < 0.001). In our investigation, only one RS identified vasopressor use as a factor linked to elevated mortality. It is noteworthy that a majority of existing RS omit vasopressor support and mechanical ventilation as variables impacting mortality, potentially limiting the predictive accuracy of severity assessments. Hence, the incorporation of these two variables into prevailing questionnaires could enhance their efficacy in predicting 30-day mortality in patients with CAP.
In the context of pneumonia, the interplay with pH is intricately tied to shifts in the body’s acid-base equilibrium. The severity of pneumonia, notably in complex cases featuring respiratory failure, can precipitate respiratory acidosis. Hypoxemia, acidosis, and cognitive disorientation serve as indicators of tissue hypoperfusion and correlate with elevated mortality rates in CAP patients [32]. Acidosis in CAP patients aligns with altered consciousness [25], identified as a risk factor for 30-day mortality in those with comorbidities [33], particularly within the initial 5 days of hospitalization [32, 33]. Addressing these acid-base imbalances holds pivotal importance in pneumonia management, especially in severe cases impacting respiratory function. Notably, risk assessment tools with enhanced discriminatory capabilities, such as SMART-COP and PSI, incorporate acidosis (pH less than 7.35) as a variable in their evaluation.
Altered consciousness is correlated with acidosis, azotemia, acute kidney injury, and chronic kidney disease [7, 26], all of which increase fatal outcomes in patients with CAP [34]. In a cohort of 1474 patients with CAP, Fernández et al. [35] demonstrated that in patients over 80 years old, 49% of those who died had altered consciousness (OR: 4.92 CI: 95%; p < 0.001). Zhang et al. showed that in the population over 85 years old who had altered consciousness, mortality increased 3.3 to 6.1 times, respectively [28]. Chronic kidney disease has been described as an independent factor for early mortality in CAP. One possible explanation for this finding is the immunosuppressive state that advanced chronic kidney disease can generate. In this scenario, there is immune deficiency caused by a decrease in dendritic cells and T and B lymphocytes, as well as a proinflammatory state [36, 37].
Limitations
Among the limitations of our study is its observational nature, with information obtained from clinical records, which may have omissions. Furthermore, we did not include a characterization of the microbiological etiology of CAP, or the treatments received by the patients. However, measures were implemented to minimize information bias, such as continuous training of personnel responsible for data collection and constructing the manuscript according to the STROBE checklist Supplementary Table 4. We believe that the achieved sample size supports the conclusions drawn. Additionally, the similarity of our population characteristics to those in other series and the use of ATS/IDSA [2, 14, 15] criteria help reduce the variability or differences with the cohorts described in other observational studies.
Conclusion
The PSI, SMART-COP and CURB 65 demonstrated the best diagnostic performances in predicting 30-day mortality in patients diagnosed with CAP. Older age, the presence of comorbidities, altered consciousness, acidosis, renal dysfunction, vasopressor support, and admission to the ICU were more prevalent in the group of deceased patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gadsby NJ, Musher DM. The Microbial etiology of community-acquired pneumonia in adults: from classical bacteriology to Host Transcriptional Signatures. Clin Microbiol Rev. 2022;35(4):e0001522.
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67.
Nair GB, Niederman MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther. 2021;217:107663.
File TM Jr, Ramirez JA. Community-Acquired Pneumonia. N Engl J Med. 2023;389(7):632–41.
Khari S, Salimi Akin Abadi A, Pazokian M, Yousefifard M. CURB-65, qSOFA, and SIRS Criteria in Predicting In-Hospital mortality of critically ill COVID-19 patients; a Prognostic Accuracy Study. Arch Acad Emerg Med. 2022;10(1):e36.
Pletz MW, Blasi F, Chalmers JD, Dela Cruz CS, Feldman C, Luna CM, et al. International Perspective on the New 2019 American Thoracic Society/Infectious Diseases Society of America Community-Acquired Pneumonia Guideline: a critical Appraisal by A Global Expert Panel. Chest. 2020;158(5):1912–8.
Bradley J, Sbaih N, Chandler TR, Furmanek S, Ramirez JA, Cavallazzi R. Pneumonia Severity Index and CURB-65 score are good predictors of mortality in hospitalized patients with SARS-CoV-2 Community-Acquired Pneumonia. Chest. 2022;161(4):927–36.
Barlas RS, Clark AB, Loke YK, Kwok CS, Angus DC, Uranga A, et al. Comparison of the prognostic performance of the CURB-65 and a modified version of the pneumonia severity index designed to identify high-risk patients using the International Community-Acquired Pneumonia collaboration cohort. Respir Med. 2022;200:106884.
Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65(10):878–83.
Kaal AG, Op de Hoek L, Hochheimer DT, Brouwers C, Wiersinga WJ, Snijders D, et al. Outcomes of community-acquired pneumonia using the Pneumonia Severity Index versus the CURB-65 in routine practice of emergency departments. ERJ Open Res. 2023;9(3):00051–2023.
Alirio B-G, Carolina A, Alexandra ADBDSE, Eduardo T-Q, Gómez C, et al. Comparison of the performance of the CURB-65, A-DROP, and NEWS scores for the prediction of clinical outcomes in Pneumonia. Infect Dis Clin Pract. 2023;31(3):e1240.
Carmo TA, Ferreira IB, Menezes RC, Telles GP, Otero ML, Arriaga MB, et al. Derivation and validation of a Novel Severity Scoring System for Pneumonia at Intensive Care Unit Admission. Clin Infect Dis. 2021;72(6):942–9.
Limapichat T, Supavajana S. Comparison between the Severity Scoring systems A-DROP and CURB-65 for Predicting Safe Discharge from the Emergency Department in patients with community-acquired pneumonia. Emerg Med Int. 2022;2022:6391141.
Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023;49(6):615–32.
Barberán J, Restrepo R, Cardinal-Fernández P. Community-acquired pneumonia: similarities and differences between European and American guidelines - a narrative review. Rev Esp Quimioter. 2021;34(2):72–80.
Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82.
España PP, Capelastegui A, Gorordo I, Esteban C, Oribe M, Ortega M, Bilbao A, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–56.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208.
Hosmer DW, Lemeshow S, Sturdivant RX. Special topics. In: Hosmer DW, Lemeshow S, Sturdivant RX, editors. Applied Logistic Regression. 3rd ed. New York. NY: John Wiley & Sons. Inc; 2013. pp. 401–8.
Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75(1):25–36.
Zaki HA, Hamdi Alkahlout B, Shaban E, Mohamed EH, Basharat K, Elsayed WAE, et al. The battle of the Pneumonia predictors: a Comprehensive Meta-Analysis comparing the Pneumonia Severity Index (PSI) and the CURB-65 score in Predicting Mortality and the need for ICU support. Cureus. 2023;15(7):e42672.
Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16(4):R141.
Saldías Peñafiel Fernando. Uribe Monasterio Javier. Gassmann Poniachik Javiera. Canelo López Alejandro. Díaz Patiño Orlando. Evaluación De Los índices predictores de eventos adversos en El adulto inmunocompetente hospitalizado por neumonía adquirida en la comunidad. Rev méd Chile. 2017;145(6):694–702.
Satici C, Demirkol MA, Sargin Altunok E, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–9.
McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202(6):812–21.
Hespanhol V, Bárbara C. Pneumonia mortality. Comorbidities Matter? Pulmonol. 2020;26(3):123–9.
Zhang ZX, Yong Y, Tan WC, Shen L, Ng HS, Fong KY. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singap Med J. 2018;59(4):190–8.
Reyes LF, Bastidas Goyes A, Tuta Quintero EA, Pedreros KD, Mantilla YF, Herrera M, et al. Validity of the ROX index in predicting invasive mechanical ventilation requirement in pneumonia. BMJ Open Respir Res. 2022;9(1):e001320.
Ferrer M, Travierso C, Cilloniz C, Gabarrus A, Ranzani OT, Polverino E, et al. Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE. 2018;13(1):e0191721.
Mermiri M, Mavrovounis G, Laou E, Papagiannakis N, Pantazopoulos I, Chalkias A. Association of vasopressors with mortality in critically ill patients with COVID-19: a systematic review and meta-analysis. APS. 2023;1(2):10.
Saleem N, Kulkarni A, Snow TAC, Ambler G, Singer M, Arulkumaran N. Effect of corticosteroids on Mortality and Clinical Cure in Community-Acquired Pneumonia: a systematic review. Meta-analysis. And Meta-regression of Randomized Control trials. Chest. 2023;163(3):484–97.
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Goto Y, Fukui Y, et al. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect Dis. 2015;15(9):1055–65.
Alfano G, Fontana F, Mori G, Giaroni F, Ferrari A, Giovanella S, et al. Modena Covid-19 Working Group (MoCo19). Acid base disorders in patients with COVID-19. Int Urol Nephrol. 2022;54(2):405–10.
Fernández-Sabé N, Carratalà J, Rosón B, Dorca J, Verdaguer R, Manresa F. el al Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159 – 69.
Al-Azzam N, Khassawneh B, Al-Azzam S, Karasneh RA, Aldeyab MA. Acid-base imbalance as a risk factor for mortality among COVID-19 hospitalized patients. Biosci Rep. 2023;43(3):BSR20222362.
Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–35.
Acknowledgements
The authors are most thankful for the Universidad de La Sabana.
Funding
This work was supported by Universidad de la Sabana Grant MED-326-2022.
Author information
Authors and Affiliations
Contributions
ETQ, ARB, GGG, MCM, DT, CS, JC, GB, DC, PR, YF, EG, DA, SR, DA, and LFRcontributed substantially to the study design, data analysis and interpretation, and manuscript writing. ETQ, ABG, GGG and DT had full access to all study data and takes responsibility for data integrity as well as for accuracy of the included data analysis and, especially, any adverse effects. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the current Helsinki Declaration, as well as local, regional, and international regulations pertaining to clinical research, including Colombian Law on Biomedical Research. Ethical approval was obtained from the Medical Ethics Committee of the Clínica Universidad de La Sabana (approval number 20220102). The confidentiality of their data was strictly maintained throughout the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tuta-Quintero, E., Goyes, A.R.B., Guerrón-Gómez, G. et al. Comparison of performances between risk scores for predicting mortality at 30 days in patients with community acquired pneumonia. BMC Infect Dis 24, 912 (2024). https://doi.org/10.1186/s12879-024-09792-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09792-1