Abstract
Background
There are few reports of longitudinal serologic responses in children following Sars-CoV-2 infection and vaccination. This study describes longitudinal SARS-CoV-2 antibody responses following infection, vaccination, or both (hybrid immunity) in a cohort of Canadian children. The objectives of our study were to compare antibody levels following SARS-CoV-2 infection, vaccination, and hybrid immunity and to examine antibody decline after final antigen exposure.
Methods
The Alberta Childhood COVID-19 Cohort (AB3C) study was a prospective longitudinal cohort study conducted from July 2020 to September 2022 with repeat sampling across 5 visits. Children under 18 years of age were enrolled for serial measurement of antibody responses to SARS-CoV-2 virus vaccine and infection.
Results
The final sample size was 919; participants were 50.5% female, 48.2% were > 12 years and 88.5% were white ethnicity. The median peak spike IgG level of those with only infection was not different from those with no vaccination or infection (233 AU/mL (IQR: 99–944 AU/mL) vs. 3 AU/mL (IQR: 1–5 AU/mL; P = 0.1765). Participants with infections after vaccination had higher IgG levels than those where infection preceded vaccination (median: 36,660 (IQR: 22,084 − 40,000 AU/mL) vs. 17,461 AU/mL (IQR: 10,617 − 33,212 AU/mL); P < 0.0001). In a linear mixed methods model, children with infection-only had low levels of antibody that stayed stable over the study duration without further antigen exposures. Those with infection after vaccination had the slowest rate of antibody decline over time at 4% (95%CI: 2-5%) per week, compared with children where infection preceded vaccine 7% (95%CI: 6-8%) per week.
Conclusions
Children with hybrid immunity conferred through vaccination (2 + doses) followed by a SARS-CoV-2 infection had the highest and longest lasting antibody levels, compared to children who had an infection followed by vaccination, vaccination-only, or infection-only. The longer-term clinical importance of these findings, related to prevention of repeated infections and severe outcomes and need for further vaccine doses, is not yet known.
Similar content being viewed by others
Background
The World Health Organization declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on March 11, 2020, which was followed by the rapid development and uptake of vaccines to protect against COVID-19 infections. The evolution of numerous variants of the original virus and very high levels of acquired infection, with or without vaccination, have made it increasingly complex to evaluate immune responses in persons with multiple exposures.
The main goal of vaccination against COVID-19 is the development of neutralising antibodies (nAbs) against the receptor binding domain of the spike protein in recipients [1]. These nAbs are strongly correlated with protection against severe COVID-19 [2, 3]. However, nAbs have been shown to wane over time, which may lead to reduced effectiveness in preventing infection and severe disease. This highlights the rationale for booster doses especially for high-risk populations [4]. Both infection and vaccination increase nAbs against the spike protein. The combination of immune responses to both infection and vaccination, often called “hybrid immunity,” results in higher levels and more gradual decay of antibodies over time compared to vaccine-only or infection-only induced immunity [5,6,7,8,9,10,11,12,13,14,15,16]. Hybrid immunity has been demonstrated to provide better protection against symptomatic and severe SARS-CoV-2 infection compared to infection-only and vaccine-only induced immunity [17,18,19].
Before the Omicron variant emerged, reported symptomatic infections were less common in children than in adults [20]; however, children more frequently have asymptomatic infections and milder disease [21,22,23,24,25,26]. Most studies examining seropositivity following vaccination and infection have focused on adults [9, 27,28,29,30,31]. Few studies have evaluated immune responses in cohorts of children on a longitudinal basis following vaccination, infection, and combinations of both, though some have looked at levels of antibodies in children mostly after acquired infection [32,33,34,35,36,37].
We conducted a longitudinal study of SARS-CoV-2 antibody levels in children over a two-year period during the COVID-19 pandemic in Calgary, Canada. During this time children experienced many different combinations of exposure to SARS-CoV-2 antigens through infection, vaccination, or both. We also examined the duration of antibody levels in children following their last antigen exposure.
Methods
Study recruitment
The Alberta Childhood COVID-19 Cohort (AB3C) enrolled 1035 children to attend up to five study visits between July 2020 and September 2022 for blood collection to measure nucleocapsid and spike IgG antibodies. Children with a previous diagnosis of COVID-19 were identified by Alberta Health Services (AHS) and invited to participate. Infection-naive children were recruited through a social media announcement. All participants were under 18 years of age at enrollment. The study received approval from the University of Calgary Conjoint Health Research Ethics Board (Ethics ID: REB20-0480). Detailed methods have been reported previously [38, 39].
At each study visit, parents, guardians, or participants (older children) completed an online survey to report on health history, demographic features, and vaccination with mRNA vaccines, with vaccinations later confirmed through the AHS vaccine registry.
Across visits there was variability in how many children completed blood draws due to missed visits and withdrawals. Participants who did not provide consent to access vaccination records and those whose self-reported vaccinations were discordant with the AHS records of vaccination were excluded as there was potential for misclassification. Results from participants who withdrew part way through the study were included up to the time of withdrawal.
Laboratory
Venous blood was collected and sent to the Alberta Precision Laboratories, Public Health Laboratory for processing. Nucleocapsid and spike IgG levels were determined using the Abbott Alinity chemiluminescent microparticle immunoassays to detect binding antibodies either to the nucleocapsid protein or spike protein receptor binding domain (RBD) of SARS-CoV-2 spike protein’s S1 subunit [40, 41]. The Abbott AdviseDx Architect SARS-CoV-2 IgG II immunoassay detects IgG against the spike RBD with a positive cut off of > 50 arbitrary units per millilitre (AU/mL) [40]. The AdviseDx IgG II test has high sensitivity and specificity for detecting IgG against the RBD [42]. This immunoassay has an upper limit of 40,000 AU/mL, therefore this is the maximum reported level of IgG for this study [40]. The Abbott SARS-CoV-2 IgG (or CoV-2 IgG) detects IgG against the nucleocapsid protein [41]. Nucleocapsid antibodies are reported as signal to cut off ratio (S/C) and samples with a value of 1.4 and greater were considered positive [41].
Definitions
Infection status was based on self-report of a laboratory conducted positive polymerase chain reaction (PCR) or rapid antigen test (RAT) test prior to or throughout the study. PCR results were confirmed through AHS records, but RAT results were based only on self-report. Those who reported an exposure and had serology results to suggest infection were also considered to have been infected. A newly positive nucleocapsid antibody test, and/or a newly positive spike IgG in an unvaccinated child, were assumed to indicate a new infection regardless of whether the participant was aware they had been infected.
All participants were included in one of 8 immune status groups defined by their SARS-CoV-2 infection and vaccination status. These included: no infection or vaccination, infection-only, vaccine only-1 dose, infection and 1 dose of vaccine, and vaccine only with 2 or more doses. There were also 3 hybrid immunity groups: infection followed by 2 vaccine doses (IVV), 2 or more vaccine doses followed by infection (VVI), and 1 vaccine dose followed by an infection and then another vaccine dose (VIV). Some children had additional exposures. Additional File 1 describes all the exposure profiles and how they were classified for the hybrid immunity groups.
Descriptive analysis
Demographic and clinical factors were tabulated as of the final visit for each participant including age, sex at birth, ethnicity, Indigenous status, comorbidities (asthma, immunosuppressive disorders, and other health conditions), SARS-CoV-2 infection status, vaccination status, and hybrid immunity status.
Peak spike IgG values were determined and children were classified into the immune group that reflects their immune status at the time of the peak spike measurement. The median spike IgG value of each immune status group was compared to all others using a Kruskal-Wallis test for ranked data with an alpha level < 0.05 considered significant. Dunn’s multiple comparison test was performed on each pair of groups to determine where significant differences occurred. Analysis was conducted using Prism 10 and STATA/SE version 18.0.
Analysis of antibody durability: Linear mixed effects Model
Last antigen exposure was defined as the date of the last confirmed SARS-CoV-2 infection or vaccine dose. The time since last antigen exposure was calculated in weeks. We compared the decline of spike IgG between the main immune response groups: infection-only, vaccine-only, IVV, and VVI. Throughout the study period, the immune status group of a participant could change, so each child could provide data to different immune status groups sequentially (e.g., vaccine only initially and then VVI at later visits). Data for the mixed-effects model was restricted to only include spike IgG values where sampling occurred when a participant was within the four main immune status groups (infection-only, vaccine-only, VVI, and IVV).
Data were excluded from children whose last antigen exposure was a new infection detected by newly positive nucleocapsid IgG or a newly positive spike IgG in an unvaccinated child. For these children the date of last antigen exposure could not be determined as infection was detected at blood draw. The analysis excluded children with no infection or vaccine (no antigen exposure), infection and 1 vaccine, and 1 vaccine only as well as the VIV group as these groups were too small to consider. Nucleocapsid-only infections that were followed by a vaccine were still included as the last exposure was the vaccine. Spike IgG values following reinfections or following a third dose of vaccine were also excluded as we wanted to look at decline over time since last exposure without other exposures increasing IgG levels.
A mixed-effects linear regression model was developed to examine change in trend for log spike antibody levels (AU/mL) since the last antigen exposure. The spike values were log transformed using a natural logarithm to improve normality of residuals and allow for simpler interpretation of slopes. The model allowed for both individual trend over time as well as an average trend over time. Models were adjusted for age group and sex at birth. Age was treated as a time-varying covariate, with individuals potentially moving between the following categories over time: <5 years, 5–12 years, and 12 + years. Sex at birth was included as a time constant covariate. Infection-only was the reference group for the model. A restricted model to compare VVI and IVV directly was also developed. The model was truncated at 40 weeks due to no data past 40 weeks for the VVI group.
Results
Population
There were 1035 children recruited into the AB3C study. We excluded children who did not consent to access vaccination records (n = 107) and children whose self-reported vaccinations were discordant with the AHS registry (n = 9) (Fig. 1). The final study population included 919 children. The number whose final blood draw was obtained during each of the 5 visit periods is noted in Fig. 1. The participants were 50.5% female, 48.2% were 12 years or older and 88.5% were white ethnicity (Table 1).
Infections and vaccinations
Throughout the study 563/919 children (61.3%) had a single SARS-CoV-2 infection; 75 (8.2%) had 2 infections, and 1 (0.15%) had 3 infections, for a total of 639 (69.5%) with 1 or more infections. Most first infections (398/563; 70.7%) occurred during the Omicron variant period after visit 3.
Although most infections were determined by positive PCR or RAT as well as serology indicating infection, 163 infections were determined by increase in nucleocapsid antibody at blood draw and 23 were based on an increase in spike antibody with no history of vaccination. None of these children reported symptomatic illnesses prior to serology testing indicating 186/639 infections (29.1%) were asymptomatic.
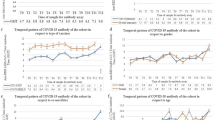
There was 1 hospital admission and 3 emergency department visits reported that were related to COVID-19 infections across all 5 study visits [38]. The proportion of children with spike IgG rose from 8.8% (94/908) tested at V1 to 94.6% (442/467) tested at V5 after infection, vaccination, or both. By study end, 85.5% (786/919) of all participants had received at least one dose of vaccine, and 79% (728/919) received 2 or more doses.
The proportion of children who received 2 or more doses of vaccine was 5/100 (5%) for children under 5 yearss, 262/404 (65%) for children 5–11 years, and 390/415 (94%) for children 12 + years.
Peak antibody levels by Immune Response groups
The mean, median, and range of peak spike antibody levels are shown for each of 8 immune status groups in Fig. 2. The median peak spike IgG level of those with infection-only, did not significantly differ from those without a history of vaccination or infection (233 AU/mL (IQR: 99–944 AU/mL) vs. 3 AU/mL (IQR: 1–5 AU/mL) (P = 0.1765)). The median IgG level of participants who had received 2 + doses of vaccine (20,581 AU/mL; IQR: 12,092 − 35,753 AU/mL) was significantly higher than those with infection-only (P < 0.0001), but significantly lower than those with hybrid immunity grouping VVI (36,660 AU/mL; IQR: 22,084 − 40,000 AU/mL) (P < 0.0001). There was no difference between those with 2 + vaccine doses and those with hybrid immunity group IVV (17,461 AU/mL; IQR: 10,617 − 33,212 AU/mL) (P > 0.9999). Participants with breakthrough hybrid immunity (VVI) had significantly higher median IgG levels than those with hybrid immunity where infection preceded vaccination (IVV) (P < 0.0001). Additional file 2 shows comparisons between the peak IgG levels for all immune groups.
Mixed-effects model
We excluded 180 children who were never exposed to antigens, received only one vaccine by study conclusion, were in the hybrid immunity VIV group, or did not have any spike values to contribute. We also excluded 81 cases as their last antigen exposures was indicated only by an increase in nucleocapsid or spike levels and so the infection date was not known. The final sample analyzed included 658 children, with 1283 spike IgG data points, including 1 to 5 data points per child (Figs. 3 and 4).
The infection-only group had lower levels of IgG and the level declined by 0.3% per week which was not significant (95%CI: -0.3-1%) (Fig. 4). IgG levels for vaccine-only (2 doses) started much higher than infection-only but declined over time by approximately 7% (95%CI: 6-8%) per week, IVV declined by 5% (95%CI: 4–6%) per week and VVI IgG levels declined at approximately 4% (95%CI: 2-5%) per week. With the model restricted to compare IVV and VVI, spike IgG for the VVI group declined at 4% (95%CI: 2-5%) per week, which was significantly slower than the IVV group at 7% (95%CI: 6-8%) per week. Although all groups except infection-only waned after the last antigen exposure, the IgG levels still remained well above the positivity threshold even up to 40 weeks post exposure. The model was adjusted for age and sex with the overall model including all children of all ages combined. Age-group specific analysis within the different immunity groups was not performed since the number of data points from children under 5 years with 2 or more doses of vaccine was very small (5/100, 5%).
Mixed-effects model showing duration of log antibody levels over time in weeks from last infection/vaccine exposure (N = 658 children, 1283 spike IgG measurements). The slope coefficients are indicated in the legend. The model was also adjusted for age and sex. * indicates a slope that is significantly different from zero. All slopes except infection-only were significant with P < 0.001
Discussion
This cohort of Canadian children and adolescents was followed longitudinally through the peak of the COVID-19 pandemic (summer 2020 to autumn 2022). At study conclusion, the participants were highly vaccinated and frequently infected mainly with mild or asymptomatic infections. The children demonstrated high levels of binding antibody, particularly after vaccination with or without infection (hybrid immunity). Those with hybrid immunity due to breakthrough infection after vaccination with at least 2 doses of mRNA vaccine (VVI) had the highest and longest lasting antibody levels, compared to children who had an infection followed by vaccination (IVV), vaccination-only, or infection-only. Numerous adult studies have focused on studying antibody responses towards COVID-19, but there have been fewer studies examining antibody response in children [43,44,45,46].
Hybrid immunity has been demonstrated to result in greater antibody response than vaccine or infection alone [16, 44, 47]. The order of these immunity generating events may also be relevant in the development of optimal immune response. Similar to our findings, adult studies found infection after vaccination had the highest median antibody levels, higher than the groups with vaccination after infection, as well as the vaccine-only group and infection-only group [16, 48]. In children, vaccine after infection also confers high levels of antibodies [49, 50].
In children, two or more mRNA vaccine doses led to high median IgG levels, as did vaccination preceded (IVV) or followed (VVI) by infection. The highest peak IgG response was in the VVI group and antibody decline over time was slowest in this group. Yamamoto et al. also found hybrid immunity to have the slowest waning of antibody levels over time but did not distinguish between infection prior to or following vaccination [51]. They demonstrated that 3 doses of vaccine waned more slowly than 2 doses, which was something we were unable to assess due to a limited number of measurements that occurred after a third vaccine dose [51]. Waning antibody levels are expected with time [8, 44, 52]. We observed considerable diversity in antibody waning to the end of the current study period, with some children maintaining spike IgG levels above 20,000 AU/mL up to 40 weeks after their last antigen exposure with no documented subsequent exposures. Further, we found antibody levels did not wane significantly in those with infection-only, although the levels were much lower to start. Another study also showed no significant waning of naturally acquired antibodies over 6–8 months in children [35]. We found spike IgG remained well above the positivity threshold even at 40 weeks after last antigen exposure which others have also found in adults [53] and children [37].
Measurement of serum IgG binding antibodies provides important information about the immune response to SARS-CoV-2 vaccines and infections but does not provide information about other humoral or the cell-mediated responses. One study suggested that children may mount a more robust innate immune response (as evidenced by higher levels of inflammatory cytokines) than adults and lower levels of neutralizing antibody after infection [54]. In addition, while our study described much higher antibody levels in vaccine recipients than those with infection only, it is unclear what additional protective benefit may have been provided. In this study of generally healthy children, there were very few SARS-CoV-2 infections that required emergency department visits or hospital admissions. Infection-only immunity may result in lower antibody levels but, with concurrent cell-mediated immunity, may still provide adequate protection against severe outcomes. A meta-analysis by Bobrovitz et al. showed that infection-only immunity provided good protection against hospitalization and severe disease but lower protection against reinfection and the protection waned over time [19]. Several studies have shown that hybrid immunity had the strongest and most durable protection against reinfection, severe disease, and hospitalization in adults [13, 17, 19], and reinfection in children [55].
We found 29% of children in this study had serology results indicating a recent infection without a symptomatic illness and without a positive RAT or PCR test. This may have been an underestimate of the frequency of asymptomatic infections as other children had nucleocapsid antibody increases that did not reach the threshold for positivity but were suggestive of infection. Two other studies reported approximately 44% of asymptomatic infections in children [33, 56]. Other studies have shown seropositivity to be similar in children and adults but with fewer symptoms in children, suggesting mild and asymptomatic infections in children often go undetected [21, 36, 57].
This study had some limitations. We examined binding antibody levels only, not neutralizing antibody levels or cellular immunity. However, binding antibody and neutralizing antibody measurements are highly correlated, and both can be used as correlates of protection against future infection and severe disease [58,59,60,61].
We previously reported that the AB3C study enrolled limited numbers of participants from lower income families; however, in our previous analysis income was not a significant factor in choice to vaccinate when other factors were considered [39]. Similarly, visible minorities and Indigenous groups were not fully represented in the study. In Alberta, the 2021 national census found that 6.8% of the population identified as Indigenous people. Within the study population 4.2% identified as Indigenous [62]. In the 2016 national census 33.7% of the population of Calgary identified as a visible minority with the most common being South Asian and Chinese [63]. Within the study population, 9.7% identified as a visible minority. Therefore, these results may not be completely generalizable to visible minorities or lower income children.
Due to the staggered age-specific rollout of COVID-19 vaccines in Canada, starting with adolescents and later including younger age groups, we could not conduct age group-specific examination of the effect of age on antibody levels. Nearly all children aged 12 + years were vaccinated with 2 or more doses of vaccine, whereas nearly all children under 5 years received no vaccine doses. For children 5–11 years, 65% received 2 or more vaccine doses. However, age (and sex) were included as covariates in the mixed effects model which provided results for all children combined. This enabled analysis of the effects of both vaccination and infection on antibody levels.
The time span of this study included the periods of multiple SARS-CoV-2 variants although most infections occurred later in the study during the Omicron variant period. Those first infected prior to vaccination most often had ancestral or Alpha variant infections and those first infected after vaccinations most often had Omicron variant infections. We cannot distinguish whether the difference in humoral response before or after vaccination is related to the variants causing infection, as well as the order of antigen exposures.
Children in this observational study had a multitude of exposures to SARS-CoV-2 infections and vaccines. Restricting the groups to comparable combinations of infection and/or vaccines within each group reduced the number of observations, but ensured each group was comprised of children with uniform exposures.
Conclusions
This longitudinal cohort of children followed through the COVID-19 pandemic were highly vaccinated and frequently infected, and demonstrated high levels of binding antibody, particularly after vaccination with or without infection. Those with hybrid immunity conferred through vaccination with at least 2 doses of mRNA vaccines followed by a SARS-CoV-2 infection had the highest and longest lasting antibody levels, compared to children who had an infection followed by vaccination, vaccination-only, or infection-only. The longer-term clinical relevance of these findings, related to prevention of repeated infections and severe outcomes, is not yet known.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Funk CD, Laferrière C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020;11:937.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11.
Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Kent SJ, Triccas JA, Khoury DS, Davenport MP. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–61.
Evans JP, Zeng C, Carlin C, Lozanski G, Saif LJ, Oltz EM, Gumina RJ, Liu SL. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14(637):eabn8057.
Crotty S. Hybrid Immunity. In: Science vol. 372: Science; 2021: 1392–1393.
Ebinger JE, Joung S, Liu Y, Wu M, Weber B, Claggett B, Botting PG, Sun N, Driver M, Kao YH, et al. Demographic and clinical characteristics associated with variations in antibody response to BNT162b2 COVID-19 vaccination among healthcare workers at an academic medical centre: a longitudinal cohort analysis. BMJ Open. 2022;12(5):e059994.
Tonnetti L, Dodd RY, Burke DD, Saá P, Spencer BR, Xu M, Haynes JM, Stramer SL. A longitudinal study of severe Acute Respiratory Syndrome Coronavirus 2 antibody response in a subset of United States blood donors. Open Forum Infect Dis. 2023;10(2):ofac697.
Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022;310(1):27–46.
Tauzin A, Nicolas A, Ding S, Benlarbi M, Medjahed H, Chatterjee D, Dionne K, Gong SY, Gendron-Lepage G, Bo Y, et al. Spike recognition and neutralization of SARS-CoV-2 Omicron subvariants elicited after the third dose of mRNA vaccine. Cell Rep. 2023;42(1):111998.
Siracusano G, Ruggiero A, Bisoffi Z, Piubelli C, Carbonare LD, Valenti MT, Mayora-Neto M, Temperton N, Lopalco L, Zipeto D. Different decay of antibody response and VOC sensitivity in naïve and previously infected subjects at 15 weeks following vaccination with BNT162b2. J Transl Med. 2022;20(1):22.
García-Pérez J, Bermejo M, Ramírez-García A, De La Torre-Tarazona HE, Cascajero A, Castillo de la Osa M, Jiménez P, Aparicio Gómez M, Calonge E, Sancho-López A, et al. Longer intervals between SARS-CoV-2 infection and mRNA-1273 doses improve the neutralization of different variants of concern. J Med Virol. 2023;95(3):e28679.
Chen Y, Tong P, Whiteman N, Sanjari Moghaddam A, Zarghami M, Zuiani A, Habibi S, Gautam A, Keerti, Bi C, et al. Immune recall improves antibody durability and breadth to SARS-CoV-2 variants. Sci Immunol. 2022;7(78):eabp8328.
Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, Wellington E, Khawam J, Munro K, Cole M, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–20.
Moghnieh R, El Hajj C, Abdallah D, Jbeily N, Bizri AR, Sayegh MH. Immunogenicity and effectiveness of primary and Booster Vaccine combination strategies during periods of SARS-CoV-2 Delta and Omicron variants. Vaccines (Basel) 2022, 10(10).
Pernet O, Balog S, Kawaguchi ES, Lam CN, Anthony P, Simon P, Kotha R, Sood N, Hu H, Kovacs A. Quantification of severe Acute Respiratory Syndrome Coronavirus 2 binding antibody levels to assess infection and Vaccine-Induced immunity using WHO standards. Microbiol Spectr. 2023;11(1):e0370922.
Epsi NJ, Richard SA, Lindholm DA, Mende K, Ganesan A, Huprikar N, Lalani T, Fries AC, Maves RC, Colombo RE, et al. Understanding hybrid immunity: comparison and predictors of Humoral Immune responses to severe Acute Respiratory Syndrome Coronavirus 2 infection (SARS-CoV-2) and Coronavirus Disease 2019 (COVID-19) vaccines. Clin Infect Dis. 2023;76(3):e439–49.
Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, Al-Khatib HA, Smatti MK, Coyle P, Al-Kanaani Z, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34.
Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–90.
Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, Selemon A, Whelan M, Premji Z, Issa H, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–67.
Mitchell R, Cayen J, Thampi N, Frenette C, Bartoszko J, Choi KB, Comeau JL, Conly J, Ellis C, Ellison J, et al. Trends in severe outcomes among Adult and Pediatric patients hospitalized with COVID-19 in the Canadian Nosocomial Infection Surveillance Program, March 2020 to May 2022. JAMA Netw Open. 2023;6(4):e239050–239050.
Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, Selvaraju S, Rade K, Kumar CPG, Sabarinathan R, Turuk A, et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int J Infect Dis. 2021;108:145–55.
Karron RA, Hetrich MK, Na YB, Knoll MD, Schappell E, Meece J, Hanson E, Tong S, Lee JS, Veguilla V, et al. Assessment of Clinical and virological characteristics of SARS-CoV-2 infection among children aged 0 to 4 years and their Household members. JAMA Netw Open. 2022;5(8):e2227348.
Spielberger BD, Goerne T, Geweniger A, Henneke P, Elling R. Intra-household and Close-Contact SARS-CoV-2 transmission among children - a systematic review. Front Pediatr. 2021;9:613292.
Wang JG, Zhong ZJ, Mo YF, Wang LC, Chen R. Epidemiological features of coronavirus disease 2019 in children: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(2):1146–57.
Xu T, Huang R, Zhu L, Wang J, Cheng J, Zhang B, Zhao H, Chen K, Shao H, Zhu C, et al. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J Med Virol. 2020;92(10):1884–9.
Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, Jaffe A, Homaira N. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. 2021;39(4):667–77.
Zheng Y, Pan J, Jin M, Wang J, Tung TH, Chen S, Bi X, Zhou K, Chen M, Wang D, et al. Efficacy of the neutralizing antibodies after the booster dose on SARS-CoV-2 Omicron variant and a two-year longitudinal antibody study on wild type convalescents. Int Immunopharmacol. 2023;119:110151.
Yalcin D, Bennett SJ, Sheehan J, Trauth AJ, Tso FY, West JT, Hagensee ME, Ramsay AJ, Wood C. Longitudinal variations in antibody responses against SARS-CoV-2 Spike epitopes upon serial vaccinations. Int J Mol Sci 2023, 24(8).
Underwood AP, Sølund C, Fernandez-Antunez C, Villadsen SL, Mikkelsen LS, Fahnøe U, Bollerup S, Winckelmann AA, Schneider UV, Binderup A, et al. Durability and breadth of neutralisation following multiple antigen exposures to SARS-CoV-2 infection and/or COVID-19 vaccination. EBioMedicine. 2023;89:104475.
Suarez Castillo M, Khaoua H, Courtejoie N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill 2022, 27(16).
Pradenas E, Marfil S, Urrea V, Trigueros M, Pidkova T, Pons-Grífols A, Ortiz R, Rovirosa C, Tarrés-Freixas F, Aguilar-Gurrieri C, et al. Impact of hybrid immunity booster vaccination and omicron breakthrough infection on SARS-CoV-2 VOCs cross-neutralization. iScience. 2023;26(4):106457.
Leone V, Meisinger C, Temizel S, Kling E, Gerstlauer M, Frühwald MC, Burkhardt K. Longitudinal change in SARS-CoV-2 seroprevalence in 3-to 16-year-old children: the Augsburg Plus study. PLoS ONE. 2022;17(8):e0272874.
Han MS, Um J, Lee EJ, Kim KM, Chang SH, Lee H, Kim YK, Choi YY, Cho EY, Kim DH, et al. Antibody responses to SARS-CoV-2 in Children with COVID-19. J Pediatr Infect Dis Soc. 2022;11(6):267–73.
Méndez-Echevarría A, Sainz T, Falces-Romero I, de Felipe B, Escolano L, Alcolea S, Pertiñez L, Neth O, Calvo C. Long-term persistence of Anti-SARS-CoV-2 antibodies in a Pediatric Population. Pathogens 2021, 10(6).
Bonfante F, Costenaro P, Cantarutti A, Di Chiara C, Bortolami A, Petrara MR, Carmona F, Pagliari M, Cosma C, Cozzani S et al. Mild SARS-CoV-2 infections and neutralizing antibody titers. Pediatrics 2021, 148(3).
Yang HS, Costa V, Racine-Brzostek SE, Acker KP, Yee J, Chen Z, Karbaschi M, Zuk R, Rand S, Sukhu A, et al. Association of Age with SARS-CoV-2 antibody response. JAMA Netw Open. 2021;4(3):e214302.
Raineri A, Radtke T, Rueegg S, Haile SR, Menges D, Ballouz T, Ulyte A, Fehr J, Cornejo DL, Pantaleo G, et al. Persistent humoral immune response in youth throughout the COVID-19 pandemic: prospective school-based cohort study. Nat Commun. 2023;14(1):7764.
Doucette EJ, Gray J, Fonseca K, Charlton C, Kanji JN, Tipples G, Kuhn S, Dunn J, Sayers P, Symonds N, et al. A longitudinal seroepidemiology study to evaluate antibody response to SARS-CoV-2 virus infection and vaccination in children in Calgary, Canada from July 2020 to April 2022: Alberta COVID-19 Childhood Cohort (AB3C) study. PLoS ONE. 2023;18(4):e0284046.
Doucette EJ, Ricketson L, Tarannum T, Alatorre I, Gray J, Constantinescu C, Kuhn S, Dunn JKE, Kellner JD. COVID-19 vaccine confidence, concerns, and uptake in children aged 5 and older in Calgary, Alberta: a longitudinal cohort study. Paediatrics & Child Health; 2023.
Abbott. AdviseDx SARS-CoV-2 IgG II ARCHITECT - Instructions for Use. In.; 2022.
Abbott. SARS-CoV-2 IgG ARCHITECT - Instructions for Use. In.; 2022.
Maine GN, Krishnan SM, Walewski K, Trueman J, Sykes E, Sun Q. Clinical and analytical evaluation of the Abbott AdviseDx quantitative SARS-CoV-2 IgG assay and comparison with two other serological tests. J Immunol Methods. 2022;503:113243.
Skowronski DM, Kaweski SE, Irvine MA, Kim S, Chuang ESY, Sabaiduc S, Fraser M, Reyes RC, Henry B, Levett PN, et al. Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. CMAJ. 2022;194(47):E1599–609.
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, Alroy-Preis S, Huppert A, Milo R. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–12.
Suntronwong N, Vichaiwattana P, Klinfueng S, Puenpa J, Kanokudom S, Assawakosri S, Chansaenroj J, Srimuan D, Thatsanatorn T, Songtaisarana S, et al. SARS-CoV-2 infection- induced seroprevalence among children and associated risk factors during the pre- and omicron-dominant wave, from January 2021 through December 2022, Thailand: a longitudinal study. PLoS ONE. 2023;18(4):e0279147.
Sharma P, Basu S, Mishra S, Singh MM. Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in children and adolescents in Delhi, India, from January to October 2021: a repeated cross-sectional analysis. Osong Public Health Res Perspect. 2022;13(3):184–90.
Bates TA, McBride SK, Leier HC, Guzman G, Lyski ZL, Schoen D, Winders B, Lee JY, Lee DX, Messer WB, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7(68):eabn8014.
Buckner CM, Kardava L, El Merhebi O, Narpala SR, Serebryannyy L, Lin BC, Wang W, Zhang X, Lopes de Assis F, Kelly SEM, et al. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell. 2022;185(23):4333–e43464314.
Li J, Li J, Dai S, Dang L, Wang L, Cao L, Chen X, Wang Y, Ge M, Liu W, et al. Pediatric population (aged 3–11 years) received primary inactivated SARS-CoV-2 vaccination prior to infection exhibiting robust humoral immune response following infected with Omicron variant: a study conducted in Beijing. Front Immunol. 2023;14:1269665.
Saraban K, Suntarattiwong P, Chantasrisawad N, Boonsathorn S, Kosalaraksa P, Phongsamart W, Tangsathapornpong A, Jaruampornpan P, Srisarang S, Puthanakit T. Hybrid immunity from SARS-CoV-2 infection and mRNA BNT162b2 vaccine among Thai school-aged children. Vaccine: X. 2023;15:100414.
Yamamoto S, Oshiro Y, Inamura N, Nemoto T, Horii K, Okudera K, Konishi M, Ozeki M, Mizoue T, Sugiyama H, et al. Durability and determinants of anti-SARS-CoV-2 spike antibodies following the second and third doses of mRNA COVID-19 vaccine. Clin Microbiol Infect. 2023;29(9):e12011201–5.
Bloise S, Marcellino A, Testa A, Dilillo A, Mallardo S, Isoldi S, Martucci V, Sanseviero MT, Del Giudice E, Iorfida D, et al. Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults. Eur J Pediatr. 2021;180(11):3335–42.
Srivastava K, Carreño JM, Gleason C, Monahan B, Singh G, Abbad A, Tcheou J, Raskin A, Kleiner G, van Bakel H, et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity. 2024;57(3):587–99.
Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, Garforth SJ, Herrera NG, Jangra RK, Morano NC et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 2020, 12(564).
Gazit S, Saciuk Y, Perez G, Peretz A, Ben-Tov A, Stuart EA, Patalon T. Hybrid immunity against reinfection with SARS-CoV-2 following a previous SARS-CoV-2 infection and single dose of the BNT162b2 vaccine in children and adolescents: a target trial emulation. Lancet Microbe. 2023;4(7):e495–505.
Messiah SE, Talebi Y, Swartz MD, Sabharwal R, Han H, Bergqvist E, Kohl HW 3rd, Valerio-Shewmaker M, DeSantis SM, Yaseen A et al. Long-term immune response to SARS-CoV-2 infection and vaccination in children and adolescents. Pediatr Res 2023.
Buonsenso D, Valentini P, De Rose C, Pata D, Sinatti D, Speziale D, Ricci R, Carfì A, Landi F, Ferrari V, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in children with household exposure to adults with COVID-19: preliminary findings. Pediatr Pulmonol. 2021;56(6):1374–7.
Gallais F, Gantner P, Bruel T, Velay A, Planas D, Wendling MJ, Bayer S, Solis M, Laugel E, Reix N, et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine. 2021;71:103561.
Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–30.
Goldblatt D, Fiore-Gartland A, Johnson M, Hunt A, Bengt C, Zavadska D, Snipe HD, Brown JS, Workman L, Zar HJ, et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40(2):306–15.
Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A Covid-19 milestone attained - A correlate of Protection for vaccines. N Engl J Med. 2022;387(24):2203–6.
2021 Census of Canada. Indigenous people [https://open.alberta.ca/publications/2021-census-of-canada-indigenous-people] Accessed on June 26, 2024.
2016 Census of Canada. Visible minorities [https://www.alberta.ca/census-canada-highlights] Accessed on June 26, 2024.
Acknowledgements
The authors gratefully thank all children and families who participated in the study and acknowledge LeeAnn Turnbull and the research staff at Alberta Precision Laboratories-Public Health Laboratory for completing serology testing. We acknowledge contributions by the ACHIEVE Research Team in sample collection (Anna Maria Ang-Becker, Isabelle Banks, Kyu Hwa Lim, Nathalie Uy, and Shairoz Lallany) and participant recruitment and study facilitation (Albert Michael, Candice Jay, Charisse Dominski, Conné Lategan, Julie-Anne Lemay, Nicole McMillan, Sardar (Shahzeb) Khan, Shannon Pyra, Taylor Cave, and Vivian Ly). We acknowledge non-author members of the Alberta Childhood COVID-19 Cohort Study Team for conceptualization of the seroepidemiology study: Susanne Benseler, Byron Berenger, Francois Bernier (AB3C Study Co-Principal Investigator).
Funding
This study was funded by the Government of Alberta and the Alberta Children’s Hospital Research Institute (ACHRI).
Author information
Authors and Affiliations
Contributions
EJD, IA, JG, and JDK were involved in acquisition of data. JG, GT, CC, JNK, KF, and JDK were responsible for conception and design of the study. LJR, EJD, IA, TT, and JDK performed the analysis and interpretation of data. LJR, EJD, IA, WB, and JDK drafted the manuscript. LJR, EJD, IA, TT, JG, WB, GT, CC, JNK, KF, and JDK gave critical revision of the manuscript for important intellectual content. JG and JDK obtained funding and accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and received approval from the University of Calgary Conjoint Health Research Ethics Board (REB20-0480). In accordance with the Tri-Council Policy Statement2 (TCPS2 2014), informed consent was obtained from the parents or legal guardians of all participating children, or directly from mature minors with decision-making capacity, prior to participation in the study.
Consent for publication
Not applicable.
Competing interests
JDK has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, and Pfizer, outside the submitted work. All funds have been paid to his institution, and he has not received any personal payments. He has been an unpaid member of the Canadian COVID-19 Immunity Task Force (Leadership Group member, Field Studies Working Party Co-Chair and Pediatric Network Lead), and of the Alberta Advisory Committee on Immunizations. All other authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12879_2024_9615_MOESM2_ESM.docx
Additional file 2: Comparison of median peak IgG levels between different immune response groups using Kruskal-Wallis Test with Dunn’s Multiple Comparison test
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ricketson, L.J., Doucette, E.J., Alatorre, I. et al. Pediatric antibody responses to SARS-CoV-2 after infection and vaccination in Calgary, Canada. BMC Infect Dis 24, 705 (2024). https://doi.org/10.1186/s12879-024-09615-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09615-3