Abstract
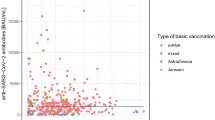
The first two vaccines administered in the COVID-19 vaccination campaign of India were Covaxin (BBV152) and Covishield (ChAdOx1-nCoV-19). In this study, we evaluate the longevity and sustainability of the humoral immune response after vaccination and various factors influencing it. An observational study was conducted in individuals who received both doses of Covaxin or Covishield vaccine, and their blood samples were analyzed for total-antiRBD-SARS-CoV-2 antibodies. Then, antibody titers were classified based on monthly time-intervals up to 360 days and their trend was analyzed. In addition, the correlation between antibody titers and factors such as previous SARS-CoV-2-infection status, vaccine type and presence of comorbidities was examined. Of the 2069 participants, most (1767;85.4%) had been vaccinated with Covaxin, but the higher antibody titers were induced by Covishield vaccine at all time points. However overall, antibodies persisted for at least 1 year, although a drop in antibody titers occurred in the 3rd and 6th months. In addition, 430 (20.8%) participants had prior SARS-CoV-2 infection (hybrid immunity) with a significantly higher humoral immune response compared with vaccine-induced immunity (naive immunity). No significant differences were observed in antibody titers related to age, sex and presence of comorbidities. We concluded that vaccine-mediated immunity lasts for at least one year. However, antibody titers decrease over time, which may be more pronounced in certain groups such as Covaxin vaccine, vaccine-induced-immunity, presence of comorbidities and > 60 years which should be considered when recommending booster vaccination, as these individuals may have a stronger and longer-lasting immune response to the virus.

Similar content being viewed by others
References
World Health Organization. India rolls out the world’s largest COVID-19 vaccination drive. WHO 2021. Available online at: https://www.who.int/india/news/feature-stories/detail/india-rolls-out-the-world-s-largest-covid19-vaccination-drive [Accessed 5 August 2023].
World Health Organization. Background document on the Bharat Biotech BBV152 COVAXIN® (COVID-19) vaccine. Available online at: WHO/2019-nCoV/vaccines/SAGE_recommendation/BBV152/background/2021.1 [Accessed 5 August 2023].
MOHFW. SOPs on administration of second dose of Covishield vaccine prior to prescribed time interval (after 28 days but before 84 days) to persons intending to undertake international travel for education purpose, for joining employment in foreign countries and for India’s contingent to Tokyo Olympics. Available online at:https://www.mohfw.gov.in/pdf/AdministrationofSecondDoseofCovishieldVaccinePriortoPrescribedTimeInterval.pdf [Accessed 5August 2023].
Approved for restricted use in emergency situations of ChAdOx1 nCoV- 19 Coronavirus vaccine (recombinant) COVISHIELD™ in prevention of COVID-19 disease in individuals 18 years of age and older. Available online: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/en/Factsheetof-ChAdSerum.pdf [Accessed 5 August 2023].
Fact sheet for vaccine recipients and caregivers restricted use of CovaxinTM under clinical trial mode the Bharat biotech COVID-19 vaccine (COVAXIN TM) to prevent coronavirus disease 2019 (COVID-19) prioritized groups of individuals who have been informed by the ministry of health and family welfare to attend a booth specified for COVAXIN TM based vaccination. Available online: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/en/FactSheet_Whole-virion-inacttivated-corona-virus-vaccine-_Bharat-Biotech-.pdf [Accessed 5 August 2023].
Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–84. https://doi.org/10.1016/S0140-6736(21)02000-6.
Kulkarni PS, Padmapriyadarsini C, Vekemans J, Bavdekar A, Gupta M, Kulkarni P, et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. EClinicalMedicine. 2021;42:101218. https://doi.org/10.1016/j.eclinm.2021.101218.
World Health Organization.India marks one year of COVID vaccination. WHO 2022. Available online at: https://www.who.int/india/news/feature-stories/detail/india-marks-one-year-of-covid-vaccination#:~:text=India%20crossed%20the%20one%2Dyear,vaccination%20drive%20in%20the%20world [Accessed on 5 August, 2022].
Li D, Sempowski GD, Saunders KO, Acharya P, Haynes BF. SARS-CoV-2 neutralizing antibodies for COVID-19 prevention and treatment. Annu Rev Med. 2022;73:1–16. https://doi.org/10.1146/annurev-med-042420-113838.
Morens DM, Folkers GK, Fauci AS. The concept of classical herd immunity may not apply to COVID-19. J Infect Dis. 2022;226:195–8. https://doi.org/10.1093/infdis/jiac109.
Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–7. https://doi.org/10.1016/S0140-6736(21)01642-1.
Choudhary HR, Parai D, Dash GC, Peter A, Sahoo SK, Pattnaik M, et al. IgG antibody response against nucleocapsid and spike protein post-SARSCoV-2 infection. Infection. 2021;49:1045–8. https://doi.org/10.1007/s15010-021-01651-4.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. https://doi.org/10.1136/bmj.39335.541782.
WHO. Interim Statement on COVID-19 Vaccine Booster Doses. Available online: https://www.who.int/news/item/10-08-2021-interim-statement-on-COVID-19-vaccine-booster-doses [Accessed 5 August 2023].
Galipeau Y, Greig M, Liu G, Driedger M, Langlois MA. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol. 2020;11:610688. https://doi.org/10.3389/fimmu.2020.610688.
Hamady A, Lee J, Loboda ZA. Waning antibody responses in COVID-19: what can we learn from the analysis of other coronaviruses? Infection. 2022;50(1):11–25. https://doi.org/10.1007/s15010-021-01664-z.
Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–8. https://doi.org/10.1038/s41591-020-0897-1.
Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Humoral antibody kinetics with ChAdOx1-nCOV (Covishield™) and BBV-152 (Covaxin™) vaccine among Indian Healthcare workers: a 6-month longitudinal cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Diabetes Metab Syndr. 2022;16(2):102424. https://doi.org/10.1016/j.dsx.2022.102424.
Campo F, Venuti A, Pimpinelli F, Abril E, Blandino G, Conti L, et al. Antibody persistence 6 months post-vaccination with BNT162b2 among health Care workers. Vaccines (Basel). 2021;9(10):1125. https://doi.org/10.3390/vaccines9101125.
Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. https://doi.org/10.1016/j.lanepe.2021.100208.
Kumar NP, Padmapriyadarsini C, Uma Devi KR, Banurekha VV, Nancy A, Girish Kumar CP, et al. Antibody responses to the BBV152 vaccine in individuals previously infected with SARS-CoV-2: a pilot study. Indian J Med Res. 2021;153(5&6):671–6. https://doi.org/10.4103/ijmr.IJMR_2066_21.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. https://doi.org/10.1016/S0140-6736(20)31604-4.
Wheeler SE, Shurin GV, Yost M, Anderson A, Pinto L, Wells A, et al. Differential antibody response to mRNA COVID-19 vaccines in healthy subjects. Microbiol Spectr. 2021;9(1):e0034121. https://doi.org/10.1128/Spectrum.00341-21.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dwivedi, T., Raj, A., Das, N. et al. Persistence of SARS-CoV-2 Antibodies for a Year Following SARS-CoV-2 Vaccinations (BBV152 and ChAdOx1 nCoV-19). Ind J Clin Biochem (2023). https://doi.org/10.1007/s12291-023-01149-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-023-01149-w