Abstract
Background
Recently, linezolid-resistant staphylococci have become an emerging problem worldwide. Understanding the mechanisms of resistance, molecular epidemiology and transmission of linezolid-resistant CoNS in hospitals is very important.
Methods
The antimicrobial susceptibilities of all isolates were determined by the microdilution method. The resistance mechanisms and molecular characteristics of the strains were determined using whole-genome sequencing and PCR.
Results
All the strains were resistant to oxacillin and carried the mecA gene; 13 patients (36.1%) had prior linezolid exposure. Most S. epidermidis and S. hominis isolates were ST22 and ST1, respectively. MLST typing and evolutionary analysis indicated most linezolid-resistant CoNS strains were genetically related. In this study, we revealed that distinct CoNS strains have different mechanisms of linezolid resistance. Among ST22-type S. epidermidis, acquisition of the T2504A and C2534T mutations in the V domain of the 23 S rRNA gene, as well as mutations in the ribosomal proteins L3 (L101V, G152D, and D159Y) and L4 (N158S), were linked to the development of linezolid resistance. In S. cohnii isolates, cfr, S158Y and D159Y mutations in the ribosomal protein L3 were detected. Additionally, emergence of the G2576T mutation and the cfr gene were major causes of linezolid resistance in S. hominis isolates. The cfr gene, G2576T and C2104T mutations, M156T change in L3 protein, and I188S change in L4 protein were found in S. capitis isolates.
Conclusion
The emergence of linezolid-resistant CoNS in the environment is concerning because it involves clonal dissemination and frequently coexists with various drug resistance mechanisms.
Similar content being viewed by others
Background
Coagulase-negative staphylococci (CoNS) have become important pathogens in patients with health care-associated infections caused by indwelling medical devices or immunocompromised patients [1, 2]. According to the monitoring data from the China Antimicrobial Resistance Surveillance System (CARSS, www.carss.cn/), the frequency of methicillin-resistant coagulase-negative staphylococci (MRCoNS) was extremely high, and the prevalence of MRCoNS was 74.5% in 2021.
Linezolid is an oxazolidinone antibiotic that is a last-resort antibiotic for the treatment of serious infections caused by gram-positive bacteria, including drug resistant organisms, such as methicillin-resistant staphylococci and vancomycin-resistant enterococci [3, 4]. The first clinical isolates of linezolid-resistant staphylococci and enterococci were reported in 2001 [5, 6]. Since then, linezolid-resistant organisms have been sporadically reported worldwide [7,8,9,10]. However, the detection rate of linezolid-resistant coagulase-negative staphylococci has progressively increased in recent years in China.
Point mutation in the domain V region of the 23 S rRNA gene was the most common mechanism of linezolid resistance, and the most frequent mutation was G2576T. In addition, T2500A, T2604C, C2532T, C2551T, G2603T, G2614T, C2190T, and G2447T mutations have been reported [11,12,13]. Furthermore, other mechanisms of linezolid resistance in staphylococci have also been reported, such as the presence of the cfr gene, which encodes an rRNA methyltransferase, and mutations in the ribosomal proteins L3, L4 and L22 [14,15,16].
In China, the prevalence of linezolid-resistant staphylococci has gradually increased in recent years; however, very few studies have investigated linezolid resistance. In this study, the clinical characteristics, antibiotic susceptibility, and resistance mechanisms of linezolid-resistant MRCoNS isolates that were recovered from 2019 to 2023 in a Chinese tertiary hospital were investigated.
Materials and methods
Bacterial isolates
Thirty-seven linezolid-resistant MRCoNS isolates, including thirteen Staphylococcus capitis isolates, nine Staphylococcus hominis isolates, eight Staphylococcus epidermidis isolates, six Staphylococcus cohnii isolates and one Staphylococcus haemolyticus isolate, were collected from December 2019 to March 2023 in a large tertiary teaching hospital (The First Affiliated Hospital of Zhengzhou University, Zhengzhou, with 8,000 beds located in east-central China). Two strains were isolated from pleural fluid, two from catheter tips, one from abdominal dropsy fluid, and one from cerebrospinal fluid; the remaining strains were recovered from blood cultures. Thirty-seven linezolid-resistant MRCoNS isolates were isolated from 36 patients, 25 of whom were male and 11 of whom were female. Linezolid-resistant S. epidermidis-5 and S. capitis-33 were isolated from the same patient, a 76-year-old man who was admitted to the respiratory intensive care unit. In addition, all patients were administered antibiotics, and 13 patients (36.1%) had received prior linezolid treatment. The characteristics of isolates and associated clinical data are listed in Table 1. Identification of the organisms was carried out using a VITEK®2 Compact system and VITEK® MS (bioMérieux, Marcy-l’Étoile, France).
Antimicrobial susceptibility testing
The antimicrobial susceptibilities of all the isolates were determined using the microdilution method. S. aureus ATCC 29213 was used as a for quality control strain for susceptibility testing. The breakpoint for tigecycline treatment was interpreted according to the European Committee on Antimicrobial Susceptibility Testing Guidelines (http://www.eucast.org/clinical_breakpoints/). The results of susceptibility testing for linezolid, penicillin, oxacillin, chloramphenicol, clindamycin, erythromycin, gentamicin, trimethoprim/sulfamethoxazole, ciprofloxacin, levofloxacin, rifampin, tetracycline, vancomycin and teicoplanin were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) M100-S33 breakpoints.
Genomic DNA extraction and whole-genome sequencing
Genomic DNA was extracted using the Puregene Yeast/Bacteria Kit (QIAGEN) according to the manufacturer’s instructions for gram-positive bacteria. Whole-genome sequencing (WGS) was performed using the Illumina HiSeq PE150 platform (Novogene Bioinformatics Technology Co., Ltd., Beijing, China). The acquired antibiotic resistance genes (ARGs) carried by the isolates were analyzed using KmerResistance v 2.2 [17] with raw reads. The results were reported for reads with ≥ 90% nucleotide identity, ≥ 90% coverage of the query, and a sequence depth of ≥ 10×. The raw reads were assembled into scaffolds using SPAdes v 3.13.1 [18]. Multilocus sequence typing (MLST) of the isolates was performed using MLST 2.0 (https://cge.food.dtu.dk/services/MLST/) and further validated with PubMLST (https://pubmlst.org) for S. epidermidis, S. hominis, and S. haemolyticus. Additionally, the phylogenetic tree of S. capitis and S. cohnii isolates was constructed using BacWGSTdb 2.0 (http://bacdb.cn/BacWGSTdb/index.php).
Molecular detection of resistance genes and mutations
Domain V of the 23 S rRNA gene and cfr gene was amplified and sequenced as previously described [19, 20], and the rplC, rplD, and rplV genes, which encode the ribosomal proteins L3, L4 and L22, respectively, were tested using previously described conditions and primers [10, 21].
Results
Antimicrobial susceptibility
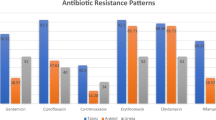
All 37 isolates displayed varying degrees of resistance to penicillin, oxacillin, and linezolid, and the mecA gene was detected in all isolates. Most of the isolates were resistant to chloramphenicol, levofloxacin, ciprofloxacin, clindamycin, erythromycin, and gentamicin, with resistance rates of 94.6% (35/37), 89.2% (33/37), 89.2% (33/37), 91.9% (34/37), 67.6% (25/37), and 54.1% (20/37), respectively. Interestingly, only 1 of the 8 S. epidermidis isolates was resistant to erythromycin; however, all thirteen S. capitis strains were resistant to erythromycin. The resistance rates of the linezolid-resistant MRCoNS strains to trimethoprim/sulfamethoxazole, tetracycline and rifampicin were 27.0% (10/37), 16.2% (6/37), and 10.8% (4/37), respectively; however, six S. cohnii isolates were sensitive to trimethoprim/sulfamethoxazole. No resistance to tigecycline, vancomycin or teicoplanin was detected(Table 2).
Molecular characteristics and the mechanisms of linezolid resistance
Seven of the eight S. epidermidis isolates belonging to the same clone, ST22, had two point mutations (T2504A and C2534T) in domain V of the 23 S rRNA gene, and exhibited L101V, G152D, and D159Y changes in the amino acid sequences of the L3 protein and a N158S change in the L4 protein. No cfr genes were detected in the seven ST22-type S. epidermidis isolates. However, another ST2-type S. epidermidis isolate with positive carriage of the cfr gene but no point mutation in domain V of the 23 S rRNA gene was found(Table 3).
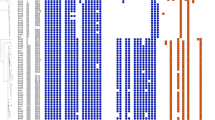
The six S. cohnii isolates were found to belong to the same clone by phylogenetic analysis (Fig. 1) and carried the cfr gene, as well as had S158Y and D159Y changes in the L3 protein. No mutation in domain V of the 23 S rRNA gene was detected in any of the S. cohnii isolates.
The nine S. hominis isolates were divided into 3 distinct clones: ST1 (n = 5), ST2 (n = 2) and ST85 (n = 2). A novel ST type, ST85, was found for the first time in this study. The cfr gene was identified among six S. hominis isolates, while the other three S. hominis isolates without the cfr gene had a 23 S rRNA G2576T mutation.
In the present study, clone spread was found among those S. capitis isolates by phylogenetic analysis (Fig. 1), and G2576T and C2104T 23 S rRNA mutations were identified. Additionally, 8 S. capitis isolates (61.5%) harbored the cfr gene. Furthermore, M156T and I188S changes were identified in the L3 and L4 proteins in most S. capitis strains. S. haemolyticus-37 harbored the cfr gene and had an additional R138V change in the L3 protein.
No mutation in the L22 protein was detected in any of the linezolid-resistant MRCoNS isolates in this study.
In addition to linezolid-related resistance genes, other resistance genes were also detected in our study, and the mecA gene was detected in all linezolid-resistant MRCoNS isolates (Table 3).
Discussion
In the present study, 37 linezolid-resistant MRCoNS isolates were obtained from a large tertiary teaching hospital from December 2019 to March 2023. In this hospital linezolid-resistant MRCoNS strains were first found in 2016; however, the detection rate of linezolid-resistant MRCoNS strains has steadily increased in recent years. Linezolid is an important alternative for the management of MRCoNS infections. The rapid emergence of linezolid-resistant MRCoNS is alarming and requires ongoing surveillance. Previous studies have indicated that linezolid administration is a significant risk factor for linezolid-resistant gram-positive cocci during hospital outbreaks [22, 23]. Our data showed that 13 patients (36.1%) had received prior linezolid therapy, thus, we speculated that increasing selective pressure most likely contributed to drug resistance. In addition, all 37 isolates were resistant to multiple antibiotics, and various resistance genes were detected, which indicated that the treatment options were limited.
Multilocus sequence typing indicated that ST22 was the dominant clone among the S. epidermidis isolates. The results of the present study were consistent with studies on the S. epidermidis lineage in Greece and Spain [24, 25] and different from the findings in Germany and France (ST2) [26, 27]. ST2, ST5, and ST22 are clustered into the CC5 clone, which is the most prevalent clonal complex among the nosocomial S. epidermidis population according to the literature [28]. Although linezolid-resistant CoNS strains have been reported sporadically worldwide, S. hominis pathogens that are resistant to linezolid are uncommon. Nine S. hominis strains were isolated in the present study, ST1 was the predominant clone, and ST85, a novel ST type, was found for the first time in our study.
MLST typing of S. epidermidis and S. hominis as well as phylogenetic analysis of S. capitis and S. cohnii suggested the transmission of resistant clones from patient to patient and clonal spread within the intensive care unit in our hospital.
The primary causes of linezolid resistance among staphylococci include modification of the target site of 23 S rRNA, acquisition of the cfr gene, and mutations of the ribosomal proteins L3 and L4 [11,12,13,14,15,16]. G2576T was the most frequently detected mutation in domain V of the 23 S rRNA gene, In addition, mutations in 23 S rRNA at positions G2534T, G2603T, T2504A and T2500A are also associated with reduced linezolid susceptibility [11,12,13]. In the present study, three ST1-type S. hominis isolates had a G2576T mutation, and thirteen S. capitis isolates coharbored G2576T and C2104T mutations, similar results have also been reported in previous studies [29, 30]. Furthermore, T2504A and C2534T mutations were found among seven ST22-type S. epidermidis isolates, yet no mutation in domain V of the 23 S rRNA gene was detected in S. cohnii or S. haemolyticus.
Acquisition of the cfr gene constitutes the second mechanism of target site mutation in staphylococci, and the cfr gene is usually located on a plasmid and confers resistance to linezolid [15, 27]. In the present study, the cfr gene was detected in one S. epidermidis, one S. haemolyticus, five S. cohnii, six S. hominis and eight S. capitis strains. Interestingly, the cfr gene was found in ST2-type S. epidermidis but not in ST22-type S. epidermidis, and the findings indicated that strains of distinct clones had diverse mechanisms of linezolid resistance.
Mutations of the ribosomal proteins L3, L4 and L22 were also analyzed in this study, and S. epidermidis and S. capitis isolates showed a series of alterations in the ribosomal proteins L3 and L4. Notably, the M156T mutation in the L3 protein and the I188S mutation in the L4 protein were also detected among S. capitis isolates for the first time. Mutations in the ribosomal L3 and L4 proteins were not detected among the seven S.hominis isolates. Notably, our data demonstrated that the mechanism of linezolid resistance in S. epidermidis and S. capitis was complex and involved simultaneous acquisition of the MDR gene cfr, as well as mutations of the target site 23 S rRNA and ribosomal proteins L3 and L4, and the linezolid MIC was greater than that in S. cohnii and S. hominis. These multiple resistance mechanisms could contribute to more high-level linezolid resistance than a single resistance mechanism.
In conclusion, there has been an increase in the prevalence of linezolid resistance among CoNS in our hospital’s intensive care units in recent years. Additionally, many of the isolates were clonally related, suggesting the intrahospital dissemination of resistant clones. Resistance is related to the presence of the cfr gene, a point mutation in the V domain of the 23 S rRNA gene and/or a mutation in the ribosomal L3 and L4 proteins, and multiple drug resistance mechanisms often coexist. Notably, distinct CoNS have different mechanisms of linezolid resistance. Taken together, these findings on the spread of linezolid-resistant CoNS in our setting highlight the importance of monitoring linezolid resistance in MRCoNS. Strict control measures should be taken to prevent further dissemination, and the relevant use of antibiotics needs to be emphasized.
Data availability
The sequence data generated in this study have been submitted to the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/) under accession numbers PRJNA1077915.
Abbreviations
- CoNS:
-
coagulase-negative staphylococci
- MRCoNS:
-
methicillin-resistant coagulase-negative staphylococci
- MLST:
-
multilocus sequence typing
- CARSS:
-
China Antimicrobial Resistance Surveillance System
- CLSI:
-
Clinical and Laboratory Standards Institute
- WGS:
-
whole-genome sequencing
References
Decousser JW, Desroches M, Bourgeois-Nicolaos N, Potier J, Jehl F, Lina G, et al. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int J Antimicrob Agents. 2015;46(6):622–30. https://doi.org/10.1016/j.ijantimicag.2015.07.022.
Angela França. The Role of Coagulase-Negative Staphylococci Biofilms on Late-Onset Sepsis: Current Challenges and Emerging Diagnostics and Therapies. Antibiotics (Basel)2023, 10;12(3):554. https://doi.org/10.3390/antibiotics12030554.
Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther2018, 18;12:1759–67. https://www.dovepress.com/by129.130.89.83.
Meka VG, Gold HS. Antimicrobial resistance to linezolid. Clin Infect Dis. 2004;39(7):1010–5. https://doi.org/10.1086/423841.
Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to Vancomycin resistant Enterococcus faecium resistant to linezolid. Lancet. 2001;357(9263):1179.
Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358:207–8.
Brescini L, Fioriti S, Coccitto SN, Cinthi M, Mingoia M, Cirioni O, et al. Genomic analysis of a Linezolid-Resistant Staphylococcus capitis causing bacteremia: report from a University Hospital in Central Italy. Microb Drug Resist. 2023. https://doi.org/10.1089/mdr.2022.0330.
Dadashi M, Sharifian P, Bostanshirin N, Hajikhani B, Bostanghadiri N, Khosravi-Dehaghi N, et al. The global prevalence of Daptomycin, Tigecycline, and linezolid-resistant Enterococcus faecalis and Enterococcus faecium strains from human clinical samples: a systematic review and Meta-analysis. Front Med (Lausanne). 2021. https://doi.org/10.3389/fmed.2021.720647.
Kevin Bouiller D, Ilic PH, Wicky P, Cholley C, Chirouze X, Bertrand. Spread of clonal linezolid-resistant Staphylococcus epidermidis in an intensive care unit associated with linezolid exposure. Eur J Clin Microbiol Infect Dis. 2020;39(7):1271–7. https://doi.org/10.1007/s10096-020-03842-7.
Sadowy MK-SE, Gawryszewska I, Klepacka J, Tomasik T, Michalik M, et al. Emergence of linezolid-resistant Staphylococcus epidermidis in the tertiary children’s hospital in Cracow, Poland. Eur J Clin Microbiol Infect Dis. 2020;39(9):1717–25. https://doi.org/10.1007/s10096-020-03893-w.
Cidral TA, Carvalho MC, Figueiredo AM, de Melo MC. Emergence of methicillin-resistant coagulase-negative staphylococci resistant to linezolid with rRNA gene C2190T and G2603T mutations. Apmis. 2015;123(10):867–71. https://doi.org/10.1111/apm.
Lincopan N, de Almeida LM, Elmor de Araújo MR, Mamizuka EM. Linezolid resistance in Staphylococcus epidermidis associated with a G2603T mutation in the 23S rRNA gene. Int J Antimicrob Agents. 2009;34(3):281–2. https://doi.org/10.1016/j. ijantimicag.2009.02.023.
Gajanand M et al. Vasundhra Bhandari,Rajni Gaind,Vandana Rani,Shimpi Chopra,Reetika Dawar,. Linezolid resistant coagulase negative staphylococci (LRCoNS) with novel mutations causing blood stream infections (BSI) in India. BMC Infect Dis. 2019,19(1):717.
Mendes RE, Deshpande LM, Jones RN. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat. 2014;17:1–12.
Giessing AMB, Søren, Jensen S, Rasmussen A, Hansen LH, Gondela A et al. Katherine Long, Identification of 8-methyladenosine as the modification catalyzed by the radical SAM methyltransferase Cfr that confers antibiotic resistance in bacteria. RNA. 2009, 15:327–36.
Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56:603–12. https://doi.org/10.1128/AAC.05702-11.
Clausen PT, Zankari E, Aarestrup FM, Lund O. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J Antimicrob Chemother. 2016;71(9):2484–8.
Nurk S, Bankevich A, Antipov D, Gurevich AA, Korobeynikov A, Lapidus A, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol. 2013;20(10):714–37.
Li Ding, Pei Li,Yang Yang, Dongfang Lin, Xiaogang Xu. The epidemiology and molecular characteristics of linezolid-resistant Staphylococcus capitis in Huashan Hospital, Shanghai. J Med Microbiol. 2020;69(8):1079–88.
Kehrenberg C, Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob Agents Chemother. 2006;50:1156–63.
Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother. 2010;65:2329–35.
Kevin Bouiller D, Ilic PH, Wicky P, Cholley C, Chirouze X, Bertrand. Spread of clonal linezolid-resistant Staphylococcus epidermidis in an intensive care unit associated with linezolid exposure. Eur J Clin Microbiol Infect Dis. 2019;39(7):1271–7. https://doi.org/10.1007/s10096-020-03842-7.
Kévin Bouiller J, Bador Rémi, Bruyère A, Toitot Sébastien, Prin J-P, Quenot. Pierre-Emmanuel Charles. Recent exposure to linezolid is strongly associated with the isolation of linezolid-resistant coagulase-negative staphylococcus species in patients with related infection or colonization: a case–control study in an intensive care unit. Int J Antimicrob Agents. 2017;50(5):693–4. https://doi.org/10.1016/j.ijantimicag.2017.08.026.
Karavasilis V, Zarkotou O, Panopoulou M, Kachrimanidou M, Themeli-Digalaki K, Stylianakis A, et al. On behalf of the Greek Study Group on Staphylococcal Linezolid Resistance Wide dissemination of linezolid-resistant Staphylococcus epidermidis in Greece is associated with a linezolid-dependent ST22 clone. J Antimicrob Chemother. 2015;70(6):1625–9. https://doi.org/10.1093/jac/dkv028.
Lozano C, Ruiz-García M, Gómez-Sanz E, López-García P, Royo García G, Zarazaga M, Torres C. Characterization of a cfr positive methicillin-resistant Staphylococcus epidermidis strain of the lineage ST22 implicated in a life-threatening human infection. Diagn Microbiol Infect Dis. 2012;73(4):380–2. https://doi.org/10.1016/j.diagmicrobio.2012.04.013.
Dortet L, Glaser P, Kassis-Chikhani N, Girlich D, Ichai P, Boudon M, et al. Long-lasting successful dissemination of resistance to oxazolidinones in MDR Staphylococcus epidermidis clinical isolates in a tertiary care hospital in France. J Antimicrob Chemother. 2018;73(1):41–51. https://doi.org/10.1093/jac/dkx370.
Bender J, Strommenger B, Steglich M, Zimmermann O, Fenner I, Lensing C, et al. Linezolid resistance in clinical isolates of Staphylococcus epidermidis from German hospitals and characterization of two cfr carrying plasmids. J Antimicrob Chemother. 2015;70(6):1630–8. https://doi.org/10.1093/jac/dkv025.
Thomas JC, Vargas MR, Miragaia M, Peacock SJ, Archer GL, Enright MC. Improved multilocus sequence typing scheme for Staphylococcus epidermidis. J Clin Microbiol. 2017;45:616–9. https://doi.org/10.1128/JCM.01934-06.
Jia CCYY, Hu R, Zhang HW, Zhou G-X, Chen. Linezolid-resistant clinical isolates of meticillin-resistant coagulase-negative staphylococci and Enterococcus faecium from China. J Med Microbiol. 2012;61(Pt 11):1568–73.
Ding L, Li P, Yang Y, Lin D, Xu X. The epidemiology and molecular characteristics of linezolid-resistant Staphylococcus capitis in Huashan Hospital, Shanghai. Med Microbiol. 2020;69(8):1079–88.
Acknowledgements
Not applicable
Funding
This work was supported by grants from the National Natural Science Foundation of China (Project Number: 82102438).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Cailin Liu, Jing Yu, Chunguang Chen, Xiaogai Li, Yafei Ye, Yani Dong, Xinxin Ying, Haijun Li, and Wanhai Wang. The first draft of the manuscript was written by Cailin Liu, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscrip.
Corresponding author
Ethics declarations
Ethical approval
The study protocol was approved by the First Affiliated Hospital of Zhengzhou University Ethics Committee for Research in Health. The First Affiliated Hospital of Zhengzhou University Ethics Committee also approved the waiver of informed consent to participate in this study. All patient data were anonymized prior to analysis(2020-KY-173).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., Yu, J., Chen, C. et al. Characterization of linezolid- and methicillin-resistant coagulase-negative staphylococci in a tertiary hospital in China. BMC Infect Dis 24, 486 (2024). https://doi.org/10.1186/s12879-024-09376-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09376-z