Abstract
Objective
In the present study, we aimed to compare the clinical efficacy and safety of omadacycline (OMC) with its comparators for the treatment of complicated skin and soft tissue infections (cSSTIs) in adult patients.
Methods
Randomized controlled trials (RCTs) evaluating OMC for cSSTIs were searched in databases of PubMed, Embase, Cochrane, Web of Science, and Clinical Trial, up to July 2022. The primary outcomes were clinical efficacy and microbiological response, with secondary outcome was safety.
Results
Four RCTs consisting of 1,757 patients were included, with linezolid (LZD) as a comparator drug. For clinical efficacy, OMC was not inferior to LZD in the modified intent-to-treat (MITT) (OR: 1.24, 95% Cl: [0.93, 1.66], P = 0.15) and clinically evaluable (CE) populations (OR: 1.92, 95% Cl: [0.94, 3.92], P = 0.07). For microbiological response, OMC was numerically higher than LZD in the microbiologically evaluable (ME) (OR: 1.74, 95% Cl: [0.81, 3.74], P = 0.16) and microbiological MITT (micro-MITT) populations (OR: 1.27, 95% Cl: [0.92, 1.76], P = 0.14). No significant difference was found in subpopulations of monomicrobial or polymicrobial mixed infection populations. The mortality and adverse event rates were similar between OMC and LZD.
Conclusions
OMC was as good as LZD in terms of clinical efficacy and microbiological response, and has similar safety issues in treating cSSTIs. OMC might be a promising option for treating cSSTIs in adult patients.
Similar content being viewed by others
Background
Skin and soft tissue infections (SSTIs) are caused by bacteria invading the skin and surrounding tissues, which is a common problem in hospitals. In the United States, there are more than 14 million outpatients with SSTIs and almost 900,000 hospitalized patients yearly [1].
According to the extent of infected skin, the Infections Diseases Society of America (IDSA) divides SSTIs into complicated SSTIs (cSSTIs) and uncomplicated SSTIs [2]. cSSTIs include deep soft tissue infections, such as necrotizing infections, infected ulcers, infected burns, and severe abscesses [3]. Uncomplicated SSTIs refer to superficial infections, including cellulitis, simple abscesses, impetigo, and furuncles [3]. Gram-positive bacteria are the primary pathogens of cSSTIs, among which Staphylococcus aureus is the most common one, and methicillin-resistant Staphylococcus aureus (MRSA) accounts for 46% of S. aureus isolates [4]. Other Gram-positive bacteria include Streptococcus pyogenes, Enterococcus faecalis, etc. In contrast, Gram-negative bacteria are less common in cSSTIs [4].
The most recent guideline of the Surgical Infection Society (SIS) on the management of cSSTIs suggests that vancomycin, linezolid (LZD), daptomycin, ceftaroline, and telavancin are first-line agents for cSSTIs caused by MRSA [5]. Vancomycin has always been the standard treatment for MRSA-caused cSSTIs, while LZD has been proven to be an effective substitute for vancomycin [6]. However, these drugs have limitations in their clinical application. Vancomycin has low tissue penetration and nephrotoxicity risk during treatment. Moreover, only intravenous dosage forms of vancomycin can be used. Long-term use of LZD may cause thrombocytopenia, as well as peripheral and central neuropathies [7, 8]. Since cSSTIs are characterized by deep tissue involvement and diverse pathogens, long-term antimicrobial therapy is usually required. Therefore, the optimal antibiotics for cSSTIs must have good tissue distribution, a broad antibacterial spectrum, and long-term medication safety and compliance.
Omadacycline (Nuzyra, PTK 0796, OMC) is a third-generation tetracycline derivative, but the first aminomethylcycline. It inhibits the synthesis of bacterial proteins by binding to 30 s ribosomal subunits and blocking the binding of aminoacyl tRNA [9, 10]. The structural modification of OMC makes it overcome the common tetracycline resistance mechanisms, such as the increasing number of efflux pumps and the production of ribosomal protective proteins [11, 12]. OMC has been proven to have good antibacterial activity against common clinical Gram-positive bacteria, Gram-negative bacteria, and anaerobes, showing low minimum inhibitory concentrations [13, 14]. OMC has high oral bioavailability, allowing intravenous injection and oral administration [15], which is convenient for outpatients and discharged patients with medicine. The dosage of once a day improves the patient's compliance with medication [16]. In addition, OMC has the characteristics of a large steady-state distribution volume and a high tissue penetration rate [10, 17, 18]. For patients with liver and kidney impairments, there is no need to adjust the dosage of OMC [19]. The FDA approved OMC to treat acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia in October 2018 [20].
The present meta-analysis included all available randomized controlled trials (RCTs) to comprehensively evaluate the efficacy and safety of OMC in the treatment of cSSTIs. Collectively, our current findings provided valuable insights into the treatment for cSSTIs in clinical practice.
Methods
Study search and selection
This study was conducted in accordance with the Preferred Reporting Program for Systematic Review and Meta-Analysis (PRISMA) statement [21]. PubMed, Embase, Cochrane, Web of Science, and Clinical Trial databases were searched from the establishment of the database to July 2022, regardless of language, using the following search terms: “omadacycline” OR “nuzyra” OR “PTK 0796”. Duplicate records were eliminated using Endnote X9, and two reviewers (Liang and Yin) independently monitored the records to avoid bias according to the inclusion and exclusion criteria. If any disagreement arose during the review process, a third reviewer (Xu) would decide. Inclusion criteria were set as follows: ① RCTs of efficacy and safety, ② patients over 18 years old with SSTIs, and ③ the patients in the test group were treated with OMC, and the patients in the control group were treated with other drugs. Exclusion criteria were set as follows: ① republished literature, ② review, case report, etc., and ③ data from the same RCT.
Data extraction and quality assessment
In all included studies, the data were extracted primarily by two researchers independently, and if there was disagreement during extraction, it was examined and determined by a third investigator. The following information was extracted from included studies, such as the first author, publication year, research place, start and end time, intervention measures, sample size, outcome indicators, pathogenic microorganisms, infection types, size of lesion and drainage procedures. According to the items in the Cochrane Collaboration Risk of Bias Tool, the risk of bias in all included studies was rated as “low risk”, “unclear”, or “high risk” [22]. The quality of the included studies was evaluated using the Jadad scale [23]. A total score of 0 ~ 5 points, including 0 ~ 2 for randomization, 0 ~ 2 for blinding, and 0 ~ 1 for withdrawal.
Definitions
The modified intent-to-treat (MITT) population included all randomized patients who did not have a sole Gram-negative causative pathogen at baseline. The clinically evaluable (CE) population included patients in the MITT population who had a qualifying infection as per study-entry criteria, received the study drug, did not take other antibiotics that may confound with results, and had an assessment of outcome during the protocol-defined window.
The microbiologically evaluable (ME) population consisted of the CE population who had at least one Gram-positive pathogen at baseline. The microbiological MITT (micro-MITT) population was composed of MITT patients with at least one Gram-positive bacterial pathogen identified from blood culture or the sample obtained from the cSSTI site at baseline.
Outcome measurement
Clinical efficacy endpoints were defined as the infection was fully resolved, and no further antimicrobial treatment was required at the end of treatment and post-treatment evaluation (PTE) in the MITT and CE populations [24]. The microbiological response was determined in the ME and micro-MITT population as eradication (absence of original baseline pathogen) or presumed eradication (no source specimen to culture in a subject assessed with a clinical success) of baseline pathogens [24]. Adverse events (AEs) were defined as AEs that emerged during or after administration, increased in severity, or were associated with the study drug during the study period.
Data analysis
Review Manager 5.3 software was used for meta-analysis. Qualitative data were expressed by odds ratio (OR) and its 95% Cl, and quantitative data were described by mean difference (MD) and its 95% Cl. The heterogeneity test was evaluated by the Cochrane I2 statistics. When P < 0.10 or I2 > 50%, it was considered that there was statistical heterogeneity among the studies, and the random effects model was used for meta-analysis; otherwise, the fixed effects model was used. P < 0.05 was considered statistically significant.
This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD 42022362152).
Results
Study selection and characteristics
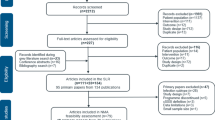
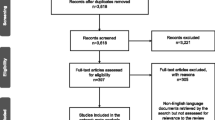
A total of 768 references were obtained (PubMed: 215, Embase: 261, Cochrane: 58, Web of Science: 223, Clinical Trial: 11), while 446 duplicates were excluded. By reading topics and abstracts, 302 references were excluded. Finally, 20 studies remained. By reading the full text, four RCTs [25,26,27,28] consisting of 1,757 patients were included. Figure 1 illustrates the process of literature search and screening. Table 1 shows the essential characteristics, and all compared drugs included in this meta-analysis were LZD. Moreover, all included studies were high-quality RCTs, and Fig. 2 shows the risk chart of bias.
Clinical efficacy
The results of the meta-analysis showed that the efficacy of OMC was not inferior to LZD in both the MITT population (OR: 1.24, 95% Cl: [0.93, 1.66], P = 0.15) and CE population (OR: 1.92, 95% Cl: [0.94, 3.92], P = 0.07) (Fig. 3).
Microbiological response
Two studies reported microbiological responses in the micro-MITT and ME populations under the same baseline. Generally, the microbiological response of the OMC group was numerically higher compared with the LZD group (micro-MITT population: OR: 1.27, 95% Cl: [0.92, 1.76], P = 0.14; ME population: OR: 1.74, 95% Cl: [0.81, 3.74], P = 0.16) (Fig. 4).
The microbiological response of OMC in the micro-MITT population at PTE was not inferior to LZD among patients with monomicrobial Gram-positive infection (OR: 1.38, 95% Cl: [0.92, 2.06], P = 0.11), polymicrobial Gram-positive infection (OR: 1.26, 95% Cl: [0.59, 2.68], P = 0.55), and polymicrobial mixed Gram-positive and Gram-negative infection (OR: 1.03, 95% Cl: [0.45, 2.35], P = 0.94) (Fig. 5A). Next, we compared the ability of OMC and LZD to eradicate common Gram-positive bacteria that caused cSSTIs. The results suggested that OMC was not inferior to LZD in eradicating Gram-positive pathogens (S. aureus: OR: 1.12, 95% Cl: [0.77, 1.63], P = 0.55; S. pyogenes: OR: 1.06, 95% Cl: [0.38, 3.00], P = 0.91; S. anginosus group: OR:1.64, 95% Cl: [0.83, 3.27], P = 0.16; E. faecalis: OR: 2.47, 95% Cl: [0.36, 16.97], P = 0.36) (Fig. 5B).
Safety
There was no significant difference in treatment-emergent adverse events (TEAEs) (OR: 1.17, 95% Cl: [0.74, 1.86], P = 0.50), serious AEs (OR: 1.40, 95% Cl: [0.72, 2.70], P = 0.32), treatment discontinuation for AEs (OR: 1.24, 95% Cl: [0.59, 2.63], P = 0.57), and treatment related TEAEs (OR: 1.29, 95% Cl: [0.56, 3.00], P = 0.55) between OMC and LZD (Fig. 6). In addition, no significant difference was found in nausea (OR: 1.88, 95% Cl: [0.79, 4.44], P = 0.15), vomiting (OR: 1.53, 95% Cl: [0.46, 5.09], P = 0.49), diarrhea (OR: 0.35, 95% Cl: [0.09, 1.43], P = 0.14), and blood and lymphatic system disorders (OR: 0.63, 95% Cl: [0.24, 1.64], P = 0.34) (Fig. 7).
In terms of mortality, there was no significant difference between OMC and LZD (OR: 0.76, 95% Cl: [0.17, 3.40], P = 0.72) (Fig. 8).
Discussion
Our meta-analysis consisting of four RCTs showed that the clinical efficacy of OMC was as good as LZD. A previous meta-analysis has evaluated the efficacy and safety of OMC in the treatment of acute bacterial infection, which includes three studies on cSSTIs and one study on CABP. The results show that the clinical efficacy of OMC is numerically higher compared with the comparator antibiotics, and OMC shows similar clinical efficacy to LZD in treating cSSTIs [29]. However, the study did not appear to unify the criteria for the MITT and CE populations included in the study (two studies excluded patients with Gram-negative bacteria only; one study did not and added aztreonam against Gram-negative bacteria). After intravenous injection of OMC in rats, the skin-to-blood concentration ratio is increased by 6.6 times [30]. In healthy subjects, the steady-state distribution volumes of OMC are 190 ~ 204 L [17, 18], and the plasma protein binding rate is about 21% [10]. In contrast, the steady-state distribution volumes of LZD are 40 ~ 50 L [31], and the plasma protein binding rate is about 31% and has poor penetration into adipose tissue [32]. These findings may indicate that OMC has a high rate of tissue penetration than LZD.
The microbiological response of OMC was numerically higher than LZD, which might be related to its lower MIC. S. aureus is the most common pathogen causing cSSTIs, accounting for 81% of isolated pathogens [4]. Compared with LZD, OMC has a lower MIC against common Gram-positive bacteria in vitro. For example, for S. aureus (including MRSA and MSSA), S. pyogenes, and E. faecalis, the MICs are ≤ 0.015 ~ 2 mg/L for OMC and ≤ 0.12 ~ 8 for LZD [33]. The tissue distribution concentration of OMC and LZD is 378 mg/L and 15.5 mg/L, respectively [30, 34]. Free concentrations of OMC and LZD at the skin and soft tissue sites could cover common Gram-positive pathogen bacteria.
Although Gram-positive bacteria are the most common pathogens, Gram-negative bacteria or mixed infections still account for about 12 ~ 21% of severe skin infections [6, 35]. Among Gram-negative pathogens, Enterobacteriaceae is the most common isolated bacteria [36]. OMC shows activity against Gram-negative bacteria in vitro, such as Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), and Haemophilus influenzae, with MIC50/90 values of 0.5/2, 1/4, and 0.5/1 mg/L, respectively [33]. In recent years, multidrug-resistant (MDR)/extensively drug-resistant (XDR) bacteria have also been isolated from cSSTIs (such as MDR/XDR Enterobacteriaceae) [35]. A multicenter, observational study involving nine patients has found that oral OMC (450 mg loading dose plus 300 mg maintenance dose) is effective against MDR E. coli and K. pneumoniae-induced bone/joint or intra-abdominal infection [37]. Since LZD is not active against Gram-negative pathogens [38, 39], patients with sole Gram-negative pathogenic bacterial infections were excluded from RCTs. Therefore, we could not analyze how effective OMC was in treating cSSTIs caused by Gram-negative pathogens. In this analysis that integrated OASIS-1 and OASIS-2, the microbiological response of mixed infection with Gram-negative strains of OMC and LZD was similar in the micro-MITT population at PTE, and this finding might be attributed to the small number of cases. Considering that OMC is effective against Gram-negative bacteria or mixed infections, it might be preferred for treating cSSTIs.
Regarding safety, the incidence of adverse drug reactions in OMC was similar to that in LZD, and most of them were transient AEs. Unsurprisingly, since OMC is structurally similar to tetracycline antibiotics, the most common AEs are gastrointestinal events [40]. It was observed that the incidence of nausea and vomiting in the OMC group was slightly higher compared with the LZD group in this meta-analysis. Some studies have shown that the gastrointestinal AEs of OMC are dose-dependent in healthy and diseased subjects [41, 42]. A higher incidence of gastrointestinal AEs in the OASIS-2 study with a higher dosage applied is also observed. Studies have shown that oral OMC after meals can reduce gastrointestinal AEs. However, compared with fasting, taking meals 2 to 4 h before the administration will reduce the bioavailability of OMC [43]. Therefore, to obtain an excellent therapeutic effect, oral OMC should be taken on a fasting state and avoided in combination with dairy products. Some AEs associated with the long-term use of LZD include myelosuppression, peripheral and optic neuropathy, serotonin syndrome, and so on [44]. Only a trend of a higher number of AEs in the hematological system was observed in the present meta-analysis (1.60% in LZD vs. 1.01% in OMC). This finding might be related to the short duration of treatment (the treatment time was 7 ~ 14 days). Studies have shown that the hematological system response of LZD is increased with the prolongation of medication time (treatment duration ≤ 14 days reported 1.9%; 5.1% for 15 ~ 28 days; 7.4% for > 28 days) [45]. Although the recommended duration of cSSTIs treatment is 7 to 10 days, the treatment time should be extended if the infection is not improved during treatment [46]. Therefore, it is still necessary to pay attention to the hematological toxicity of LZD.
This study has several limitations. Firstly, the study failed to demonstrate that OMC was superior to LZD against Gram-positive infection. The ME population included a CE population with at least one Gram-positive pathogen at baseline, but there was no significant difference between OMC and LZD. Moreover, although no restrictions were set on the types of control drugs, only LZD was included in this meta-analysis. Comparisons between OMC and other antibiotics, such as vancomycin, for the treatment of cSSTIs could not be evaluated here. Lastly, because LZD was ineffective against Gram-negative bacteria, we could not assess the efficacy of OMC against infections caused by Gram-negative bacteria.
Conclusions
This meta-analysis showed that OMC was as good as LZD regarding clinical efficacy and microbiological response, and has a similar safety profile. Therefore, OMC might be a promising option for treating cSSTIs in adult patients. However, we need to further study the hepatorenal impact and low immune function populations before applying OMC in Gram-negative or mixed cSSTIs.
Availability of data and materials
The data sets generated during and/ or analyzed during the current study are available from the corresponding author on reasonable request.
References
Burnham JP, Kollef MH. Treatment of severe skin and soft tissue infections: a review. Curr Opin Infect Dis. 2018;31(2):113–9.
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147–59.
Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58.
Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022;17(1):3.
Duane TM, Huston JM, Collom M, et al. Surgical Infection Society 2020 updated guidelines on the management of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2021;22(4):383–99.
Golan Y. Current treatment options for acute skin and skin-structure infections. Clin Infect Dis. 2019;68(Suppl 3):206-S212.
Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. 2015;48(4):508–19.
Esposito S, Bassetti M, Concia E, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother. 2017;29(4):197–214.
Gallagher JC. Omadacycline: a modernized tetracycline. Clin Infect Dis. 2019;69(Suppl 1):1-S5.
Watkins RR, Deresinski S. Omadacycline: a novel tetracycline derivative with oral and intravenous formulations. Clin Infect Dis. 2019;69(5):890–6.
Bidell MR, Lodise TP. Use of oral tetracyclines in the treatment of adult outpatients with skin and skin structure infections: focus on doxycycline, minocycline, and omadacycline. Pharmacotherapy. 2021;41(11):915–31.
Barber KE, Bell AM, Wingler MJB, et al. Omadacycline enters the ring: a new antimicrobial contender. Pharmacotherapy. 2018;38(12):1194–204.
Huband MD, Pfaller MA, Shortridge D, et al. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme, 2017. J Glob Antimicrob Resist. 2019;19:56–63.
Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and preclinical review of omadacycline. Clin Infect Dis. 2019;69(Suppl 1):6-S15.
Honeyman L, Ismail M, Nelson ML, et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother. 2015;59(11):7044–53.
Sun H, Ting L, Machineni S, et al. Randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother. 2016;60(12):7431–5.
Gotfried MHHK, Garrity-Ryan L, et al. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother. 2017;61(9):e01135-01117.
Berg JK, Tzanis E, Garrity-Ryan L, et al. Pharmacokinetics and safety of omadacycline in subjects with impaired renal function. Antimicrob Agents Chemother. 2018;62:62 (2).
Rodvold KA, Burgos RM, Tan X, et al. Omadacycline: a review of the clinical pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet. 2020;59(4):409–25.
Dougherty JA, Sucher AJ, Chahine EB, et al. Omadacycline: a new tetracycline antibiotic. Ann Pharmacother. 2019;53(5):486–500.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Reviews. 2015;4(1):1.
Puljak L, Ramic I, Arriola Naharro C, et al. Cochrane risk of bias tool was used inadequately in the majority of non-cochrane systematic reviews. J Clin Epidemiol. 2020;123:114–9.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Administration UFaD acute bacterial skin and skin structure infections: developing drugs for treatment. https://www.fda.gov/media/71052/download. Accessed 16 Jul 2022.
ClinicalTrials.gov. Study the safety and efficacy of PTK 0796 in patients with Complicated Skin and Skin Structure Infection (CSSSI) (CSSI) https://www.clinicaltrials.gov/ct2/show/NCT00865280?id=NCT00865280&draw=2&rank=1&load=cart. Accessed 16 Jul 2022.
Noel GJ, Draper MP, Hait H, et al. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(11):5650–4.
O’Riordan W, Green S, Overcash JS, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med. 2019;380(6):528–38.
O’Riordan W, Cardenas C, Shin E, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080–90.
Lan SH, Chang SP, Lai CC, et al. The efficacy and safety of omadacycline in treatment of acute bacterial infection: a systemic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(51):e18426.
Lin W, Flarakos J, Du Y, et al. Pharmacokinetics, distribution, metabolism, and excretion of omadacycline following a single intravenous or oral dose of 14 C-omadacycline in rats. Antimicrob Agents Chemother. 2017;61(1):e01784-16.
Stein GE, Wells EM. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus: Vancomycin and linezolid. Curr Med Res Opin. 2010;26(3):571–88.
Rao GG, Konicki R, Cattaneo D, et al. Therapeutic drug monitoring can improve Linezolid Dosing regimens in current clinical practice: a review of linezolid pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2020;42(1):83–92.
Pfaller MA, Huband MD, Shortridge D, et al. Surveillance of omadacycline activity tested against clinical isolates from the USA: report from the SENTRY Antimicrobial Surveillance Program, 2019. J Glob Antimicrob Resist. 2021;27:337–51.
Traunmuller F, Schintler MV, Spendel S, et al. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents. 2010;36(1):84–6.
Jabbour JF, Kanj SS. Gram-negative skin and soft tissue infections. Infect Dis Clin North Am. 2021;35(1):157–67.
Falcone M, Concia E, Giusti M, et al. Acute bacterial skin and skin structure infections in internal medicine wards: old and new drugs. Intern Emerg Med. 2016;11(5):637–48.
Morrisette T, Alosaimy S, Lagnf AM, et al. Real-world, multicenter case series of patients treated with oral omadacycline for resistant gram-negative pathogens. Infect Dis Ther. 2022;11(4):1715–23.
Clemett DMA. Linezolid. Drugs. 2000;59(4):815–28.
Livermore DM. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother. 2003;51(Suppl 2):ii9-16.
Rusu A, Buta EL. The development of third-generation tetracycline antibiotics and new perspectives. Pharmaceutics. 2021;13(12):2085.
Bundrant LATE, Garrity-Ryan L, et al. Safety and pharmacokinetics of the aminomethylcycline antibiotic omadacycline administered to healthy subjects in oral multiple-dose regimens. Antimicrob Agents Chemother. 2018;62(2):e01487-01417.
Overcash JS, Bhiwandi P, Garrity-Ryan L, et al. Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study. Antimicrob Agents Chemother. 2019;63(5):e02083.
Tzanis E, Manley A, Villano S, et al. Effect of food on the bioavailability of omadacycline in healthy participants. J Clin Pharmacol. 2017;57(3):321–7.
Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–67.
Shaw KJ, Barbachyn MR. The oxazolidinones: past, present, and future. Ann N Y Acad Sci. 2011;1241:48–70.
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-52.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundations of China (81770004 and 82073894), Cultivation Project of PLA General Hospital for Distinguished Young Scientists (2020-JQPY-004), and New Medicine Clinical Research Fund (4246Z512).
Author information
Authors and Affiliations
Contributions
Wenxin Liang and Hong Yin reviewed the literature, extracted and analyzed the data, and drafted the manuscript; Huiling Chen and Juan Xu extracted and analyzed the data; as corresponding authors, Yun Cai contributed to study design, protocol, data extraction, data analysis, and writing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liang, W., Yin, H., Chen, H. et al. Efficacy and safety of omadacycline for treating complicated skin and soft tissue infections: a meta-analysis of randomized controlled trials. BMC Infect Dis 24, 219 (2024). https://doi.org/10.1186/s12879-024-09097-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09097-3