Abstract
Background
Transfusion transmissible infections (TTIs) remain a major public health problem in developing countries including Ethiopia. In Ethiopia, comprehensive information about sero-epidemiology of major TTIs is lacking at the national level. Therefore, this systematic review and meta-analysis was aimed at providing the pooled estimate of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and syphilis among blood donors in Ethiopia.
Methods
Relevant studies published until May 31, 2019 were searched through PubMed/Medline, EMBASE, SCOPUS, HINARI, Cochrane database library, Web of Science, Google Scholar and Google. The methodological quality of articles was assessed using Joanna Brigg’s Institute critical appraisal checklist for prevalence and analytical studies. The pooled sero-epidemiology of HIV, HBV, HCV and syphilis were determined using the random-effects model. Heterogeneity between the studies was assessed using the I2 statistics. Publication bias was assessed by visual inspection of the funnel plot and Egger's statistics.
Results
A total of 7921 articles were retrieved, and 7798 were screened for eligibility after duplicates removed. Forty-nine full-text articles were assessed for eligibility; of which 45 were eligible for qualitative and quantitative synthesis: categorized as 36, 34, 31 and 23 studies for estimations of HBV, HIV, HCV and syphilis, respectively. In the random-effects model, the pooled sero-epidemiology of HBV, HIV, HCV and syphilis was 5.20, 2.83, 0.93 and 1.50%, respectively. Moreover, being a male blood donor was significantly associated with HBV and syphilis infection, whereas being a replacement blood donor was significantly associated with a high burden of HIV, HBV and HCV infections.
Conclusion
The pooled sero-epidemiology of major TTIs among blood donors was high. Therefore, there is a need to design prevention and control strategies in a comprehensive approach to reduce the burden.
Similar content being viewed by others
Background
Blood is an invaluable, life-sustaining fluid. The loss of a large volume of blood due to casualties, hemorrhage, and other medical conditions lead to blood transfusion as part of the standard care to save a life. Because of this, blood transfusion is considered as an integral and essential element of a health care system [1, 2]. Though blood transfusion is one of the ways to save patients’ life in many cases, sometimes it poses the risk of TTIs. Supplying safe blood and preventing the transmission of infectious diseases are among the utmost important goals for blood transfusion organization [1]. Transfusion-transmissible infectious agents mainly HIV, HBV, HCV, and syphilis are among the greatest threats for the receivers if not rigorously screened [3].
According to the World Health Organization (WHO) 2016 report, the reactivity for markers of TTIs, outdated stock, and incomplete collection were the main reasons for the discard of donated blood. The median total discard rate was 10.9, 9.0, 6.7 and 5.7% in lower-middle-income, low-income, upper-middle-income, and high-income countries, respectively. Markers of TTIs are the most common reason for the discard, which accounted for a median discard rate of 7.4, 5.1, 3.9 and 1.1% in low-income, lower-middle-income, upper-middle-income, and high-income countries, respectively. Globally, it is estimated that 1.8 million donated blood were discarded due to TTIs reactivity in 2013 [4].
On another hand, according to the WHO report of 2020, 37.6 million people were living with HIV globally, with over two-thirds of who are living in the WHO African region. Some portion of HIV infection was through transfusion of blood and blood products. Similarly, reports from Sub-Saharan Africa showed that the median overall risk of becoming infected with HIV from a blood transfusion is 1 infection per 1000 units [5], and risk for 12.5% of post-transfusion viral hepatitis infection [6].
Viral hepatitis is the other global health burden. More than one million people die each year from HCV and HBV infections. According to the WHO 2017 report, chronic HBV infection affects over 257 million people worldwide. On the other hand, HCV is a leading cause of chronic liver disease, and persistent HCV infection is associated with cirrhosis, hepatocellular carcinoma, liver failure, and death [7]. Globally, the sero-prevalence of HCV ranges from 2 to 3%, with an estimated 71.1 million patients with active viremia, where 10.15 million are from sub-Saharan Africa [8]. Recent systematic review and meta-analysis showed that HCV was estimated to be 1.78% among blood donors in sub-Saharan Africa [9]. On the other hand, syphilis was also associated with blood donation, and its sero-prevalence among blood donors varies in Africa [10,11,12,13].
Implementing rigorous screening algorism of donated blood and blood products for markers of TTIs is the strategy to ensure the safety of blood donation. However, the risk of post-transfusion hepatitis and HIV is challenging in Sub-Saharan African countries; and patients who received blood transfusion develop post-transfusion hepatitis and HIV [5, 6]. Therefore, prevention and control of TTIs is the leading concern and priority agenda of WHO in Sub-Saharan Africa [14] and also the Ethiopian Federal Ministry of Health [15].
Systematic review and meta-analysis generates pooled and concrete evidence that would help to determine the distribution of the TTIs and contributes to planning the national strategies for containment and awareness-raising campaigns as well as to design specific preventive measures. As to the authors’ knowledge, limited systematic and meta-analysis were reported, each focusing on a single TTI type [16, 17]. Thus, systematically organized and strong evidence with regard to the sero-epidemiology and associated factors of the major TTIs among blood donors in Ethiopia is crucial. Therefore, the aim of this review was to estimate the epidemiology of TTIs among blood donors in Ethiopia by: (a) performing a systematic literature review, and (b) performing a meta-analysis on TTIs sero-prevalence and commonly reported risk factors of TTIs.
Methods
Context of the review
The context of this systematic review was limited to blood donors in Ethiopia. Ethiopia is one of the developing countries located in the sub-Saharan and horn of Africa having more 100 million people with an area of 1,100,000 km2.
Design and protocol registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P 2015 Guidelines) was used for this review [18]. The protocol had been registered in the PROSPERO with a registration number CRD42018076616.
Eligibility criteria
Primary studies with full-text original articles written in English up to May 31, 2019 and having extractable data on sero-epidemiology of and/or factors associated with HIV, HBV, HCV and syphilis among blood donors in Ethiopia were included in the review. However, studies conducted among Ethiopian blood donors living abroad, repeated studies used the same dataset, and studies focused on the molecular Epidemiology of TTIs, case–control studies, case reports, case series and editorials were excluded from this review.
Outcomes of the review
This review had two outcomes. The first outcome was to determine the pooled sero-epidemiology of HIV, HBV, HCV and syphilis among blood donors in Ethiopia. The secondary outcome was to identify factors associated with the sero-epidemiology HBV, HIV, HCV and syphilis.
Search strategy
All relevant articles published were searched in PubMed/Medline, EMBASE, HINARI, SCOPUS, Cochrane database library, Google Scholar and Web of Sciences electronic databases. In addition, we searched grey literature in Goggle. The search terms were developed in accordance with the Medical Subject Headings Thesaurus (MeSH), and Boolean operators (AND, OR) were used for searching the articles. The following terms in combination with free text key terms; “transfusion transmissible infections”, “transfusion transmissible diseases”, “transfusion transmissible viral hepatitis”, “Transfusion transmitted infections”, “Transfusion transmitted viral hepatitis”, “HIV”, “Hepatitis B virus”, “TTIs” “Hepatitis C virus” “Syphilis”, “T.pallidum”, “blood donor” and “Ethiopia” were used. Also, hand searching of articles published in the Ethiopian Journal of Health Sciences, Ethiopian Medical Journal, Ethiopian Journal of Health and Development, and Ethiopian Journal of Health and Biomedical Sciences was conducted. Reference lists of relevant retrieved articles were used to identify any studies that were not retrieved through electronic database searching.
Study selection and quality appraisal
First, retrieved articles were imported to EndNote X7 (Thomson Reuters, New York, USA), and duplicated articles were removed electronically, and manually if the citation style variation of different databases were noted. Then, articles were screened by their title and abstract independently by two groups comprising four authors each: group one (MM, ZA, MA, BB), and group two (SA, ES, BW, DG). Similarly, the two groups of authors had appraised the full-texts of the studies and in case of discrepancies, it was resolved by the third group of authors (BE, BT, TD, DD, TT, AK). In the case of articles eligible for full-text appraisal but not accessible in full text, the corresponding author communicated with the journal editorial office and/or publishers via email and/or phone. For assessment of the methodological quality of articles, the Joanna Brigg’s Institute critical appraisal tools for prevalence and analytical studies were used [19, 20].
Data extraction
The data were extracted using Microsoft Excel format. Accordingly, the following pieces of information were collected: first author’s name, year of publication, year of data collection, length of the data collection period, sampling techniques (blood donors recruitment strategy), a diagnostic method used, total population participated, the number of blood donors who were sero-reactive for HIV, HBV, HCV and syphilis, blood donors type, median/mean age of the study participants, sex of study participants, and the number of male and female blood donors who were sero-reactive and non-reactive, sero-reactivity for each TTIs by donor type, and the reported odds ratio (OR) for the respective TTIs.
Data analysis and interpretation
We used STATA version 14 software (Stata Corp LLC, Texas, USA) for analysis. The magnitude of heterogeneity between included studies was quantitatively measured by the index of heterogeneity (I2 statistics) [21]. Low, medium and high heterogeneities were noted where the I2 values are 25%, 50% and 75%, respectively. The significance of heterogeneity was determined by the P-value of I2 statistics and a P-value of < 0.05 was considered as evidence of heterogeneity. In the case of a medium and high level of heterogeneity between the included studies, Dersimonian and Laird random-effects model [22], sensitivity and subgroup analysis were used for estimating the pooled effect size. Considering the median year of sample collection as a factor, univariable random-effects meta-regression was performed to evaluate the trend of TTIs sero-prevalence over time.
Similarly, small-study effect or publication bias was evaluated using the visual funnel plot test, and Egger’s statistics. In the case of small-study effect, trim and fill analysis (Duval and Tweedie’s methods) was used to adjust the pooled estimates [23]. The ORs were extracted from the articles when reported, or calculated for each of the reported variables and pooled in a meta-analysis. Then OR with its 95% confidence interval was used to estimate the association between each TTIs and the reported factors. The results were presented using text and plots.
Results
Characteristics of included studies
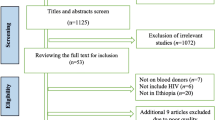
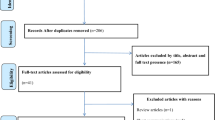
A total of 7921 records were retrieved; of which 123 articles were removed due to duplication. During title/abstract screening, 7749 articles were also removed as their titles and abstracts were not relevant to the current review. One article eligible for full-text appraisal was excluded as the full-text article was not accessible after repeated contact of the corresponding author via email, and the journal editorial office [24]. The remaining 49 full-text articles were assessed for eligibility.
Out of the total studies that underwent critical appraisal, 4 were excluded: 1 study did not report the epidemiology HBV infection [25]; 1 study the sample size is too small for epidemiological estimation [26]; 1 study did not clearly report the outcome interest [27], and the other study authored by Dessie et al. in 2008 [28] was a duplicate publication from the same dataset published in 2007 [29]. The remaining 45 studies were included in the qualitative and quantitative synthesis (Fig. 1).
Regarding the type of literature included in this review, 43 (95.6%) articles were published studies, and the rest were grey literature [30, 31], which involved a total 4,441,920 study participants. Twenty-four (53.3%) and 21 (46.7%) of the included studies were retrospective and prospective studies, respectively. Concerning the regions where these studies conducted, 17 (37.8%), 6 (13.3%), 6 (13.3%) and 5 (11.1%) of the studies were conducted in Amhara, Addis Ababa, South Nations and Nationalities, and Oromia regional state, respectively; whereas 4 (8.9%) of the studies were sub-national level studies.
Of all included studies, 36 reported HBV [3, 13, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]; 34 reported HIV [3, 13, 29,30,31,32,33, 35,36,37,38, 42, 43, 45, 46, 48,49,50,51,52,53,54,55,56,57,58, 60,61,62,63,64,65,66,67,68]; 31 reported HCV [3, 13, 29,30,31,32,33, 35,36,37,38, 41,42,43, 45,46,47,48,49,50,51, 54, 55, 57, 58, 60,61,62, 69, 70]; and 23 reported syphilis [3, 13, 29,30,31, 33, 35, 36, 38, 42, 43, 45, 46, 49,50,51,52, 54, 57, 58, 60, 68, 71] prevalence among blood donors in Ethiopia. Regarding the distribution of each TTIs with sex and donor type of the study participants, it was found as follows: of 36 studies reported HBV, 24 and 9 reported sex and donor type, respectively; of 34 studies reported HIV, 24 and 8 studies reported sex and donor type, respectively; of 31 reported HCV, 19 and 7 reported sex and donor type, respectively; and of 23 studies reported syphilis, 14 and 5 studies reported sex and donor type, respectively (Table 1).
Pooled estimates of TTIs
The Pooled estimate and trend of HIV
For the estimation of the pooled prevalence of HIV among blood donors, 34 articles with a total of 355, 026 participants’ data were used. These studies reported varying prevalence, ranging from 0.14% to 14.5%. In the random-effect model, the pooled size effect of HIV was 2.83% (95% CI 2.43, 3.23%) (Fig. 2). The trend of HIV showed that the seroprevalence showed a decrement from 1989 to 2018. The random-effects meta-regression analysis also confirmed that the pooled seroprevalence of HIV was negatively but significantly associated with the midpoint year of the data collection period (Coefficient = − 0.089; 95% CI − 0.133, − 0.045; P < 0.001). The seroprevalence estimate of HIV changed from 8.84% (95% CI 5.96, 17.73%) before 2000 to 1.20% (95% CI 0.87, 1.53%) during 2015–2018 (Table 2).
The influential analysis revealed that no single study was outside the 95% CI of the pooled estimate indicating that omitting each study did not affect the pooled estimate. Regarding the publication bias, the visual funnel plot seems symmetric, suggesting that there was no small-study effect. The objective-based test, egger’s statistics, confirmed that there was no small-study effect (Coefficient of bias = 3.69, P = 0.111). Since the degree of heterogeneity between the included studies was higher, subgroup analysis was done considering the “region” as a grouping variable. In the random-effects model, the pooled estimated prevalence of HIV was 4.41% (95% CI 3.50, 5.32%) in Amhara region, 1.72% (95% CI 1.18, 2.26%) in South Nations and Nationalities, 1.14% (95% CI 0.22, 2.06%) in Oromia, and 0.98% (95% CI 0.39, 1.58%) in Harar/Dire Dawa (Fig. 2).
HIV and its association with sex and donor type
Factors such as sex and donor type were assessed to estimate their effect size on the pooled estimate of HIV. Twenty four and 8 studies had reported the distribution of HIV by sex and donor type, respectively. In the random-effects model, being a replacement blood donor was more likely to have HIV infection among blood donors in Ethiopia [Odds ratio (OR)] = 2.09; 95% CI 1.39, 3.13; I-squared = 80.1%) (Fig. 3). However, sex had no association with HIV infection (OR = 1.09; 95% CI 0.85, 1.40; I-squared = 86.1%). In both cases, there was no publication bias as evidenced by egger’s statistics (P > 0.05) and the visual funnel plot.
The Pooled estimate and trend of HBV
Regarding HBV among blood donors in Ethiopia, 36 articles which include 391, 339 study participants were used for estimation. These studies reported varying prevalence, ranging from 1.03 to 25%. In the random-effects model, the pooled seroprevalence of HBV was 5.20% (95% CI 4.64, 5.77%; with I-squared = 98.4%). The trend analysis also showed that a decrement from 12.03% (95% CI 9.72, 14.34%) before 2000 to 4.30% (95% CI 3.35, 5.24%) during 2015–2018. Though the trend seems to decline from 1986 to 2018, the random-effects meta-regression analysis showed that the decline is not significantly associated with the midpoint year of the data collection period (Coefficient = − 0.029; 95% CI − 0.078, 0.02, P = 0.25) (Table 2). The subgroup analysis revealed that there were considerable variations of HBV estimate across regions in Ethiopia. The visual funnel plot was symmetric, suggesting no publication bias. Likewise, the egger’s statistics showed that there was no small-study effect on the pooled estimate of HBV infection (P = 0.450, coefficient of bias = 1.42) (Fig. 4).
HBV and its association with sex and donor type
As there was high-level heterogeneity between the studies included for the estimation of the effect size of sex and donor type on HBV, a random-effects model was fitted. Therefore, male blood donors (OR = 1.87; 95% CI 1.51, 2.32) (Fig. 5), and replacement blood donors (OR = 1.68; 95% CI 1.34, 2.12) (Fig. 6) were more likely to be HBV sero-reactive. Besides, the assessment of publication bias revealed that there was no evidence of a small-study effect in both factors (P > 0.05).
The Pooled estimate and trend of HCV
For the estimation of HCV among blood donors, 31 studies with a total of 380, 876 study participants were included. These studies reported varying prevalence, ranging from 0.2% to 13.3%. In the random-effects model, the pooled seroprevalence of HCV was 0.93% (95% CI 0.73, 1.13) (Fig. 7). In the random-effects meta-regression analysis, the pooled estimate of HCV showed a decrement from 1991 to 2018 but not statistically significant (Coefficient = − 0.041; 95% CI − 0.093, 0.01; P = 0.114). The pooled estimate changed from 1.40% (95% CI 0.54, 2.26%) before 2000 to 0.83% (95% CI 0.55, 1.11%) during 2015–2018 (Table 2). Egger’s statistics indicated that there was no small-study effect (coefficient of bias = 1.24; P = 0.446). About the effect size of sex and donor type on the pooled estimate of HCV, being a replacement blood donor was significantly associated with HCV (OR = 1.50; 95% CI 1.03, 2.20; I-squared = 75.7%) (Fig. 8). However, sex was not associated with HCV (OR = 1.24; 95% CI 0.95, 1.51). In both factors, there was no evidence of a small-study effect (P > 0.05).
The Pooled estimate and trend of Syphilis
With regard to syphilis, 23 studies comprising 32, 0730 study participants were eligible for estimating its pooled sero-epidemiology. These studies reported varying prevalence, ranging from 0.1% to 12.9%. In the random-effects model, the pooled estimate of syphilis was 1.50% (95% CI 1.20, 1.80%) (Fig. 9). The pooled estimate declined from 8.39% (95% CI 3.71, 13.08%) before 2000 to 1.04% (95% CI 0.67, 1.40%) during 2015–2018. The trend analysis showed a decrement from 1989 to 2018. This has been supported by the random-effects meta-regression analysis, and the analysis confirmed that the pooled seroprevalence of Syphilis was negatively but significantly associated with the midpoint year of the data collection period (Coefficient = − 0.085; 95% CI − 0.156, − 0.015; P = 0.021) (Table 2).
As there was a publication bias evidenced by egger’s statistics (P < 0.001), Duval and Tweedie’s trim and fill analysis was done to adjust the final pooled estimate of syphilis sero-epidemiology. For the adjustment of an estimate, 12 studies were filled, and it was found that the pooled estimated prevalence in the random-effects model was found to be 0.11% (95% CI 0.061, 0.19%).
Concerning the effect size of sex and donor type on the sero-epidemiology of syphilis, being a male blood donor was more likely to be sero-reactive for syphilis compared to being a female donor in the random-effects model (OR = 1.35; 95% CI 1.01, 1.79; I-squared = 68%) (Fig. 10). Unlike sex, donor type was not associated with syphilis sero-reactivity (OR = 1.84; 95% CI 0.56, 6.07; I-squared = 88.4%). No publication bias was observed for both factors (P > 0.05).
Discussion
Blood donation is becoming one of the lifesaving emergency services in medical and obstetrics services. Despite its advantage in lifesaving practice, blood transfusion is a potential risk for transmission of TTIs, such as HBV, HIV, HCV and syphilis [72]. In this review, the pooled sero-epidemiology of HBV, HIV, HCV and syphilis among blood donors in Ethiopia was 5.20% (95% CI 4.64, 5.77%), 2.83% (95% CI 2.43, 3.23%), 0.93% (95% CI 0.73, 1.13%) and 1.50% (95% CI; 1.20, 1.80%), respectively.
The pooled sero-epidemiology of HBV was higher than the estimates of a systematic review conducted in Iran (0.7%) [73], Eastern Mediterranean and Middle Eastern Countries (2.03%) [74] and European countries (0.74%) [75]. The possible reasons for the observed discrepancy might be due to the differences in cultural practices with regard to prevention of TTIs, and economic status which results in variation in vulnerability for sexually transmitted diseases. In addition, variation in the time period, the strength of preliminary screening of donors and factors related to testing algorithms used for screening might also be the possible reason for the discrepancy in the sero-epidemiology of HBV.
Moreover, our review showed that male blood donors (OR = 1.87; 95% CI 1.51, 2.32) were more likely to be HBV sero-reactive than females. This was in line with previously established studies in south Iran [76], Philippines [77], Ghana [78] and Egypt [79]. Another study also reported that females were less likely to be positive for HBV [80]. Almost 95% of HBV infected persons clear HBV by developing a protective Hepatitis B Surface Antibody (HBsAb) when HBV infection is acquired during adulthood, and only 5 to 10% of HBV infected persons will develop a persistent infection. Particularly, due to the slower plasma extinction rate in males, males are about 1.5 times more likely to develop chronic HBV infection relative to females, suggesting that sex is one of the genetic determinants that affect disease outcome [81]. On the other hand, replacement blood donors (OR = 1.68; 95% CI 1.34, 2.12) were more likely to be HBV sero-reactive compared to volunteer blood donors. This result was supported by earlier studies in which the reported prevalence of HBV among replacement donors was higher than that of voluntary donors [82, 83]. This could be the fact that during replacement donation, the donor might not consider the details of all his/her previous risky behaviours for TTIs as compared to volunteer donors.
Similarly, the pooled sero-epidemiology of HIV was higher than the estimate done in Iranian blood donors (0.0079%) [1]. The possible reasons for this difference might be attributable to the type of donors’ recruited (volunteer versus replacement), and the testing algorithms and kits used to diagnose HIV. In addition, the discrepancy was explained by the variation of the study period in which studies included for the estimation, as the Iranian included studies published since 1996, whereas our estimate included studies published since 1989.
Regarding factors associated with HIV, our review showed that being a replacement blood donor was more likely to have HIV infection compared to volunteer donors (OR = 2.09; 95% CI 1.39, 3.13). A consistent result had been reported previously in different countries [82, 83]. This might be partly explained by that, during replacement blood donation, family members of blood donors may not pass through strict preliminary screening as a result of the emergency nature of the case. Nevertheless, the prevalence of HIV was not significantly associated with sex. But other studies reported that the prevalence was high among males than females in the South of Iran [1, 76]. On the other hand, a study conducted in Iran in 2017 revealed that the seroprevalence of HIV among females was high [84]. This discrepancy in HIV report in terms of gender could be due to differences in the age status of employed donors and risky personal behaviours.
The pooled sero-epidemiology of HCV was 0.93% (95% CI 0.73, 1.13%). This estimate was consistent with estimates of first-time blood donors from European countries (0.02–3.3%). Our estimate is lower than an estimated prevalence of HCV Chinese blood donors (8.6%) [85]. The plausible reasons for the difference might be due to; firstly, the variation in the method of diagnosis, for example, the systematic review done in China included studies that used molecular techniques; secondly, the difference in population phenomena between countries like population migration, and thirdly, the difference in practices related to safe sexual practice. In addition, one of the major reasons for the variation in HCV prevalence is probably related to different safety regulations and variations in the level to which they are reinforced across countries (Additional file 1: Table S1, Additional file 2: Figure S1, Additional file 3: Figure S2, Additional file 4: Figure S3, Additional file 5: Figure S4, Additional file 6: Figure S5).
HCV was significantly associated with donor type. Replacement donors were 1.5 times more likely to be HCV sero-reactive compared to volunteer donors (OR = 1.50; 95% CI 1.03, 2.20). This might be probably because volunteer blood donors donate blood in a regular manner who know their sero-status for HCV infection before donating blood. Thus, volunteer blood donors were found to be relatively safer than replacement donors in which blood banks should follow stringent donor selection criteria with an emphasis on getting more volunteer blood donors. However, sex was not associated with HCV in this study.
Based on our review, the prevalence of syphilis was 1.50% (95% CI 1.20, 1.80%). This was almost consistent with the previous report from Kenya 1.0% [86]. On the other hand, a higher prevalence of syphilis was reported from Burkina Faso 3.96% [10] and Nigeria 4.2% [87]. In contrast to the current finding, a lower prevalence of syphilis was reported from earlier studies in Pakistan 0.43% [88] and India 0.43% [89]. The possible reason for the difference in the magnitude of syphilis across studies may be due to variation in risk behaviours in different geographical locations, or partly due to differences in socio-cultural practices.
On the other hand, male blood donors were more likely to be sero-reactive for syphilis (OR = 1.35; 95% CI 1.01, 1.79) compared to female donors. This might be due to differences in gender-related practices such as having multiple sexual partners. In addition, alcohol abuse is common among males that might expose males to high-risk behaviour which could attribute to an increased in the prevalence of syphilis. Furthermore, in some countries including Ethiopia, males make up the majority of blood donors. This could potentially be one factor as to why males had a higher odds ratio than females for TTIs. In this review, from the total 45 studies included in the review, 36 reported donor distribution by sex, and males accounted for 76.83% (324,590/422,499) of blood donors.
Conclusion
In this systematic review, the pooled estimate of HBV, HIV, HCV and syphilis was high suggesting that there is a need to design and implement strategies to reduce the burden of TTIs in the general population. Moreover, creating community awareness about the prevention of TTIs should be strengthened. Moreover, being a replacement donor was also associated with high HBV, HIV and HCV infection. In addition, advanced and vigilance screening techniques of donated blood should be strengthened prior to transfusion. Furthermore, national policies and strategies of post-donation counselling for recruitment and retention of safe regular donors are a timely need. Lastly, conducting a national population-based survey for TTIs has of utmost importance to monitor the burden and trend of TTIs in the community.
The review has some limitations. One of the limitations is a high level of heterogeneity between included studies which can be attributed to differences in methodology, study period, and geographic location. Besides, there were differences in the diagnostic methods used across the studies which might be implicated with a high level of heterogeneity. Given these limitations, the review was conducted according to the preferred reporting items for systematic review and meta-analysis (PRISMA-P 2015 statement) protocol. Besides, a comprehensive searching of databases and the involvement of experts improved the quality of evidence generated.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FMoH:
-
Federal Ministry of Health
- HBV:
-
Hepatitis B virus
- HBsAg:
-
Hepatitis B surface antigen
- HBsAb:
-
Hepatitis B surface antibody
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- OR:
-
Odds ratio
- TTI:
-
Transfusion transmitted infection
- WHO:
-
World Health Organization
References
Musavi H, Rahimi H, Kooti W, Dorostkar R, Azami M, Sharghi M, Ashrafi-Zadeh H, Firoozbakht M, Taheri RA. Prevalence of human immunodeficiency virus in Iranian blood donors: a systematic review and meta-analysis. Arch Iran Med. 2018;21(6):260–7.
Patel PA, Patel SP, Oza HV. Seroprevalence of transfusion transmitted infections (TTIs) in blood donors at western Ahmedabad-A secondary care hospital based study. Int J Biol Med Res. 2012;3(2):1806–10.
Azerefegn E, Worku M, Hailemariam M. Transfusion-transmissible infections among blood donors at Hawassa Regional Blood Bank Center, South Ethiopia. IJRSMB. 2018;4(3):10–6.
WHO: Global Status Report on Blood Safety and Availability 2016, 2017. File:, WHO 2016.
Jayaraman S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion. 2010;50(2):433–42.
Fasola FA, Otegbayo IA. Post-transfusion viral hepatitis in sickle cell anaemia: retrospective-prospective analysis. Nig J Clinical Practice. 2002;5(1):16–9.
WHO. Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/global-hepatitis-report-2017.
Sonderup MW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, Dusheiko G, Gogela N, Lohouès-Kouacou M-J, Lam P. Hepatitis C in sub-Saharan Africa: the current status and recommendations for achieving elimination by 2030. The Lancet Gastroenterol Hepatol. 2017;2(12):910–9.
Mora N, Adams WH, Kliethermes S, Dugas L, Balasubramanian N, Sandhu J, Nde H, Small C, Jose J, Scaglione S. A synthesis of hepatitis C prevalence estimates in Sub-Saharan Africa: 2000–2013. BMC Infect Dis. 2016;16(1):283.
Nagalo MB, Sanou M, Bisseye C, Kabore MI, Nebie YK, Kienou K, Kiba A, Dahourou H, Ouattara S, Zongo JD, et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011;9(4):419–24.
Bisseye C, Eko Mba JM, Ntsame Ndong JM, Kosiorek HE, Butterfield RJ, Mombo LE, M’Batchi B, Borad MJ, Nagalo BM, Allain JP. Decline in the seroprevalence of syphilis markers among first-time blood donors in Libreville (Gabon) between 2004 and 2016. BMC Public Health. 2019;19:167.
Jary A, Dienta S, Leducq V, Le Hingrat Q, Cisse M, Diarra AB, Fofana DB, Ba A, Baby M, Achenbach CJ, et al. Seroprevalence and risk factors for HIV, HCV, HBV and syphilis among blood donors in Mali. BMC Infect Dis. 2019;19:1064.
Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, Emmrich F, Sack U. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10:111.
WHO. Blood Safety in Ethiopia, WHO Regional office for Africa 2014. http://www.afro.who.int/en/ethiopia/country-programmes/topics/4466-blood-safety.html. Accessed date: 28/2/2019.
FMOH: Federal Democratic Republic of Ethiopia Ministry of Health. National Blood Transfusion Services Strategy 2005. http://www.transplant-observatory.org/download/national-blood-transfusion-services-strategy-2005-ethiopia/.
Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:761.
Mulugeta H, Dessie G, Wagnew F, Jara D, Leshargie CT, Negesse A. Seroprevalence and trend of human immunodeficiency virus among blood donors in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:383.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart L, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
JBI. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews: Checklist for Analytical Cross Sectional Studies 2016. http://joannabriggs.org/research/critical-appraisal-tools.html; accessed date: 14 March 2019.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc. 2015;13(3):147–53.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(6):1539–58.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Tweedie R, Duval S. A nonparametric, “Trim and Fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98.
Gebreselassie L. Occurrence of hepatitis B surface antigen and its antibody in Ethiopian blood donors. Ethiop Med J. 1983;21(4):205–8.
Gebreselassie L. The distribution of the subtypes of HBsAg in Ethiopia. Int J Epidemiol. 1986;15(3):392–4.
Huruy K, Kassu A, Mulu A, Worku N, Fetene T. Seroprevalence and risk factors for hepatitis B virus infection in the healthy blood donors at Jimma University Hospital, Southwest Ethiopia. Pharmacologyonline 2008; 3(32–7).
Tsega E, Mengesha B, Nordenfelt E, Hansson BG, Lindberg J. Serological survey of human immunodeficiency virus infection in Ethiopia. Ethiop Med J. 1988;26(4):179–84.
Dessie A, Abera B, Walle F, Wolday D, Tamene W. Evaluation of determine HIV-1/2 rapid diagnostic test by 4th generation ELISA using blood donors’ serum at Felege Hiwot Referral Hospital, northwest Ethiopia. Ethiop Med J. 2008;46(1):1–5.
Dessie A, Abera B, Wale F. Seroprevalence of major blood-borne infections among blood donors at Felege Hiwot referral hospital, northwest Ethiopia. Ethiop J Health Dev. 2007;21(1):68–9.
Terefe JE, Tsegaye A, Tamene M, G/Michael D, Zeleke A. Assessment of transfusion transmissible infections among blood donors (six years study) and strategy on direct laboratory testing cost of blood screening at national blood transfusion service of Addis Ababa, Ethiopia. [Unpublished article].
Birhan W, Deressa T, Enawgaw B, Abebe M, Wondiferaw W, Terefe B, Melku M. Distribution of transfusion-transmissible infection among apparently healthy blood donors in Amhara regional state, North West, Ethiopia: Cross sectional study. [Unpublished article].
Asrie F, Kiflie H, Gebremedin T. Magnitude of transfusion-transmissible infections among blood donors at North Gondar Blood Bank, northwest Ethiopia. Ethiop J Lab Med. 2016;3(3):44–53.
Teklemariam Z, Mitiku H, Weldegebreal F. Seroprevalence and trends of transfusion transmitted infections at Harar blood bank in Harari regional state, Eastern Ethiopia: eight years retrospective study. BMC Hematol. 2018;18:24.
Habte Y, Seyoum B, Alemayehu T. Hepatitis B virus infection and associated factors among blood donors at Dire Dawa, Eastern Ethiopia. J Antivir Antiretrovir. 2016;8(4):103–6.
Deressa T, Birhan W, Enawgaw E, Abebe M, Baynes HW, Desta M, Terefe B, Melku M. Proportion and predictors of transfusiontransmissible infections among blood donors in North Shewa Zone, Central North Ethiopia. PLoS ONE. 2018;13(3):0194083.
Ambachew H, Zheng M, Pappoe F, Shen J, Xu Y. Genotyping and sero-virological characterization of hepatitis B virus (HBV) in blood donors, Southern Ethiopia. PLoS ONE. 2018;13(2):0193177.
Degefa B, Gebreeyesus T, Gebremedhin Z, Melkamu G, Gebrekidan A, Hailekiros H, Tsegay E, Niguse S, Abdulkader M. Prevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus amongblood donors of Mekelle blood bank, Northern Ethiopia: a three-year retrospective study. J Med Virol. 2018;90(11):1724–9. https://doi.org/10.1002/jmv.25248.
Abate M, Wolde T. Seroprevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and syphilis among Blood Donors at Jigjiga Blood Bank, Eastern Ethiopia. Ethiop J Health Sci. 2016;26(2):153–60.
Gelaw B, Mengitsu Y. The prevalence of HBV, HCV and malaria parasites among blood donor in Amhara and Tigray regional states. Ethiop J Health Dev. 2008;22(1):3–7.
Gezahegn M, Woldemichael K, Godesso A. HIV sero-prevalence trend among blood donors in Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2012;22(1):37–43.
Hundie GB, Raj VS, GebreMichael D, Haagmans BL. Seroepidemiology of hepatitis B and C virus infections among blood donors in Ethiopia. J Med Virol. 2017;89(7):1300–3.
Kebede W, Mekonnen Z, Gerbi A, Abebe G. Transfusion-transmissible infection surveillance among blood donors in Southwest Ethiopia: a six years retrospective study. Asian Pac J Trop Dis. 2017;7(3):156–61.
Yami A, Alemseged F, Hassen A. Hepatitis B and C viruses infections and their association with Human Immunodeficiency virus: a cross-sectional study among blood donors in Ethiopia. Ethiop J Health Sci. 2011;21(1):67–75.
Tsega E, Mengesha B, Nordenfelt E, Hansson BG, Lindberg J. Prevalence of hepatitis B virus markers among Ethiopian blood donors: is HBsAg screening necessary? Trop Geogr Med. 1987;39(4):336–40.
Ataro Z, Urgessa F, Wasihun T. Prevalence and trends of major transfusion transmissible infections among blood donors in Dire Dawa Blood bank, Eastern Ethiopia: retrospective study. Ethiop J Health Sci. 2018;28(6):701–10.
Mohammed Y, Bekele A. Seroprevalence of transfusion transmitted infection among blood donors at Jijiga blood bank, Eastern Ethiopia: retrospective 4 years study. BMC Res Notes. 2016;9:129.
Assefa A, Mathewos B, Alemu A, Addis Z, Alem M, Gizachew M. Hepatitis B and C viral infections among blood donors at Bahir dar, Ethiopia. Int J Med Res Health Sci. 2013;2(3):624–30.
Biadgo B, Shiferaw E, Woldu B, Alene KA, Melku M. Transfusion-transmissible viral infections among blood donors at the North Gondar district blood bank, northwest Ethiopia: a three year retrospective study. PLoS ONE. 2017;12(7):0180416.
Birhaneselassie M. Prevalence of transfusion-transmissible infections in donors to an Ethiopian Blood Bank between 2009 and 2013 and donation factors that would improve the safety of the blood supply in underdeveloped countries. Lab Med. 2016;47(2):134–9.
Bisetegen FS, Bekele FB, Ageru TA, Wada FW. Transfusion-transmissible infections among voluntary blood donors at Wolaita Sodo University Teaching Referral Hospital, South Ethiopia. Can J Infect Dis Med Microbiol. 2016;2016:8254343.
Bonja F, Hussein M, Alemu J, Gemechu G, Birhaneselassie M. The prevalence of transfusion transmitted infections: a focus on hepatitis B virus among blood donors at Hawassa blood bank center, Southern Ethiopia. Int J Blood Transfus Immunohematol. 2017;7:7–14.
Rahlenbeck SI, Yohannes G, Molla K, Reifen R, Assefa A. Infection with HIV, syphilis and hepatitis B in Ethiopia: a survey in blood donors. Int J STD AIDS. 1997;8(4):261–4.
Zawde D, Sisay Y. National blood requirement, serum ALT and hepatitis in Ethiopian blood donors. Ethiop Med J. 1991;29(4):175–83.
Ramos JM, Tissiano G, Fano H, Yohannes T, Gosa A, Reyes F, Górgolas M. Prevalence of positive HIV, HBV, HCV and treponemal tests in blood donors in a rural hospital in southern Ethiopia. J Clin Virol 2016; 77(60–2).
Diro E, Alemu S, Asfawesen G-Y. Blood safety & prevalence of transfussion transmissible viral infections among donors at the Red Cross Blood Bank in Gondar University Hospital. Ethiop Med J. 2008;46(1):7–13.
Kabato AA, Weldearegay GM. Prevalence and associated risk factors of hepatitis B and hepatitis C virus among volunteer blood donors in Arba Minch Blood Bank SNNPR, Ethiopia. J Med Lab Diagn. 2016;7(4):20–7.
Shiferaw E, Tadilo W, Melkie I, Shiferaw M. Sero-prevalence and trends of transfusion-transmissible infections among blood donors at Bahir Dar district blood bank, northwest Ethiopia: a four year retrospective study. PLoS ONE. 2019;14(4):0214755.
Berhanu S, Abebaw S, Digissie A. Transfusion-transmissible infections among voluntary blood donors at Debre Tabor Blood Bank, North West Ethiopia: a three years retrospective study. Health Care Curr Rev. 2018;6:4.
Bialfew Y, Hailu G, Samuel T. Prevalence and associated factors of hepatitis B virus infection among blood donors in Debre Markos Blood Bank Centre, Northwest Ethiopia, 2018. Epidemiology (Sunnyvale). 2018;8:4.
Heyredin I, Mengistie B, Weldegebreal F. Sero-prevalence of transfusion transmittable infections and associated factors among blood donors in Eastern Ethiopia: an institutional-based cross-sectional study. SAGE Open Med. 2019;7:1–8.
Negash M, Ayalew M, Geremew D, Workineh M. Seroprevalence and associated risk factors for HIV, hepatitis B and C among blood Donors in South Gondar District blood Bank, Northwest Ethiopia. BMC Infect Dis. 2019;19:430.
Tigabu A, Engda T, Mekonnen F. Seroprevalence of transfusion transmissible viral infections (HIV, HBV and HCV) among voluntary blood donors at University of Gondar Comprehensive Specialized Hospital, Gondar; Northwest Ethiopia. BMC Infect Dis. 2019;19:393.
Sharew B, Mulu A, Teka B, Tesfaye T. HIV-Sero-prevalence trend among blood donors in North East Ethiopia. Afr Health Sci. 2017;21(3):712–8.
Seyoum E, Wolday D, Mekonen T, Girma M, Meselle T, Källander C, Gronowitz S, Britton S. Alternative approach to blood screening using the ExaVir reverse transcriptase activity assay. Curr HIV Res. 2005;3(4):371–6.
Kassu A, Moges F, Mekonnen F, Mengistu G, Abate E, Mekonnen E, Molla K, Zewde T, Aseffa A, Wondmikun Y, et al. Seroprevalence of human immunodeficiency virus among blood donors in Northwest Ethiopia, 1995–2002. Trop Doct. 2006;36(2):106–7.
Ramos JM, Reyes F, Tesfamariam A. Seroprevalence of human immunodeficiency virus among blood donors in a rural Ethiopian hospital. Trop Doct. 2008;38(3):182–3.
Sentjens RE, Sisay Y, Vrielink H, Kebede D, Ader HJ, Leckie G, Reesink HW. Prevalence of and risk factors for HIV infection in blood donors and various population subgroups in Ethiopia. Epidemiol Infect. 2002;128(2):221–8.
Assefa A, Rahlenbeck S, Molla K, Alemu S. Seroprevalence of HIV-1 and syphilis antibodies in blood donors in Gonder, Ethiopia, 1989–1993. J Acquir Immune Defic Syndr. 1994;7(12):1282–5.
Frommel D, Tekle-Haimanot R, Berhe N, Aussel L, Verdier M, Preux PM, Denis F. A survey of antibodies to hepatitis C virus in Ethiopia. Am J Trop Med Hyg. 1993;49(4):435–9.
Tsega E, Nordenfelt E, Hansson BG. Hepatitis C virus infection and chronic liver disease in Ethiopia where hepatitis B infection is hyperendemic. Trans R Soc Trop Med Hyg. 1995;89(2):171–4.
Sisay Y, Tegene Y. Prevalence of syphilis among Ethiopian blood donors. Ethiop J Health Dev. 1995;9(1):91103.
Garraud O, Filho LA, Laperche S, Tayou-Tagny C, Pozzetto B. The infectious risks in blood transfusion as of today—a no black and white situation. Presse Med. 2016;45(7–8 Pt 2):e303-311.
Alizadeh S, Pakzad I, Sayehmiri K, Pakzad R, Darshini P. Prevalence of hepatitis B among blood donors in Iran: a systematic review and meta-analysis. Asian J Biol Sci. 2014;7(2):35–46.
Babanejad M, Izadi N, Najafi F, Alavian SM. The HBsAg prevalence among blood donors from eastern Mediterranean and middle eastern countries: a systematic review and meta-analysis. Hepat Mon. 2016;16(3):35664.
Hahne SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar Mv. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181.
Farshadpour F, Taherkhani R, Tajbakhsh S, Tangestani MG, Hajiani G, Sharifi N, Taherkhani S, Nejadbolkheyr A. Prevalence and trends of transfusion-Transmissible viral infections among blood donors in south of Iran: an Eleven-Year retrospective study. PLoS ONE. 2016;11(6):0157615.
Yanase Y, Ohida T, Kaneita Y, Agdamag DMD, Leaño PS, Gill CJ. The prevalence of HIV, HBV and HCV among Filipino blood donors and overseas work visa applicants. Bull World Health Organ. 2007;85(2):131–7.
Walana W, Ahiaba S, Hokey P, Vicar EK, Acquah SEK, Der EM. XXXX R: Sero-prevalence of HIV, HBV and HCV among blood donors in the Kintampo Municipal Hospital, Ghana. Br Microbiol Res J. 2014;4(12):1491–9.
Nada HA, Atwa M. Seroprevalence of HBV, HCV, HIV and syphilis markers among blood donors at Suez Canal University Hospital Blood Bank. J Blood Disord Transf. 2013;5(1):177.
Apriastini NKT, Ariawati K. Risk factors of acute blood transfusion reactions in pediatric patients in Sanglah General Hospital, Bali-Indonesia. Bali Med J. 2017;6(35):534–8.
Thursz MR. Host genetic factors influencing the outcome of hepatitis. J Viral Hepat. 1997;4(4):215–20.
Bhawani Y, Rao PR, Sudhakar V. Seroprevalence of transfusion transmissible infections among blood donors in a tertiary care hospital of Andhra Pradesh. Biol Med. 2010;2(4):45–8.
Shah N, Shah J, Jhaveri P, Patel K, Shah C, Shah N. Seroprevalence of HBV, HCV, HIV, and syphilis among blood donors at a tertiary care Teaching Hospital in Western India. Gujarat Med J. 2013;68(2):35–9.
Zadsar M, Pourfathollah AA, Rasouli M, Karimi G. Trends and sero-epidemiology of human immuno deficiency virus in voluntary blood donations in Iran, 2008–2013. Arch Iran Med. 2017;20(3):135–40.
Gao X, Cui Q, Shi X, Su J, Peng Z, Chen X, Lei N, Ding K, Wang L, Yu R, et al. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: a systematic review and meta-analysis. BMC Infect Dis. 2011;11:88.
Mahuro GM, Gichangi PB, Mwangi CW, Kipkorir N. Seroprevalence of hepatitis B, hepatitis C, human immunodeficiency virus and syphilis in donated blood in Kenya, 2016: situation analysis. J Blood Disord Transfus. 2017;8(4):1000389.
Okoroiwu HU, Okafor IM, Asemota EA, Okpokam DC. Seroprevalence of transfusion-transmissible infections (HBV, HCV, syphilis and HIV) among prospective blood donors in a tertiary health care facility in Calabar, Nigeria; an eleven years evaluation. BMC Public Health. 2018;18:645.
Attaullah S, Khan S, Khan J. Trend of transfusion transmitted infections frequency in blood donors: provide a road map for its prevention and control. J Transl Med. 2012;10:20.
Makroo RN, Hegde V, Chowdhry M, Bhatia A, Rosamma NL. Seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north India. Indian J Med Res. 2015;142(4):317–22.
Acknowledgements
The authors would like to acknowledge the College of Medicine and Health Sciences, the University of Gondar for infrastructure support to conduct this systematic review. Besides, we are grateful to the authors of the studies included in this systematic review.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
MM, ES, MA, BB, SA, BW, and DG were involved in the design, literature searching, and assessment of study quality and data extraction. BE, ZA, BT, TD, DD, AK and TT revised the quality of literature. MM, ES, BW, DG, BT, BB, AK, and MA performed the statistical analysis and drafted the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA 2009 checklist.
Additional file 2: Figure S2.
A Plot of Egger’s test of publication bias for pooled estimate of HIV among Blood donors in Ethiopia.
Additional file 3: Figure S3.
A Plot of Egger’s test of publication bias for pooled estimate of HBV among Blood donors in Ethiopia.
Additional file 4: Figure S4.
A Plot of Egger’s test of publication bias for pooled estimate of HCV among Blood donors in Ethiopia.
Additional file 5: Figure S5.
A Plot of Egger’s test of publication bias for pooled estimate of Syphilis among Blood donors in Ethiopia.
Additional file 6: Figure S6.
Effect size of sex on HCV infection among blood donors in Ethiopia—a sub-group analysis by geographic region.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Melku, M., Ambachew, S., Enawgaw, B. et al. Sero-epidemiology and associated factors of HIV, HBV, HCV and syphilis among blood donors in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis 21, 778 (2021). https://doi.org/10.1186/s12879-021-06505-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06505-w