Abstract
Background
Group B streptococcus (GBS) is reported as the leading cause of neonatal sepsis and meningitis. Newborns from GBS colonized pregnant women are at high risk of infection.
Method
A Hospital based cross-sectional study was conducted at Hawassa University Comprehensive Specialized Hospital from November 05, 2014 to March 25, 2015. A total of 280 pregnant women along with their newborns were screened for GBS using standard method recommended by Center of Disease Control and Prevention. GBS strains were serotyped by using serotype specific antisera. A structured questionnaire was used to collect sociodemographic, obstetrics and clinical data of pregnant women and newborns. Data was analyzed by using chi-square and logistic regression to determine factors associated with prevalence of GBS among pregnant women and newborns. Descriptive statistics was used to determine prevalence of GBS among pregnant women and newborns. P value less than 0.05 was considered statistically significant.
Result
Prevalence of GBS among pregnant women, newborns and vertical transmission rate at Hawassa University Comprehensive Specialized Hospital were 44(15.7%), 26(8.9%) and 59.1% respectively. Among 26 GBS colonized newborns one developed sign and symptoms of early onset disease. Serotype distribution of GBS isolates collected from pregnant women and newborns was Ia 13(18.6%), Ib 9(12.9%), II 24(34.3%), III 8(11.4%), V 14(20%), and NT 2 (2.9%).
Conclusion
In our study we found relatively high prevalence of GBS among pregnant women and vertical transmission rate. The most prevalent GBS serotypes identified in this study were serotype II followed by V, Ia and Ib. Therefore, appropriate prevention strategies such as intrapartum antibiotic prophylaxis and vaccine development should be considered.
Similar content being viewed by others
Background
Neonatal disease caused by GBS is traditionally classified as early onset disease (EOD) which occurs in newborn less than 7 days of age and late onset disease (LOD) which occurs in newborn whose age is between 7 days to 90 days. The primary risk factor for EOD is recto vaginal colonization of pregnant women with GBS before delivery [1, 2]. Prevalence of GBS among pregnant women in the world is estimated to be 18% with regional variation of 11–35% [3]. Risk factors such as prolonged rupture of membrane, prematurity, chorioamnionitis, low-level of anti-GBS capsular antibody and previous newborn with EOD can increase the likelihood of EOD [1,2,3].
Intrapartum antibiotic prophylaxis (IAP) strategy is being used in some industrialized countries to prevent vertical transmission of GBS from pregnant women to their newborns. The strategy recommends administration of IAP based on universal screening of pregnant women for GBS at 35–37 weeks of gestation or those with risk factors such as preterm labor or premature rupture of membranes; prolonged rupture of membranes; intrapartum fever ≥100.4°F; history of a previous newborn disease caused by GBS and GBS bacteruria during pregnancy [4].
Penicillin is the drug of choice for prophylaxis, and ampicillin is an alternative [5]. For pregnant women who are allergic to penicillin and without a history of anaphylaxis, cefazolin is the preferred antibiotic. Vancomycin is recommended for those with a history of anaphylaxis, if GBS is resistant to erythromycin and clindamycin. Erythromycin or clindamycin can be used in some countries for IAP, if GBS are susceptible to them. However because of high resistance erythromycin is no longer recommended in the United States for pregnant women who are allergic to penicillin [6].
The prevention strategy does not eliminate all cases of EOD ceased by GBS; it does not affect LOD caused by GBS and there is concern of selection of antimicrobial resistant bacteria [3]. Above all, it is not feasible for developing countries including Ethiopia, with resource limitation in laboratory diagnosis. As an alternative, capsular based vaccine is being developed and currently vaccine formulation containing Ia, Ib, and III has completed phase II clinical trial and has been reported to be cost effective [4, 7]. To come up with comprehensive vaccine, data on GBS serotype is required from various geographic locations.
There is limited information regarding prevalence of GBS among pregnant women and newborns, vertical transmission rate, and serotype distribution in Southern parts of Ethiopia. Therefore, this study was conducted with the aim of determining prevalence of GBS among pregnant women and their newborns, vertical transmission rate of GBS from pregnant women to their newborns, serotype distribution of GBS and risk factors associated with prevalence of GBS among pregnant women and their newborns attending Hawassa University Comprehensive Specialized Hospital.
Methods
Study design
A Hospital based cross-sectional study was conducted from November 05, 2014 to March 25, 2015 at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia.
Study area
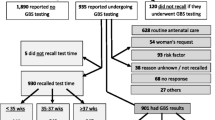
Hawassa is the capital city of South nation and nationalities region of Ethiopia on the shore of Lake Hawassa and it is located 275 km south of Addis Ababa, capital city of Ethiopia. The hospital is the largest hospital in the region; it serves as teaching, training and clinical service center. The Antenatal clinic of Hawassa University Comprehensive Specialized Hospital serves an average of 45 pregnant women per day and about 200 beds are available for both prenatal and postnatal service in the hospital. During the study period there were 1290 deliveries out of this 280 participants were included in the study, the rest were not included in the study due to various reasons (Fig. 1). Institutional delivery in the study area was 25.5% (information was obtained from health management information system of Hawassa Comprehensive Specialized Hospital.)
Study population
All pregnant women admitted for delivery at Hawassa University Comprehensive Specialized Hospital during the study period who fulfilled the inclusion criteria and provided informed consent were screened for GBS colonization. Convenience sampling technique was used to recruit the study participants. Newborns were followed for sign and symptoms of EOD through telephone for 7 days. Telephone interview was made with parents about their newborns health status by using ten questions that could indicate disease development. Sample size was calculated by using single proportion formula, margin of error = 0.05, confidence interval = 95%, prevalence from previous study conducted in Ethiopia, 20.86% [8].
Inclusion criteria
Pregnant women of all gestational age who were admitted to Hawassa University Comprehensive Specialized Hospital and were able to provide consent.
Exclusion criteria
Pregnant women who were on antibiotics for the last 3 weeks, pregnant women with cesarean section delivery.
Operational definition
Early onset disease: Newborn (age less than 7 days) with fever, hypothermia, vomiting, poor appetite, abnormal breathing, buldged anterior fontanelle as interviewed by telephone.
Premature rupture of membrane: rupture of membrane which occurs at gestation age less than 37 weeks.
Chorioamnionitis: Pregnant women with intrapartum fever, uterine tenderness, fetal tachycardia, maternal tachycardia, and foul smelling or purulent amniotic fluid.
Vertical transmission rate: Transmission of GBS from pregnant women to their newborns as confirmed by isolating the same colonizing GBS serotype from both mother and newborn.
Sample collection, handling and transport
Swab from lower vaginal and rectal area of pregnant women and swabs from different body parts of newborns were collected by attending midwifery. Two specimens were collected by rolling separate sterile swabs over lower vagina and the rectal region of pregnant women, according to CDC guideline [6]. Specimens were collected from newborns by rubbing external ear, nasal area, throat and umbilical area by using sterile swabs. All specimens were collected by attending midwifery from informed and consented pregnant women during delivery. All collected specimens were placed in Stuart transport media (BD Diagnostics, USA) and was delivered to the Microbiology Laboratory within 4 h.
Culture and identification of GBS
Vaginal and rectal swabs from pregnant women during delivery and swabs from external ear, nasal, throat and umbilical area of newborns were placed into Lim broth (BD Diagnostics, USA) supplemented with colistin (10 μg/ml) and nalidixic acid (15 μg/ml). The inoculated selective medium was incubated for 18–24 h, at 37 °C in CO2 enriched atmosphere, sub-cultured onto sheep blood agar (BD Diagnostics, USA) and further incubated in CO2 enriched atmosphere at 37 °C for 18–24 h. If GBS was not identified after incubating for 18–24 h, the blood agar plate was re-incubated and examined after 48 h to identify suspected colonies. All suspected GBS colonies (β-hemolytic, or non-hemolytic, gram positive cocci, catalase negative) were sub-cultured and isolated for confirmatory testing. A CAMP (Christite, Atkins, Munch, Petersen) test were considered presumptive identification of a positive GBS culture. Ambiguous CAMP test culture results were re-tested using a Strpt. B Grouping Latex (Remel, USA).
Capsular serotyping was done for all GBS isolates by slide agglutination tests by using type specific 10 antisera for serotypes Ia, Ib, II, III, IV, V, VI, VII, VIII and IX (Statens Serum Institute, Denmark) as previously described by Slotved et al [9].
Quality control
To maintain the quality of culture medias and reagents, control strains Staphylococcus aureus (ATCC 24923), Streptococcus pyogenes (ATCC 19615), and Streptococcus agalactiae (ATCC12403) were used during the study. To maintain the quality of antisera, the manufacture’s instruction was followed, and positive and negative controls were used. For socio-demographic, obstetric and clinical data a pretested structured questionnaire was used and data was entered in two separate computers. To ensure the viability of GBS, all samples were placed in transport media immediately after collection and processed within 4 h of collection.
Statistical analysis
Data entry and analysis was done using computer with SPSS version 20 software. Frequency distribution was used to calculate prevalence figures from the total study population and separately by age group and risk factors. Bivariate logistic regression was used to compare prevalence of GBS with various factors. P value less than 0.05 was considered significant.
Results
Socio-demographic characteristics
Among 1290 deliveries that occurred during the study period, 280 pregnant women along with their 292 newborns participated in the study. 147(52.5%), 40(14.3%) and 93(33.2%) participants were from Hawassa, Shashamane and other areas respectively. 232(82.9%) participants were housewives and 111(39.6%) participants belongs to the age group of 21–25 years. Out of 292 newborns 152(52.1%) were males and 140(47.9%) were females.
Obstetric characteristics
Pregnant women
Out of the total 280 pregnant women participating in the study, 249(88.9%) delivered at gestational age of 37–42 weeks; 134(47.9%) of participants were primigravida and 146 (52.1%) were multigravida, 115(41.1%) participants had previous vaginal delivery; 241(86.4%) had ruptured membrane of 0–5 h duration and 18(6.4%) were with premature rupture of membrane (Table 1).
Newborns
Among 292 newborns participating in the present study, 12(4.1%) were twins and 280(95.9%) were alive at birth, 248(84.9%) were in the weight range of 2500-4000 g, 161 (55.1%) had APGAR score at 5 min > 7; and those with other abnormalities were 13(4.5%). All newborn twins were alive at birth (Table 2).
Prevalence of GBS among pregnant women and their newborns and vertical transmission rate
The prevalence of GBS among pregnant women and their newborns and vertical transmission rate were 44/280(15.7%), 26/292(8.9%), and 26/44(59.1%) respectively. The serotype distribution of GBS isolated from pregnant women and newborns was: Ia 13(18.6%), Ib 9(12.9%), II 24(34.3%), III 8(11.4%), V 14(20%), and NT 2(2.9%). All GBS serotypes identified from newborns were the same as GBS serotypes identified from their respective mothers (Table 3). Out of 26 newborns colonized with GBS, 1 developed signs and symptoms of EOD.
Out of the 12 newborn twins, 2 (one pair) were colonized with GBS. Among the 12 newborns who were not alive at birth, 2 were colonized with GBS. Out of 44 mothers colonized with GBS, 2 gave birth to babies who were not alive at birth, and out of 236 mothers who were not colonized with GBS, 10 had newborns who were not alive at birth.
Factors associated with prevalence of GBS among pregnant women and newborns
Pregnant women
The prevalence of GBS among pregnant women and their newborns was not significantly associated with the majority of factors. Newborns with other disease had 4.95 time chance to be colonized with GBS (Tables 4 and 5). Other disease includes cyanosis, spinal bifida, and hydrocephaly.
Discussion
In this study, prevalence of GBS among pregnant women and newborns and vertical transmission rate from pregnant women to their newborns at Hawassa University Comprehensive Specialized Hospital, Ethiopia was 15.7, 8.9 and 59.1% respectively. Prevalence of GBS among pregnant women in this study was comparable with prevalence of GBS among pregnant women reported from Brazil 14.6% and Germany 16% [10, 11]. In contrast to our finding, some countries in Asia reported low prevalence of GBS among pregnant women [12,13,14]. Studies from South Africa, Europe and recent systematic review and meta-analysis reported higher prevalence of GBS among pregnant women than current study [2, 3, 6, 15]. Similarly, a previous study from Hawassa, Ethiopia reported high prevalence of GBS among pregnant women (20.8%) [8], while comparable result was reported from central part of Ethiopia, Addis Ababa (14.6%) [16] and western parts of Ethiopia (12.2%) [17, 18]. Unlike this study, a higher maternal GBS colonization rate was reported from Nigeria (64%) [19].
These results indicate that the prevalence of maternal GBS colonization, which is primary risk factor for EOD, differs in different countries and within the same country [3]. The difference can be due to geographical differences, laboratory methods used, time and site of specimen collection [3, 14, 15, 20]. The prevalence of GBS among newborns, 8.9%, and vertical transmission rate of GBS from pregnant women to their newborns, 59.1% identified in this study were comparable with the report from earlier studies conducted in the 1970s [21, 22]. Previous studies from developed countries indicated that about 50% newborns from GBS colonized pregnant women will be positive for GBS at birth. Among GBS colonized newborns, approximately 1–3% will develop invasive disease [22]. Vertical transmission rate of GBS from mother to newborns reported from eastern part of Ethiopia, 45.02%, was lower compared to the current study [23]. The difference observed can be due to several reasons such as sample collection, laboratory methods and factors related to study participants.
Five newborns in our study developed sign and symptoms of EOD and four were from 287 GBS non-colonized newborns and one was from 26 GBS colonized newborns. One newborn who developed sign and symptoms of EOD was colonized with GBS and the mother was also positive for GBS, this may indicated GBS was transferred from mother to newborn and was responsible for the development of EOD even though the causative agent was unconfirmed. In this study, out of 26 GBS colonized newborns two (7.7%) were not alive at birth. There was no difference in prevalence of stillbirth among GBS colonized mothers two (4.5%) and non GBS colonized mothers 10/236(4.2%) (P = 0.63); this may be due to small sample size. Among 12 mothers with stillbirth, only one showed signs and symptom of disease. Even though it is not well established, some studies indicated maternal GBS colonization could cause still birth [24]. Systematic review and meta-analysis by Seale et al [25] estimated that 1% of all stillbirth in developed countries and 4% in Africa were associated with GBS. In our study, we did not find significant association between prevalence of GBS among pregnant women and newborns and majority of factors measured (P > 0.05). The only factor that showed significant association with neonatal GBS colonization was newborn who presented with other disease (P = 0.012).
There is scarce or no data on GBS serotype distribution from the study area. In the present study, the most prevalent serotypes was serotype II followed by serotype V (20%), Ia (18.6%), Ib 12.9%, and III (11.4%). Out of the total GBS strains collected, 2.9% were non-typeable.
Group B streptococcus (GBS) serotype distribution is not uniform across different countries [20]. The prevalence of serotype II in this study was high compared to report from several countries, which is less than 15% [3, 4, 26]. Relatively higher prevalence of serotype II (19.1%) was reported from Brazil [27], even though it is still far below the finding of our study. Serotype V was the second most prevalent in our study area, in line with what was reported in USA (17%) [28]. In contrast to this study, a low prevalence of serotype V was report in Japan (12%) [29], Poland (5%) [30], Brazil (13.6%) [27], and China (14.2%) [13].
Prevalence of serotype Ia detected in this study was similar with prevalence of serotype Ia reported from China (17.9%) [13] and Malaysia (17.5%) [26]. Higher prevalence of serotype Ia was reported from Brazil (27.6%) [27] and Nigeria (23.9%) [19]. Even though serotype Ib was the third most prevalent GBS serotype in this study, it was high compared to reports from many countries such as Poland (5%) [30] and South Africa (6.7%) [31]. Unlike the present study, high prevalence of serotype Ib was reported from China (16.1%) [13].
The least prevalent serotype found in the current study was serotype III. In contrast to the current study higher prevalence of serotype III was reported from Poland (60%) [30], Korea (29.8%) [32] and China (32.1%) [13]. Several studies have indicated that GBS serotype III to be the most virulent compared to other serotypes [10].
The few numbers of GBS isolates which were non-typeable does not allow comparison with other findings with on-typeability rate reported from other countries such as 2% from South Africa [33] and 20% from Brazil [34]. There are several explanations for non-typeability of GBS such as; non-encapsulated variant, uncharacterized polysaccharide, mutation in genes essential for capsule expression, reversible non-capsular phase variation or technical problem [35].
Serotype Ia, Ib, II, III and V are frequently mentioned in Africa including in our study, while serotypes IV, VI, VII, VIII, and serotype IX were rarely reported [10]. However, Cools et al. [36] reported relatively high prevalence of serotypes VI, VII, and VIII in Kenya and serotypes IV, VI and VIII in South Africa, but serotype Ib was not detected.
Based on our study vaccine formulation containing serotype II, V, Ia, Ib and III may prevent EOD caused by GBS in the study area. The current vaccine formulation in clinical trial, which is composed of Ia, Ib and III, may not cover all the GBS serotypes circulating in the study area, particularly the major serotypes, II and V which are missing from the vaccine formulation. Therefore, it is important to have data on GBS serotypes from different regions to develop a universal vaccine.
Limitations of this study: we were unable to confirm the causative agent of EOD and stillbirth by using culture method and we were unable to confirm the causative agent of stillbirth. As we used convenience sampling technique selection bias was not avoided and the study population was not representative of all pregnant women in the study area. We used this method of sampling technique because the time and budget allocated for the study was limited. Risk factors such as sexual transmitted disease and socioeconomic status were not measured.
Conclusion
Prevalence of GBS among pregnant women and newborns and vertical transmission of GBS from pregnant women to their newborns at Hawassa University Comprehensive Specialized Hospital were 15.7, 8.9 and 59.1% respectively. The predominant GBS serotypes we found in this study were II, V, Ia, Ib, and III. Majority of the risk factors measured did not show significant association with prevalence of GBS among pregnant women and newborns. Based on the findings of the current study we recommend that appropriate prevention strategy should be implemented to reduce neonatal disease that can be caused by GBS in the study area. GBS serotypes which are prevalent in the study area need to be considered during vaccine development. Most importantly, future studies in Ethiopia should focus on measuring the burden of EOD and LOD caused by GBS.
Abbreviations
- APGAR:
-
Appearance, pulse, grimace, activity, respiration
- CDC:
-
Center of Disease Control and Prevention
- EOD:
-
Early onset disease
- GBS:
-
Group B streptococcus
- HUSH:
-
Hawassa University Comprehensive Specialized Hospital
- IAP:
-
Intrapartum antibiotic prophylaxis
- LOD:
-
Late onset disease
- PROM:
-
Premature rupture of membrane
References
Schrag S, Growitz R, Fuitz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease revised guideline from CDC. MMWR. 2002;51:1–22 PubMed Google scholar.
American College of Obstetricians and Gynecologists Committee on Obstetric Practice (ACOG). Prevention of early-onset group B streptococcal disease in newborns [ACOG committee opinion]. Washington DC: ACOG; 1996. GoogleScholar https://www.acog.org/Clinical-Guidance
Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianehi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(S2):S100–11 PubMed, PMCID: PMC5848259.
Kim S-Y, Russell LB, Park J, Verani JR, Madhi S, Cutland CL, et al. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine. 2014;32:10 PubMed, GoogleScholar.
Verani JR, Spina NL, Lynfield R, Schaffner W, Harrison LH, Holst A, et al. Early-onset group B streptococcal disease in the United States: potential for further reduction. ObstetGynecol. 2014;123(4):828–37 PubMed, PMID: 24785612.
Sinha A, Russell LE, Tomczyk S, Verani J, Schrag S, Berkly JA et al and the GBS cost-effectiveness Analysis in Sub-Sahran Africa working group (2016). Disease burden of group B Streptococcus among infants in sub-Sahran Africa. A systematic literature review and meta-analysis. Pediatr Infect Dis 35(9):933–942. http://www.who.int/immunization/documents/en/
Verani JR, McGee L, Schrag SJ. Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from CDC. MMWR. 2010;59:RR10 https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm.
Mohammed M, Asrat D, Woldeamanuel Y, Assegu D. Prevalence of group B Streptococcus colonization among pregnant women attending antenatal clinic of Hawassa health center, Hawassa, Ethiopia. Ethiop J Health Dev. 2012;26:37–41 https://www.ajol.info/index.php.
Slotved HC, Elliott J, Thompson T, Konradsen HB. Latex assay for serotyping of group B Streptococcus isolates. J Clin Microbiol. 2003;41(9):4445–7 PMCID: PMC193831 PMID: 1295828.
Simoes JA, Alves VM, Fracalanzza SE, de Camarg RP, Mathias L, Milanez HM, et al. Phenotypical characteristics of group B Streptococcus in Parturients. Braz J Infect Dis. 2007;11(2):261–6 PMCID: PMC3008495, PMID: 20881175.
Brimil N, Barthell E, Heindrichs U, Kuhn M, Lutticken R, Spellerberg B, et al. Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol. 2006;296:39–44 PubMed, GoogleScholar.
Kulkarni A, Pawar S, Dharmadhikari C, Kulkarni R. Colonization of pregnant women and their newborn infants with group B streptococci. Indian J Med Microbiol. 2001;12(9):97–100. jpgmonline.com
Wang P, Tong JJ, Ma XJ, Song FL, Fan L, Guo CMa. Serotypes, antibiotic susceptibilities, and multi-locus sequence type profiles of Streptococcus agalactiae isolates circulating in Beijing, China. PLoS One. 2015;10(3) PubMed PMID:25781346 PMCID:PMC4363692.
Darabi R, Tadi S, Mohit M, Sadeghi E, Hatamizadeh G, Kardeh B, et al. The prevalence and risk factors of group B streptococcus colonization in Iranian pregnant women. Electronic Physician. 2017;9(5):399–4404. https://doi.org/10.19082/4399.
Dauby N, Adler C, Miendje Deyi VY, Sacheli R, Busson L, Chamekh M, et al. Prevalence, risk factors, and serotype distribution of group B Streptococcus colonization in HIV-infected pregnant women living in Belgium: a prospective cohort study. Open Forum Infectious Diseases. 2018;5(12). https://doi.org/10.1093/ofid/ofy320.
Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. 2018;18:135 https://www.ncbi.nlm.nih.gov/pubmed/29728084.
Mengist HM, Zewdie O, Belaw A, Dabsu R. Prevalence and drug suseptibility pattern of group B streptococcus (GBS) among pregnant women antenatal care (ANC) in Nekemte referral hospital (NRH), Nekemte, Ethiopia. BMC Res Notes. 2017;10:388 https://www.ncbi.nlm.nih.gov/pubmed/28797286.
Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. 2016;9:351 https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-016-2158-4.
Elikwu CJ, Oduyebo O, Ogunsola FT, Anorlu RI, Okoromah CN, König B. High group B streptococcus carriage rates in pregnant women in a tertiary institution in Nigeria. Pan Afr Med J. 2016;25:249. https://doi.org/10.11604/pamj.2016.25.249.9433.
Dagnew AF, Cunnington MC, Dube Q, Edwards MS, French N, Heyderman RS, et al. Variation in reported neonatal group B streptococcal disease incidence in developing countries. Clin Infect Dis. 2012;55(1):91–102. https://doi.org/10.1093/cid/cis395.
Boyer K, Gotoff S. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986;314:1665–9 https://www.ncbi.nlm.nih.gov/PubMed/3520319.
Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine. 2016;34(2016):2876–9 PMID:26988258 PubMed.
Yadeta TA, Worku A, Egata G, Seyoum B, Marami D, Berhane Y. Vertical transmission of group B Streptococcus and associated factors among pregnant women: a cross-sectional study, eastern Ethiopia. Infect Drug Resist. 2018;11:397–404 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5856028.
Nan C, Dangor Z, Cutland C, Edwards M, Madhi S, Cunnington M, et al. Maternal group B Streptococcus-related stillbirth: a systematic review. BJOG. 2015;122:1437–45 https://www.ncbi.nlm.nih.gov/PubMed/26177561.
Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin Infect Dis. 2017;65(S2):S200–19. https://doi.org/10.1093/cid/cix664.
Eskandarian N, Ismail Z, Neela V, van Belkum A, Desa MN, Nordin SA. Antimicrobial susceptibility profiles, serotype distribution and virulence determinants among invasive, non-invasive and colonizing Streptococcus agalactiae (group B streptococcus) from Malaysian patients. Eur J Clin Microbiol Infect Dis. 2015;34:579–84 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4356882/.
Dutra VG, Alves VM, Olendzki AN, Dias CA, Bastos AF, Santos GO, et al. Streptococcus agalactiae in Brazil: serotype distribution, virulence determinants and antimicrobial susceptibility. BMC Infect Dis. 2014;14:323 https://www.ncbi.nlm.nih.gov/pubmed/24919844.
Ippolito DL, James WA, Tinnemore D, Huang RR, Dehart MJ, Williams J, et al. Group B Streptococcus serotype prevalence in reproductive-age women at a tertiary care military medical center relative to global serotype distribution. BMC Infect Dis. 2010;10:336 https://www.ncbi.nlm.nih.gov/pubmed/21106080.
Matsubara K, Baba K, Katayama K, Nigami H, Sugiyama M. Seroepidemiologic studies of serotype VIII group B Streptococcus in Japan. J Infect Dis. 2002;186:855–8 https://www.ncbi.nlm.nih.gov/pubmed/12198624.
Brzychczy-Wloch M, Gosiewski T, Bulanda M. Multilocus sequence types of invasive and colonizing neonatal group B streptococci in Poland. Med Princ Pract. 2014;23(4):323–30 https://www.karger.com/Article/Abstract/362368.
Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B Streptococcus isolates causing disease in infants and colonizing maternal-newborn. PLoS One. 2011;6(3):e17861 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0017861.
Soo YS, Srinivassan U, Oh K-Y, Shin H-H, Chae JD, Kim MY, et al. Changing molecular epidemiology of group B Streptococcus in Korea. J Korean Med Sci. 2010;25:817–23 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2877223/.
Madhi SH, Padebe K, Crewe-brown H, Frasch CE, Arkane G, Mokhachare M, et al. High burden of invasive Streptococcus agalactiae disease in south African infants. Ann Trop Pediatr. 2003;23:15–23 https://www.ncbi.nlm.nih.gov/pubmed/12648320.
Pinto TC, Costa NS, Souza AR, da Silva LG, de Almeida AB, Fernandes FG, et al. Distribution of serotypes and evaluation of antimicrobial susceptibility among human and bovine Streptococcus agalactiae strains isolated in Brazil between 1980 and 2006. Braz J Infect Dis. 2013;17(2):131–6 http://www.scielo.br/pdf/bjid/v17n2/v17n2a03.pdf.
Rosini R, Campisi E, De Chiara M, Tettelin H, Rinaudo D, Toniolo C, et al. Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS One. 2015;10(5):e0125985 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0125985.
Cools P, Jespers V, Hardy L, Crucitti T, Delany-Moretlwe S, Mwaura M, et al. A multi-country cross-sectional study of vaginal carriage of group B streptococci (GBS) and Escherichia coli in resource-poor settings: prevalence and risk factors. PLoS One. 2016;11(1) http://www.ncbi.nlm.nih.gov/pubmed/9655542. Google scholar.
Acknowledgments
We would like to acknowledge staffs of Midwifery, Pediatrics, Laboratory Department of Hawassa University, College of Medicine and Health Sciences for facilitating the study during sample collection and processing.
Ethical approval and consent to participate
The study was approved by the Institutional Review Board of the College of Health Sciences, Addis Ababa University (Ref No: 069/13/DMIP) and the National Ethics and Research Committee (Ref No: 3.10/795/06). Written informed consent was obtained from all study participants. The parents also consented for permission to collect samples from their newborns.
Funding
The budget needed for study design, data collection, processing and analysis in this study was covered by Addis Ababa University College of Health Science and Hawassa University.
Availability of data and materials
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
MMA Conceived, designed the experiments, laboratory work, data analysis and write up YW review, supervision, analysis and manuscript preparation DAW review, supervision, analysis and manuscript preparation DAF laboratory work, designed the experiments, analysis and manuscript preparation TEC facilitate data collection SJI Facilitated during specimen collection BTT managed newborns who developed sign and symptoms of early onset disease EKW facilitated data collection MTD facilitated data collection. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ali, M.M., Woldeamanuel, Y., Woldetsadik, D.A. et al. Prevalence of group B streptococcus among pregnant women and newborns at Hawassa University comprehensive specialized hospital, Hawassa, Ethiopia. BMC Infect Dis 19, 325 (2019). https://doi.org/10.1186/s12879-019-3859-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-3859-9