Abstract
Background
Group B Streptococcus (GBS) serotype (Ia, Ib, II-IX) correlates with pathogen virulence and clinical prognosis. Epidemiological studies of seroprevalence are an important metric for determining the proportion of serotypes in a given population. The purpose of this study was to evaluate the prevalence of individual GBS serotypes at Madigan Healthcare System (Madigan), the largest military tertiary healthcare facility in the Pacific Northwestern United States, and to compare seroprevalences with international locations.
Methods
To determine serotype distribution at Madigan, we obtained GBS isolates from standard-of-care anogenital swabs from 207 women of indeterminate gravidity between ages 18-40 during a five month interval. Serotype was determined using a recently described molecular method of polymerase chain reaction by capsular polysaccharide synthesis (cps) genes associated with pathogen virulence.
Results
Serotypes Ia, III, and V were the most prevalent (28%, 27%, and 17%, respectively). A systematic review of global GBS seroprevalence, meta-analysis, and statistical comparison revealed strikingly similar serodistibution at Madigan relative to civilian-sector populations in Canada and the United States. Serotype Ia was the only serotype consistently higher in North American populations relative to other geographic regions (p < 0.005). The number of non-typeable isolates was significantly lower in the study (p < 0.005).
Conclusion
This study establishes PCR-based serotyping as a viable strategy for GBS epidemiological surveillance. Our results suggest that GBS seroprevalence remains stable in North America over the past two decades.
Similar content being viewed by others
Background
Streptococcus agalactiae (Group B Streptococcus [GBS]) was first identified as a significant public health concern in maternal fetal medicine in the 1970s. Since this time, more than 7500 cases of GBS-associated neonatal sepsis and meningitis have been reported annually, with a financial burden of more than $350 million per year in neonatal costs [1]. Population-specific surveillance studies provide an important vehicle for evaluating the public health risks posed by changes in distribution of GBS serotypes [2].
Anogenital colonization with GBS is usually asymptomatic in immunocompetent adults [3]. Perinatal transmission of GBS from infected mothers during delivery can cause potentially fatal sepsis and meningitis [4]. Classification of GBS serotype is based on 10 immunologically unique capsular polysaccharides associated with pathogen virulence and encoded in the capsular gene cluster (cps) (Ia, Ib, II-IX) [5]. In the United States civilian population the predominant GBS serotypes are Ia, III, Ib, and V. Current multivalent vaccine development depends on accurate population data of serotype distribution [6]. Thus, epidemiological studies of seroprevalence are important in assessing changes in GBS distribution.
Accurate epidemiological characterization of GBS isolates is dependent on unequivocal classification of serotypes. Molecular methods have improved the accuracy and feasibility of serotyping, but these methods have only recently been applied to large cohorts [7].
Global serotyping distribution studies have documented that the prevalence of a given GBS serotype varies according to geographical location and time of study conductance [2]. Surveillance data in the United States come largely from civilian hospitals, with the Active Bacterial Core surveillance system within the Emerging Infections Program Network providing a mechanism for routine population-based surveillance [8]. Military communities are unique in their demographically diverse population including transients from military bases across the United States and around the world. It is unknown how the geographical mobility of the military might affect GBS seroprevalences in military cohorts.
Madigan Healthcare System (Madigan) is a tertiary care military medical center providing obstetric care to military families, with more than 2100 deliveries reported in 2009. Women between the ages of 18-40 are screened for GBS by urine cultures and vaginal/rectal swabs as standard of care. The objective of this study was to apply a two-tiered polymerase chain reaction (PCR) approach to determine the prevalence and distribution of the GBS serotypes in the Madigan population during the period covering July - December 2009 and to compare Madigan seroprevalences with global distributions.
Methods
Study population
This study was performed with the approval of the Madigan Healthcare System Institutional Review Board. Of the 1129 patients screened during the study period (July 2009 - December 2009), 207 were scored as GBS positive (18.3%). Clinical GBS isolates from these patients were obtained from vaginal/rectal swabs routinely conducted on women of indeterminate gravidity between ages 18-40. GBS isolates were disassociated from any personal health information and assigned a unique study number correlating with the date of specimen collection.
Bacterial culture and banking
GBS isolates were cultured from vaginal/rectal swabs. GBS positive diagnoses were confirmed by conventional morphologic and phenotypic methods. Isolated GBS colonies were obtained taking vaginal/rectal swabs and placing them directly into 5 ml of Lim enrichment broth (Fisher Scientific, Pittsburgh, PA) and grown overnight at 35°C in an 8% CO2 incubator. One microliter loop of culture was quadrant streaked on blood neomycin plates (Fisher Scientific, Pittsburgh, PA) in order to obtain isolated colonies. Single colonies were then inoculated in 5 ml of LB broth and grown overnight under the same conditions stated above. Cultures (700 μl) were placed in 1.5 ml cryovial tubes with 300 μl of 50% glycerol to make glycerol stocks. Samples were then flash frozen with liquid nitrogen and stored at -80°C. Glycerol stocks were streaked overnight onto blood neomycin plates and colonies with a clear zone of hemolysis were selected for PCR amplification.
Serotyping of GBS Isolates
Classification of GBS samples was done by PCR-based restriction fragment length polymorphism (RFLP) capsular typing to distinguish among nine of the ten known GBS serotypes (Ia, Ib, II-IX). We used PCR primers and conditions published by Manning et al (cpsG-F97, cpsL-R200, cpsR8-F40) [9]. These primers were used to amplify DNA fragments using Pure Taq Ready-to-Go PCR Beads (GE Healthcare, Pittsburgh, PA) followed by digestion with DdeI (New England Biolabs, Ipswich, MA) to generate restriction fragments. Digested amplicons were separated on 1.8% agarose gels and analyzed using a Typhoon 9410 variable mode imager (GE Healthcare, Piscataway, NJ). Serotype classification was determined by comparison of the molecular weight banding pattern of the resulting restriction fragment length polymorphisms (RFLPs) with published DNA fragment sizes of the GBS cps gene cluster [9].
Serotyping by multiplex polymerase chain reaction
GBS-specific primers (DLTS) were used to identify those isolates that were nontypeable (NT) by RFLP analysis of the cps gene cluster. Multiplex PCR confirmed serotype classification and serotyped GBS isolates that yielded ambiguous results by the RFLP analysis of the cps gene cluster. Multiplex PCR was performed on single colonies using Pure Taq PCR Ready-to-Go PCR beads using the cps primers and PCR conditions published by Poyart et al [10]. Isolates classified as NT by both methods were serotyped using the primers and methods detailed in Imperi et al [7].
Statistical analysis, systematic review, and multiple comparisons analysis
An a priori power analysis indicated that a minimum of 400 patients should be enrolled in the study, assuming a 20% rate of colonization with 5% error to determine prevalence with a precision (α) of 5% [11]. Based on this estimation, a total of 1129 patients were screened, of which 207 (18.3%) were determined to be GBS positive. To compare the Madigan cohort to the global serotype distributions, a systematic review was conducted of 426 primary literature references evaluating serotype distribution in discrete geographical regions. These studies were identified in a Pub Med keyword search for references reporting population serotyping data for GBS (through February 2010). Studies were excluded if GBS was obtained from normally sterile sites (i.e., blood, synovial fluid, etc.). Studies reporting serodistribution in neonates were likewise excluded. Of the papers surveying predominantly colonized women, we excluded studies predating the emergence of serotype V in the general population. Where raw numbers were available, we confirmed the investigator-reported serotype percentages by our own independent calculations. In two instances, the frequencies we calculated did not match the percentages reported in the article due to obvious typographical errors. We relied solely on data in article abstracts where the full text articles were not written in the English language or if the full text article could not be procured. Meta-data were compiled by calculating proportions of serotype distribution among all GBS-positive diagnoses segregated by geographical region (Table 1). Studies included in the global statistical analysis are presented in systematic review in Table 2 (United States and Canada) [12–26] and Table 3 (global data) [11, 27–64]. The proportional z-test was used to determine significance between serotype rates of the Madigan cohort and other regions. The statistical significance level was stringently set by the Bonferroni correction method to be p < 0.005.
Results
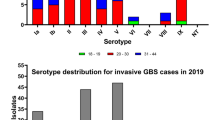
Four isolates were classified as NT by PCR amplification and DdeI restriction endonuclease digest alone. These isolates were confirmed to be GBS antigen B by GBS-specific primers dlts-F and dlts-R. Isolates were further screened by a multiplex approach to verify CPS serotype [10]. The single isolate not typeable by either method was further screened by GBS IX-specific primers [7], but results were negative. The serotype distribution results of the multi-tiered typing strategies are presented in Figure 1. Our results show that serotypes Ia, III, and V were the most abundant of the screened GBS isolates (28.5%, 27.1%, and 16.9%, respectively), followed by the less prevalent serotypes Ib, II, IV, and VI (12.1%, 12.1%, 1.9%, and 1.0%, respectively). GBS serotypes VII, VIII, and IX were not detected in our population group and one isolate was NT.
Prevalence of GBS serotypes at Madigan Healthcare System July 2009 - December 2009. Serotype was determined for GBS isolates collected from vaginal/rectal swabs from women of indeterminate gravidity at Madigan Healthcare System (Joint Base Lewis-McChord, Washington, USA). Percentages refer to serotype frequency in 207 total isolates screened. Total numbers of patients testing positive for each isolate are listed in parentheses. NT, non-typeable.
A systematic review of the literature compared Madigan seroprevalences with those reported within the rest of the United States (Table 2) and internationally (Table 3). Global proportions were combined by geographical region into a composite multiple comparisons analysis to compare Madigan serotype distributions with the rest of the world (Table 1). Because most studies conducted in Mexico did not segregate serotype I into Ia and Ib, these studies were included in the systematic review (Table 3) but not in the final analysis (Table 1).
Discussion
To determine the serotype of our Madigan population GBS isolates, we used a two-tiered PCR-based approach to identify type-specific capsular polysaccharides (CPS), epidemiologic markers used to classify serotypes according to prevalence for colonization and disease. The most common mechanism for identifying serotypes is serotype-specific latex agglutination analysis, but this method is prohibitively expensive when used on large patient populations [65]. Further, these tests have lower accuracy, and result in numerous NT isolates (Tables 2 and 3). In contrast, the polymerase chain reaction (PCR) based capsular typing techniques used in this study (i.e., RFLP analysis and multiplex PCR) are reproducible, specific, and easy to perform with fewer NT isolates reported [9, 10]. We chose a two-tiered PCR approach, first using a restriction enzyme digest fingerprinting strategy followed by multiplex PCR for serotypes classified as NT by the RFLP methods [9, 10]. There was 100% concordance between the two methods used to screen isolates, confirming accuracy of the classification scheme used in determining seroprevalences at Madigan.
The percentage of NT isolates reported in our study is significantly lower than that reported in international civilian GBS serotype surveillance studies using primarily the serotype-specific latex agglutination method (2-13%; Tables 1, 2, and 3) [65–67]. The 0.5% (n = 1 NT isolate) could result from an uncharacterized capsule [68], mutation in the capsular genes [69], or reversible nonencapsular phase variations [70]. Thus, the PCR-based method has proven an effective screening strategy for rapid and effective screening in a military population, and is anticipated to improve typing accuracy in larger cohorts as these methods become more widespread in surveillance studies.
To our knowledge, this is the first GBS serotype surveillance study targeting a population comprised solely of military beneficiaries. The primary mission of infectious disease research initiatives at military operated facilities is to identify and reduce the impact of infectious diseases affecting military populations. In accordance with this objective, this study reports a strikingly similar GBS distribution profile at Madigan relative to United States and Canadian studies (Tables 1 and 2). In comparing seroprevalences in the US with the Madigan cohort, our data suggest that seroprevalences have remained relatively stable in the United States over the past two decades since the emergence of serotype V in the mid-1990s (Table 2). Subtle regional differences in demographic makeup have been reported to affect GBS serodistribution within the same country [31]. Serotypic shifts have been reported in regional distributions in Korean study populations, for example, with a shift in prevalence of serotypes III and V depending on study site [42]. Regional data in early onset invasive GBS strains in neonates in the US indicate that GBS serotypes vary significantly according to study site, with more cases of serotype V reported in New Jersey and New York populations than in Florida, Texas, and Alabama [71]. However, combining study populations by geographical location led to a striking similarity between Madigan and the civilian sector in North America (Tables 1 and 2). It is currently unknown how rapidly seroconversion occurs in the general population. Our data plausibly reflect a stabilization of seroprevalence in the US over the past 15 years. However, very recent data indicate that serotype IV may be increasing in prevalence in the United States (8.4% of the 1160 patients enrolled from 2004 - 2008 in a multi-site United States study) [72].
Relative to global serodistribution studies, our statistical analysis indicates that North American populations have a higher representation of serotype Ia relative to other geographical locations (Tables 1 and 3). Since the emergence of serotype V in the general population, distributions of serotype proportion have shifted to accommodate the rise in serotype V prevalence in recent years in pregnant patients and neonates with invasive GBS disease [73]. Our global surveillance analysis suggests that Africa and South America have significantly greater serotype V representation relative to North American populations (Table 1).
The rate of GBS colonization rate at Madigan is comparable to the civilian-sector United States and Canada but differs from colonization rate reported in global surveillance studies (Tables 2 and 3). Data from the Emerging Pathogens Network places the overall GBS colonization incidence among U.S. women at approximately 10-30% [1, 4]. At Madigan, GBS colonization rate was 18.3% during the study interval. East Asian countries report incidences as low as 0.3-5.9% [42], while African countries such as Zimbabwe report greater than 60% colonization in pregnant patients (Table 3) [74]. Ethnicity could account for some of these differences. African Americans and Hispanic Americans, for example, are GBS-colonized at higher rates than Caucasians [4, 75]. Further, African American heritage is a recognized risk factor for early onset sepsis in neonates born to colonized mothers [1]. Finally, the mode of specimen collection (i.e., vaginal, rectal, or vaginal/rectal) has been reported to vary significantly depending on geographical location [1]. We recognize that mode of collection may bias estimates of global colonization and/or serodistribution.
Besides ethnicity and vertical transmission between colonized mother and neonate, colonization has also been associated with sexual contact and diet [22, 76–79]. All modes are relevant to the Madigan population of highly mobile military beneficiaries and could account for the differences in GBS colonization rate observed between the Madigan cohort and the global studies.
Our systematic review and statistical comparison of the Madigan cohort with global epidemiological studies indicates variability in the geographical distribution of GBS serotypes (Tables 1 and 3). Studies with populations originating in Europe, Asia, Africa, Australia, and North and South America have reported significant differences in prevalence and emergence of novel serotypes over time (Table 3). When comparing Madigan seroprevalences with the global distributions, the Madigan cohort most closely resembles studies conducted in the continental United States and Canada (Table 2) [23].
Analysis of Tables 1 and 3 prompts the following conclusions regarding the seroprevalence of GBS in the Madigan cohort relative to international cohorts: (1) Ia is higher in North America relative to the rest of the world; (2) Ia and Ib are considerably higher in Mexico and South America; (3) III is lower in the Middle East and Eastern Europe than North America but higher in Africa and Australia/New Zealand; (4) IV is comparable across continents; (5) V is higher in Africa but much lower in Australia/New Zealand than North American populations; and (6) VI/VIII are most prevalent in Japan while (7) VII is least represented across the globe with occasional isolates reported in the Middle East/Eastern Europe and Asia. Limited population seroprevalance data are available for serotype IX given its very recent emergence in the general population [5, 7]. Given that serotypes III and V (and to a lesser extent Ia) are most commonly associated with late onset neonatal illness, trends in locations such as Korea, Australia/New Zealand, and African countries with proportionally higher III seroprevalences underscore the need for continuing surveillance. An important caveat when interpreting the meta-data is the high NT rate reported in some studies (11.4-13.88%). The molecular methods more accurately assess serotype, potentially biasing the reporting of the serodistribution in the global comparative analyses.
Given the limited data available on GBS transmission [22, 76–79], surveillance studies are warranted in military hospitals located in regions reporting unique seroprevalences. Serotypes typical of discrete geographical regions (i.e., VI and VIII) were less prevalent in our Madigan population (Table 1). Serotypes VIII and VI are most prevalent in Japan [41], a country which hosts numerous U.S. military bases and associated military hospitals. However, we report no incidences of VIII and only a small percentage of VI serotypes in our Madigan population. Similarly, neonatal GBS type IV disease has been reported as more prevalent in countries such as the United Arab Emirates than the United States [28]. Interestingly, our serodistribution data indicate that IV is the most stable serotype across all countries in the world, with no statistically significant differences reported (Table 1). Surveillance studies of seroconversion in female military operatives are warranted in military medical facilities located in Japan and the Middle East.
The implementation of CDC guidelines for anovaginal swab screening at 35-37 weeks gestation and subsequent intrapartum chemoprophylaxis in culture-positive individuals has reduced the incidence of GBS disease by 70% [1, 8, 80]. However, more than 25% of women now receive intrapartum antibiotics [81], raising public health concerns over the possibility of increasing antibiotic resistance of other common pathogens affecting neonatal health [2]. Further, although GBS readily succumbs to penicillin regimens, resistance is increasing in antibiotic alternatives such as clindamycin and erythromycin in women with penicillin allergy [82, 83]. One strategy currently underway to combat this problem is the development of multivalent vaccines. Although vaccination efficacy and implementation feasibility remain controversial, understanding serotype distribution as a function of population is recognized as a critical component of vaccine development [6, 84].
Conclusions
In conclusion, the two-tiered molecular approach to GBS serotype analysis proved a viable strategy for assessing GBS serodistribution in the Madigan cohort, with fewer NT isolates than other methods employed in large population serodistribution studies. The ethnic diversity and geographical mobility of the United States military classify the military as a unique epidemiologic unit relative to US regional and global surveillance populations surveyed in our systematic review. However, the serodistributions reported in our study are remarkably comparable to those reported in civilian sector hospitals in the United States and Canada. Significant discrepancies exist between Madigan distributions and global epidemiology. Investigating seroprevalence in US military medical facilities abroad is necessary to determine whether military-specific populations will require specialized risk analysis for emerging GBS pathogens when alternatives to chemoprophylaxis come into clinical practice.
Funding
This project did not receive external financial support.
Abbreviations
- GBS:
-
Group B Streptococcus
- Madigan:
-
Madigan Healthcare System
- PCR:
-
Polymerase Chain Reaction
- RFLP:
-
Restriction Fragment Length Polymorphism.
References
Schrag S, Gorwitz R, Fulz-Butts K, Schuchat A: Prevention of perinatal Group B Streptococcal disease. MMWR. 2002, Center for Disease Control, 51: 1-22.
Schuchat A: Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998, 11 (3): 497-513.
Schrag SJ, Whitney CG, Schuchat A: Neonatal group B streptococcal disease: how infection control teams can contribute to prevention efforts. Infect Control Hosp Epidemiol. 2000, 21 (7): 473-483. 10.1086/501791.
Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ, Active Bacterial Core Surveillance/Emerging Infections Program Network: Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008, 299 (17): 2056-2065. 10.1001/jama.299.17.2056.
Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL: Serotype IX, a Proposed New Streptococcus agalactiae Serotype. J Clin Microbiol. 2007, 45 (9): 2929-2936. 10.1128/JCM.00117-07.
Johri AK, Paoletti LC, Glaser P, Dua M, Sharma PK, Grandi G, Rappuoli R: Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol. 2006, 4 (12): 932-942. 10.1038/nrmicro1552.
Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R: A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods. 2010, 80 (2): 212-214. 10.1016/j.mimet.2009.11.010.
Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, Gershman K, Schaffner W, Petit S, Zansky SM, Morin CA, Spina NL, Wymore K, Harrison LH, Shutt KA, Bareta J, Bulens SN, Zell ER, Schuchat A, Schrag SJ: Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009, 360 (25): 2626-2636. 10.1056/NEJMoa0806820.
Manning SD, Lacher DW, Davies HD, Foxman B, Whittam TS: DNA polymorphism and molecular subtyping of the capsular gene cluster of group B streptococcus. J Clin Microbiol. 2005, 43 (12): 6113-6116. 10.1128/JCM.43.12.6113-6116.2005.
Poyart C, Tazi A, Reglier-Poupet H, Billoet A, Tavares N, Raymond J, Trieu-Cuot P: Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol. 2007, 45 (6): 1985-1988. 10.1128/JCM.00159-07.
Whitney CG, Daly S, Limpongsanurak S, Festin MR, Thinn KK, Chipato T, Lumbiganon P, Sauvarin J, Andrews W, Tolosa JE: The international infections in pregnancy study: group B streptococcal colonization in pregnant women. J Matern Fetal Neonatal Med. 2004, 15 (4): 267-274. 10.1080/14767050410001668617.
Borchardt SM, Foxman B, Chaffin DO, Rubens CE, Tallman PA, Manning SD, Baker CJ, Marrs CF: Comparison of DNA dot blot hybridization and lancefield capillary precipitin methods for group B streptococcal capsular typing. J Clin Microbiol. 2004, 42 (1): 146-150. 10.1128/JCM.42.1.146-150.2004.
Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ: Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol. 2000, 96 (4): 498-503. 10.1016/S0029-7844(00)00977-7.
Croak A, Abate G, Goodrum K, Modrzakowski M: Predominance of serotype V and frequency of erythromycin resistance in Streptococcus agalactiae in Ohio. Am J Obstet Gynecol. 2003, 188 (5): 1148-1150. 10.1067/mob.2003.293.
Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, Kowalsky L, Tyrell G, Baker CJ: Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis. 2001, 184 (3): 285-291. 10.1086/322029.
Ferrieri P, Baker CJ, Hillier SL, Flores AE: Diversity of surface protein expression in group B streptococcal colonizing & invasive isolates. Indian J Med Res. 2004, 119 (Suppl): 191-196.
Ferrieri P, Cleary PP, Seeds AE: Epidemiology of group-B streptococcal carriage in pregnant women and newborn infants. J Med Microbiol. 1977, 10 (1): 103-114. 10.1099/00222615-10-1-103.
Hickman ME, Rench MA, Ferrieri P, Baker CJ: Changing epidemiology of group B streptococcal colonization. Pediatrics. 1999, 104 (2 Pt 1): 203-209. 10.1542/peds.104.2.203.
Manning SD: Molecular epidemiology of Streptococcus agalactiae (group B Streptococcus). Front Biosci. 2003, 8: s1-18. 10.2741/985.
Manning SD, Ki M, Marrs CF, Kugeler KJ, Borchardt SM, Baker CJ, Foxman B: The frequency of genes encoding three putative group B streptococcal virulence factors among invasive and colonizing isolates. BMC Infect Dis. 2006, 6: 116-10.1186/1471-2334-6-116.
McKenna DS, Matson S, Northern I: Maternal group B streptococcal (GBS) genital tract colonization at term in women who have asymptomatic GBS bacteriuria. Infect Dis Obstet Gynecol. 2003, 11 (4): 203-207. 10.1080/10647440300025522.
Meyn LA, Moore DM, Hillier SL, Krohn MA: Association of sexual activity with colonization and vaginal acquisition of group B Streptococcus in nonpregnant women. Am J Epidemiol. 2002, 155 (10): 949-957. 10.1093/aje/155.10.949.
Paoletti LJ, Bradford J, Paoletti LC: A serotype VIII strain among colonizing group B streptococcal isolates in Boston, Massachusetts. J Clin Microbiol. 1999, 37 (11): 3759-3760.
Shet A, Ferrieri P: Neonatal & maternal group B streptococcal infections: a comprehensive review. Indian J Med Res. 2004, 120 (3): 141-150.
Smith JM, Rexroth JA, Chaffin DG, Jackman SH: Serotyping group B streptococci in a small community hospital: an analysis of distribution and site of isolation. Infect Dis Obstet Gynecol. 2002, 10 (4): 165-169. 10.1155/S1064744902000182.
Ferrieri P, Hillier SL, Krohn MA, Moore D, Paoletti LC, Flores AE: Characterization of vaginal & rectal colonization with multiple serotypes of group B streptococci using multiple colony picks. Indian J Med Res. 2004, 119 (Suppl): 208-212.
Al-Sweih N, Hammoud M, Al-Shimmiri M, Jamal M, Neil L, Rotimi V: Serotype distribution and mother-to-baby transmission rate of Streptococcus agalactiae among expectant mothers in Kuwait. Arch Gynecol Obstet. 2005, 272 (2): 131-135. 10.1007/s00404-004-0705-z.
Amin A, Abdulrazzaq YM, Uduman S: Group B streptococcal serotype distribution of isolates from colonized pregnant women at the time of delivery in United Arab Emirates. J Infect. 2002, 45 (1): 42-46. 10.1053/jinf.2001.0990.
Berg S, Trollfors B, Lagergard T, Zackrisson G, Claesson BA: Serotypes and clinical manifestations of group B streptococcal infections in western Sweden. Clin Microbiol Infect. 2000, 6 (1): 9-13. 10.1046/j.1469-0691.2000.00007.x.
Brimil N, Barthell E, Heindrichs U, Kuhn M, Lutticken R, Spellerberg B: Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol. 2006, 296 (1): 39-44. 10.1016/j.ijmm.2005.11.001.
Dhanoa A, Karunakaran R, Puthucheary SD: Serotype distribution and antibiotic susceptibility of group B streptococci in pregnant women. Epidemiol Infect. 2009, 1-3.
Dore N, Bennett D, Kaliszer M, Cafferkey M, Smyth CJ: Molecular epidemiology of group B streptococci in Ireland: associations between serotype, invasive status and presence of genes encoding putative virulence factors. Epidemiol Infect. 2003, 131 (2): 823-833. 10.1017/S0950268803008847.
Ellis S, Kotiw M, Garland SM: Restriction endonuclease analysis of group B streptococcal isolates from two distinct geographical regions. J Hosp Infect. 1996, 33 (4): 279-287. 10.1016/S0195-6701(96)90014-6.
Eren A, Kucukercan M, Oguzoglu N, Unal N, Karateke A: The carriage of group B streptococci in Turkish pregnant women and its transmission rate in newborns and serotype distribution. Turk J Pediatr. 2005, 47 (1): 28-33.
Gherardi G, Imperi M, Baldassarri L, Pataracchia M, Alfarone G, Recchia S, Orefici G, Dicuonzo G, Creti R: Molecular epidemiology and distribution of serotypes, surface proteins, and antibiotic resistance among group B streptococci in Italy. J Clin Microbiol. 2007, 45 (9): 2909-2916. 10.1128/JCM.00999-07.
Gonzalez Pedraza Aviles A, Ortiz Zaragoza MC, Mota Vazquez R: Serotypes and antimicrobial susceptibility of group B Streptococcus from pregnant women in Mexico. Rev Latinoam Microbiol. 2002, 44 (3-4): 133-136.
Grimwood K, Stone PR, Gosling IA, Green R, Darlow BA, Lennon DR, Martin DR: Late antenatal carriage of group B Streptococcus by New Zealand women. Aust N Z J Obstet Gynaecol. 2002, 42 (2): 182-186. 10.1111/j.0004-8666.2002.00182.x.
Hakansson S, Axemo P, Bremme K, Bryngelsson AL, Wallin MC, Ekstrom CM, Granlund M, Jacobsson B, Kallen K, Spetz E, Tessin I, Swedish Working Group for the Prevention of Perinatal Group B Streptococcal Infections: Group B streptococcal carriage in Sweden: a national study on risk factors for mother and infant colonisation. Acta Obstet Gynecol Scand. 2008, 87 (1): 50-58. 10.1080/00016340701802888.
Jones N, Oliver K, Jones Y, Haines A, Crook D: Carriage of group B streptococcus in pregnant women from Oxford, UK. J Clin Pathol. 2006, 59 (4): 363-366. 10.1136/jcp.2005.029058.
Kieran E, Matheson M, Mann AG, Efstratiou AA, Butler K, Gorman W: Group B streptococcus (GBS) colonisation among expectant Irish mothers. Ir Med J. 1998, 91 (1): 21-22.
Lachenauer CS, Kasper DL, Shimada J, Ichiman Y, Ohtsuka H, Kaku M, Paoletti LC, Ferrieri P, Madoff LC: Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J Infect Dis. 1999, 179 (4): 1030-1033. 10.1086/314666.
Lee BK, Song YR, Kim MY, Yang JH, Shin JH, Seo YS, Oh KY, Yoon HR, Pai SY, Foxman B, Ki M: Epidemiology of group B streptococcus in Korean pregnant women. Epidemiol Infect. 2010, 138 (2): 292-298. 10.1017/S0950268809990859.
Marchaim D, Hallak M, Gortzak-Uzan L, Peled N, Riesenberg K, Schlaeffer F: Risk factors for carriage of group B streptococcus in southern Israel. Isr Med Assoc J. 2003, 5 (9): 646-648.
Martins ER, Pessanha MA, Ramirez M, Melo-Cristino J: Analysis of group B streptococcal isolates from infants and pregnant women in Portugal revealing two lineages with enhanced invasiveness. J Clin Microbiol. 2007, 45 (10): 3224-3229. 10.1128/JCM.01182-07.
Matsubara K, Katayama K, Baba K, Nigami H, Harigaya H, Sugiyama M: Seroepidemiologic studies of serotype VIII group B Streptococcus in Japan. J Infect Dis. 2002, 186 (6): 855-858. 10.1086/342411.
Motlova J, Strakova L, Urbaskova P, Sak P, Sever T: Vaginal & rectal carriage of Streptococcus agalactiae in the Czech Republic: incidence, serotypes distribution & susceptibility to antibiotics. Indian J Med Res. 2004, 119 (Suppl): 84-87.
Moyo SR, Maeland JA, Bergh K: Typing of human isolates of Streptococcus agalactiae (group B streptococcus, GBS) strains from Zimbabwe. J Med Microbiol. 2002, 51 (7): 595-600.
Moyo SR, Mudzori J, Tswana SA, Maeland JA: Prevalence, capsular type distribution, anthropometric and obstetric factors of group B Streptococcus (Streptococcus agalactiae) colonization in pregnancy. Cent Afr J Med. 2000, 46 (5): 115-120.
Namavar Jahromi B, Poorarian S, Poorbarfehee S: The prevalence and adverse effects of group B streptococcal colonization during pregnancy. Arch Iran Med. 2008, 11 (6): 654-657.
Palacios GC, Gonzalez MN, Beltran M, Arredondo JL, Torres J, Solorzano F: Serotypes of 286 group B streptococci isolated from asymptomatic carriers and invasive disease cases in Mexico. Rev Latinoam Microbiol. 2005, 47 (1-2): 21-24.
Savoia D, Gottimer C, Crocilla C, Zucca M: Streptococcus agalactiae in pregnant women: phenotypic and genotypic characters. J Infect. 2008, 56 (2): 120-125. 10.1016/j.jinf.2007.11.007.
Sensini A, Tissi L, Verducci N, Orofino M, von Hunolstein C, Brunelli B, Mala GL, Perocchi F, Brunelli R, Lauro V, Ferrarese R, Gilardi G: Carriage of group B Streptococcus in pregnant women and newborns: a 2-year study at Perugia General Hospital. Clin Microbiol Infect. 1997, 3 (3): 324-328. 10.1111/j.1469-0691.1997.tb00621.x.
Seoud M, Nassar AH, Zalloua P, Boghossian N, Ezeddine J, Fakhoury H, Abboud J, Melki I, Araj G, Nacouzi G, Sanyoura M, Yunis K: Prenatal and neonatal Group B Streptococcus screening and serotyping in Lebanon: incidence and implications. Acta Obstet Gynecol Scand. 89 (3): 399-403. 10.3109/00016340903560008.
Simoes JA, Alves VM, Fracalanzza SE, de Camargo RP, Mathias L, Milanez HM, Brolazo EM: Phenotypical characteristics of group B streptococcus in parturients. Braz J Infect Dis. 2007, 11 (2): 261-266. 10.1590/S1413-86702007000200019.
Suara RO, Adegbola RA, Mulholland EK, Greenwood BM, Baker CJ: Seroprevalence of antibodies to group B streptococcal polysaccharides in Gambian mothers and their newborns. J Natl Med Assoc. 1998, 90 (2): 109-114.
Terakubo S, Ichiman Y, Takemura H, Yamamoto H, Shimada J, Nakashima H: [Serotypes and antibody levels of group B streptococci in pregnant women]. Kansenshogaku Zasshi. 2003, 77 (3): 121-126.
Toresani I, Limansky A, Bogado I, Guardati MC, Viale A, Sutich EG: Phenotypic and genotypic study of Streptococcus agalactiae in vagina of pregnant women in Argentina. Medicina (B Aires). 2001, 61 (3): 295-300.
Tsolia M, Psoma M, Gavrili S, Petrochilou V, Michalas S, Legakis N, Karpathios T: Group B streptococcus colonization of Greek pregnant women and neonates: prevalence, risk factors and serotypes. Clin Microbiol Infect. 2003, 9 (8): 832-838. 10.1046/j.1469-0691.2003.00662.x.
Uh Y, Kim HY, Jang IH, Hwang GY, Yoon KJ: Correlation of serotypes and genotypes of macrolide-resistant Streptococcus agalactiae. Yonsei Med J. 2005, 46 (4): 480-483. 10.3349/ymj.2005.46.4.480.
van der Mee-Marquet N, Jouannet C, Domelier AS, Arnault L, Lartigue MF, Quentin R: Genetic diversity of Streptococcus agalactiae strains and density of vaginal carriage. J Med Microbiol. 2009, 58 (Pt 2): 169-173. 10.1099/jmm.0.005827-0.
van Elzakker E, Yahiaoui R, Visser C, Oostvogel P, Muller A, Ho YR, Wu JJ, van Belkum A: Epidemiology of and prenatal molecular distinction between invasive and colonizing group B streptococci in The Netherlands and Taiwan. Eur J Clin Microbiol Infect Dis. 2009, 28 (8): 921-928. 10.1007/s10096-009-0726-4.
Villasenor Sierra A, Morales Velazquez P, Palacios Saucedo G, Solorzano Santos F: [Prevalence of Streptococcus algalactiae serotype III in pregnant women]. Ginecol Obstet Mex. 2004, 72: 103-108.
von Both U, John A, Fluegge K, Siedler A, Berner R: Molecular epidemiology of invasive neonatal Streptococcus agalactiae isolates in Germany. Pediatr Infect Dis J. 2008, 27 (10): 903-906. 10.1097/INF.0b013e318178d1ff.
Walsh JA, Hutchins S: Group B streptococcal disease: its importance in the developing world and prospect for prevention with vaccines. Pediatr Infect Dis J. 1989, 8 (5): 271-277.
Elliott JA, Thompson TA, Facklam RR, Slotved HC: Increased sensitivity of a latex agglutination method for serotyping group B streptococcus. J Clin Microbiol. 2004, 42 (8): 3907-10.1128/JCM.42.8.3907.2004.
Ferrieri P, Flores AE: Surface protein expression in group B streptococcal invasive isolates. Adv Exp Med Biol. 1997, 418: 635-637.
Ramaswamy SV, Ferrieri P, Madoff LC, Flores AE, Kumar N, Tettelin H, Paoletti LC: Identification of novel cps locus polymorphisms in nontypable group B Streptococcus. J Med Microbiol. 2006, 55 (Pt 6): 775-783. 10.1099/jmm.0.46253-0.
Slotved HC, Sauer S, Konradsen HB: False-negative results in typing of group B streptococci by the standard lancefield antigen extraction method. J Clin Microbiol. 2002, 40 (5): 1882-1883. 10.1128/JCM.40.5.1882-1883.2002.
Sellin M, Olofsson C, Hakansson S, Norgren M: Genotyping of the capsule gene cluster (cps) in nontypeable group B streptococci reveals two major cps allelic variants of serotypes III and VII. J Clin Microbiol. 2000, 38 (9): 3420-3428.
Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE: Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect Immun. 2005, 73 (5): 3096-3103. 10.1128/IAI.73.5.3096-3103.2005.
Harrison LH, Elliott JA, Dwyer DM, Libonati JP, Ferrieri P, Billmann L, Schuchat A: Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program. J Infect Dis. 1998, 177 (4): 998-1002.
Diedrick MJ, Flores AE, Hillier SL, Creti R, Ferrieri P: Clonal Analysis of Colonizing Group B Streptococcus, Serotype IV, a Potential Emerging Pathogen in the United States. J Clin Microbiol. 2010
Puopolo KM, Madoff LC: Type IV neonatal early-onset group B streptococcal disease in a United States hospital. J Clin Microbiol. 2007, 45 (4): 1360-1362. 10.1128/JCM.02487-06.
Mavenyengwa RT, Afset JE, Schei B, Berg S, Caspersen T, Bergseng H, Moyo SR: Group B Streptococcus colonization during pregnancy and maternal-fetal transmission in Zimbabwe. Acta Obstet Gynecol Scand. 89 (2): 250-255. 10.3109/00016340903398029.
Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R: Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand. 2008, 87 (3): 260-271. 10.1080/00016340801908759.
Evans JJ, Klesius PH, Pasnik DJ, Bohnsack JF: Human Streptococcus agalactiae isolate in Nile tilapia (Oreochromis niloticus). Emerg Infect Dis. 2009, 15 (5): 774-776. 10.3201/eid1505.080222.
Foxman B, Gillespie B, Manning SD, Howard LJ, Tallman P, Zhang L, Marrs CF: Incidence and duration of group B Streptococcus by serotype among male and female college students living in a single dormitory. Am J Epidemiol. 2006, 163 (6): 544-551. 10.1093/aje/kwj075.
Foxman B, Gillespie BW, Manning SD, Marrs CF: Risk factors for group B streptococcal colonization: potential for different transmission systems by capsular type. Ann Epidemiol. 2007, 17 (11): 854-862. 10.1016/j.annepidem.2007.05.014.
Manning SD, Springman AC, Million AD, Milton NR, McNamara SE, Somsel PA, Bartlett P, Davies HD: Association of Group B Streptococcus colonization and bovine exposure: a prospective cross-sectional cohort study. PLoS One. 5 (1): e8795-10.1371/journal.pone.0008795.
Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, Harrison LH, Reingold A, Stefonek K, Smith G, Gamble M, Schuchat A, Active Bacterial Core Surveillance Team: A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002, 347 (4): 233-239. 10.1056/NEJMoa020205.
Apgar BS, Greenberg G, Yen G: Prevention of group B streptococcal disease in the newborn. Am Fam Physician. 2005, 71 (5): 903-910.
Andrews JI, Diekema DJ, Hunter SK, Rhomberg PR, Pfaller MA, Jones RN, Doern GV: Group B streptococci causing neonatal bloodstream infection: antimicrobial susceptibility and serotyping results from SENTRY centers in the Western Hemisphere. Am J Obstet Gynecol. 2000, 183 (4): 859-862. 10.1067/mob.2000.108839.
de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A: Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob Agents Chemother. 2001, 45 (12): 3504-3508. 10.1128/AAC.45.12.3504-3508.2001.
Baker CJ, Edwards MS: Group B streptococcal conjugate vaccines. Arch Dis Child. 2003, 88 (5): 375-378. 10.1136/adc.88.5.375.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/10/336/prepub
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting views of the Department of Defense, or Department of the Army.
We thank Mr. Paul Kulvi, Ms. Nancy Copper, Ms. Barbara Chickering, and SPC Edmond Flores in the Department of Pathology for initial specimen culturing and preparation. We gratefully acknowledge Ms. Cindy Kirker for library consultation and article procurement. We thank Mr. Mike Smith for technical assistance in PCR, bacterial stock preparations. We thank Mr. Troy Patience for statistical consultation and LTC Steven Mahlen and CPT Karen Thomas for critical manuscript review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DLI contributed to experimental design, study conception, pilot experiments, manuscript drafting, systematic review, and statistical analysis. WAJ, MJD, and JW assisted in data collection (PCR, glycerol stock preparation, and specimen banking), interpretation, and manuscript review. RRH conducted the meta-analysis, statistical interpretation, manuscript review. DT and MAW contributed to the systematic review, manuscript draft and revisions. STD contributed to experimental design, study conception, data collection and interpretation (PCR, glycerol stock preparation, specimen banking), manuscript drafting, systematic review, and statistical analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ippolito, D.L., James, W.A., Tinnemore, D. et al. Group B streptococcusserotype prevalence in reproductive-age women at a tertiary care military medical center relative to global serotype distribution. BMC Infect Dis 10, 336 (2010). https://doi.org/10.1186/1471-2334-10-336
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-10-336