Abstract
Background
Three diagnostic criteria have been proposed used for invasive pulmonary aspergillosis (IPA) diagnosis, namely EORTC/ MSG criteria, Bulpa criteria and intensive care unit (ICU) criteria. The Bulpa criteria were proposed to diagnose IPA in chronic obstructive pulmonary disease (COPD) patients specially. Our aim is to verify that whether the Bulpa criteria are the most suitable for diagnosing probable IPA in critically ill COPD patients compared with the other two criteria.
Methods
We included critically ill COPD patients admitted to the ICU from April 2006 to August 2013. Patients were classified into four populations: population one (n1 = 59) comprised all included patients; population two (n2 = 24) comprised patients with positive mycological findings (both positive cultures and positive serologic tests); population three (n3 = 18) comprised patients with positive lower respiratory tracts (LRTs) isolation; and population four (n4 = 5) comprised proven IPA patients with histopathology. Patients in four groups were diagnosed as probable IPA using three criteria respectively, and the “diagnostic rate” of each criteria were compared with each other. Then, the reasons for differences in “diagnostic rate” were analyzed in population two. Finally, the modified Bulpa criteria were proposed.
Results
Bulpa criteria yielded the highest “diagnostic rate” of probable IPA followed by the ICU criteria, while the EORTC/ MSG criteria provided the lowest rates in four populations (the “diagnostic rate” of probable IPA was 33.9%, 16.9% and 6.8% in population one, p = 0.001; 83.3%, 41.7% and 16.7% in population two, p < 0.001; 100%, 55.6% and 22.2% in population three, p < 0.001; 100%, 60% and 20% in population four, p = 0.036). The reasons for the highest “diagnostic rate” by Bulpa criteria were its less strict requirements regarding the doses/courses of steroid use and typical computed tomography (CT) findings. Finally, the modified Bulpa criteria for probable IPA were proposed for critically ill COPD patients admitted to ICU, mainly involving revised interpretations of microbiological findings.
Conclusions
Among the existing three criteria, the Bulpa criteria are the most suitable for diagnosing probable IPA in critically ill COPD patients admitted to ICU. A modified criteria maybe proposed for better diagnosis,and its clinical validity need to be verified in future studies.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) has been recognized as a potential risk factor of invasive pulmonary aspergillosis (IPA) [1–3]. The average mortality of IPA in COPD patients has been found to be as high as 50-100% [1, 4, 5], and is especially in patients with delayed diagnosis.
Tremendous challenges in early diagnosis of IPA existed among specialists in pulmonary and critical care medicine. Therefore, appropriate diagnostic criteria are crucial for IPA at the early stage.
Three diagnostic criteria have been proposed used for IPA diagnosis. The EORTC/ MSG criterion was proposed by a consensus group of the European Organization for the Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC) and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (MSG) in the year 2002 for use with haematological patients, and this criteria were revised in 2008 [6, 7]. Bulpa proposed another set of criteria in 2007 specifically for use with COPD patients in respiratory ward [8]. According to both sets of criteria, IPA is categorized as proven, probable or possible. A third set of criteria was put forwarded by Vandewoude use with intensive care unit (ICU) patients, with an entry criterion of positive lower respiratory tract (LRT) specimens for Aspergillus; this set categorized IPA as proven or putative, where putative IPA could be regarded as probable IPA in the first two criteria sets mentioned above [9, 10]. Since the critically ill COPD patients admitted to ICU had their particular characteristics which were huge different with severe immunocompromised patients, we made a clinical assumption that Bulpa criteria are the most suitable for diagnosing probable IPA in critically ill COPD patients admitted to ICU compared with the other two criteria.
Methods
Study population and inclusion criteria
All of the patients were admitted to our respiratory ICU (RICU) due to respiratory failure from April 2006 to August 2013.
Inclusion criteria
We included patients based on the following criteria:
-
1)
Older than 50 years old.
-
2)
Severe COPD with a pulmonary functional level of stage III or IV according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD). We made the corresponding changes based on the updated GOLD guidelines [11–13].
-
3)
At least one computed tomography (CT) recorded during their hospitalization. CT(s) were provided within 1 week prior to admission to the RICU.
Exclusion criteria
We excluded patients based on the following criteria:
-
1)
Patients with solid organ transplantation (SOT) and haematopoietic stem cell transplantation (HSCT), which included allergenic and autologous HSCT.
-
2)
Patients with neutropenia, blood system diseases, malignant tumors and human immunodeficiency virus (HIV) infection.
-
3)
Patients who accepted a high dosage of immunosuppressant because of connective tissue disease (CTD) in the previous 3 months.
Diagnostic criteria
Definitions for proven IPA
Items required for proven IPA were same in the three criteria [7–10].
Microscopic analysis of sterile material: histopathological, cytopathological, or direct microscopic examination of a specimen obtained by needle aspiration or sterile biopsy in which hyphae are observed and are accompanied by evidence of associated tissue damage.
Culture on sterile material: recovery of Aspergillus by culturing of a specimen obtained by lung biopsy.
Definitions for Probable/Putative IPA
The definition for probable/ putative IPA can be concluded based on the following three types of information: host factors, clinical data and microbiological findings.
The following is a brief description of the three parts in each diagnostic criterion (Table 1). The main differences among three criteria were marked with italic and underline.
Additional instructions in EORTC/ MSG Criteria [7]:
Because of the exclusion criteria adopted in our study, our patients may only satisfy condition iii).
Critically ill COPD patients who met host factor iii), one of the clinical data and one of the microbiological findings were diagnosed as probable IPA.
Additional instructions in Bulpa Criteria [8]:
In our hospital, the detection of serum antibody or precipitin for A. fumigatus was unavailable.
The cut-off values of the serum galactomannan (GM) and broncho-alveolar lavage fluid (BALF) GM tests are 0.5 ng/ml and 0.8 ng/ml [14, 15], respectively. Serum GM levels greater than 0.5 ng/ml and BALF GM levels greater than 0.8 ng/ml were defined as positive results.
Critically ill COPD patients who met all host factors, all clinical criteria and one of the microbiological findings were diagnosed as probable IPA.
Additional instructions in ICU Criteria [9, 10]
If the patients did not present any host factors, a semi-quantitative Aspergillus-positive culture of BALF (+ or ++), without bacterial growth together but with a positive cytological smear showing branching hyphae is considered as alternative. Unfortunately, the semi-quantitative Aspergillus culture of BALF was not routinely available at our institution during the study period.
The same as the EORTC criteria, because of the exclusion criteria adopted in our study, our patients may only present condition iii).
Critically ill COPD patients who met host factor iii), all clinical data and microbiological findings were diagnosed as putative IPA.
Data collection and analysis
Patient characteristics, including age, sex, the usage, dosage and duration of steroids prior toadmission to the RICU, microbiological examinations (including cultures of the sputum, endotracheal aspiration (ETA) and BALF, and serum GM and BALF GM tests), chest radiological data (chest X rays (CXRs) and CT scans) and clinical outcomes were collected.
Two experienced respiratory physicians and a radiologist who were not aware of the clinical outcomes of the patient analysed the radiological data.
Methods for population divisions
All patients were classified into four populations. The scope of each group gradually reduced.
Population one comprised all included COPD patients; population two comprised patients with positive mycological findings (both positive cultures and positive serologic tests); population three comprised patients with the positive LRTs isolation, which including sputum, ETA and BALF; and population four comprised proven cases of IPA based on histopathology.
Usually, the validity of diagnostic criteria is assessed according to a gold standard, which depends on the use of biopsy or necropsy. However, it was difficult to obtain tissue specimen for critically ill COPD patients; therefore, we evaluated the “diagnostic rate” of three criteria in different populations, and verified the result based on a proven IPA population.
Patients from each population were diagnosed as probable/ putative IPA using the above criteria, and the “diagnostic rate” were compared among them. Patients who did not fall into the probable/ putative IPA group were considered as non-classifiable. The reasons for differences in the “diagnostic rate” among the three criteria were then analysed in population two (the population with positive mycological findings). Finally, the modified Bulpa criteria for probable IPA in critically ill COPD patients who have been admitted to ICU were proposed.
Statistical analysis
Our data were categorical variables; therefore, comparative analyses were performed using a Pearson Chi-Square (x 2 test). For sample sizes of less than 40 or theoretical frequencies (T) of less than 1, Fisher’s Exact Test was used. P values < 0.05 were considered to indicate statistical significance.
Results
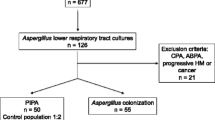
From April 2006 to August 2013, 171 COPD patients were admitted to the RICU because of respiratory failure. The numbers of patients who suffered from malignancy, used a high doses of immunosuppressant in the previous 3 months or for whom integrated radiological imaging was not available were 3,6,2 and 101, respectively; these patients were excluded from the study. 59 patients who met the inclusion criteria were enrolled.
All 59 critically ill COPD patients were classified into four populations according to the previously described methods. Population one included all 59 critically ill COPD patients; population two included 24 patients with positive mycological findings (both positive cultures and positive serologic tests); population three included 18 patients with positive LRTs isolation; and population four included 5 proven IPA cases based on bronchial mucosal biopsy (Fig. 1).
All 59 patients were classified into four populations. Population one comprised all 59 included patients; population two comprised 24 patients with positive mycological findings (both positive cultures and positive serologic tests); population three comprised 18 patients with the positive LRTs isolation; and population four comprised 5 proven cases of IPA based on histopathology
General information
The history of steroids use, typical CT manifestations and microbiological findings of the patients were provided in Additional file 1.
Estimate of the “ Diagnostic rate” for the three criteria in each population
The patients in the four populations were diagnosed as probable/ putative IPA using the Bulpa criteria, ICU criteria and EORTC/ MSG, which were recorded in Table 2. The respect “diagnostic rates” were 33.9%, 16.9% and 6.8% in population one, p = 0.001; 83.3%, 41.7% and 16.7% in population two, p < 0.001; 100%, 55.6% and 22.2% in population three, p < 0.001; 100%, 60% and 20% in population four, p = 0.036. The Bulpa criteria resulted in the highest “diagnostic rate” of probable IPA, followed by the ICU criteria and the EORTC/ MSG criteria, in that order; this trend was confirmed by population four (the proven IPA patients).
Analysis of the reasons for the non-classification of patients by each set of diagnostic criteria
The findings regarding “diagnostic rate” followed the trend: Bulpa criteria, ICU criteria and EORTC/ MSG criteria; therefore, we attempted to determine the reasons for these differences by comparing any two criteria in population two.
Comparison between EORTC/ MSG criteria and Bulpa criteria
We found that all probable IPA diagnosed by the EORTC criteria were also diagnosed by the Bulpa criteria; 16 (80%) patients who were not classified by EORTC/ MSG could be diagnosed as probable IPA by the Bulpa criteria, and the remaining 4 (20%) patients remained non-classified even based on the Bulpa criteria (Fig. 2).
Comparison between the EORTC/ MSG and Bulpa criteria. We found that all probable IPA diagnosed by the EORTC/ MSG criteria were also diagnosed by the Bulpa criteria; 16 (80%) patients who were not classified by EORTC could be diagnosed as probable IPA by the Bulpa criteria, and the remaining 4 (20%) patients remained non-classified even based on the Bulpa criteria
Comparison between ICU criteria and Bulpa criteria
We found that all cases that were diagnosed as probable IPA by the ICU criteria were also diagnosed by the Bulpa criteria; 10 (71.4%) cases that were not classified by ICU were diagnosed as probable IPA by the Bulpa criteria, and the remaining 4 (28.6%) patients remained non-classified even based on the Bulpa criteria (Fig. 3).
Comparison between the ICU and Bulpa criteria. We found that all cases that were diagnosed as probable IPA by the ICU criteria were also diagnosed by the Bulpa criteria; 10 (71.4%) cases that were not classified by ICU were diagnosed as probable IPA by the Bulpa criteria, and the remaining 4 (28.6%) patients remained non-classified even based on the Bulpa criteria
Comparison between EORTC/ MSG criteria and ICU criteria
We found that only 1 (25%) case that was diagnosed as probable IPA based on the EORTC/ MSG criteria was diagnosed by the ICU criteria, and the remaining 3 (75%) cases were not classified; 9 (45%) cases that were not classified by EORTC were diagnosed as probable IPA based on the ICU criteria, and the remaining 11 (55%) patients remained non-classified by the ICU criteria (Fig. 4).
Comparison between the EORTC/ MSG and ICU criteria. We found that only 1 (25%) case that was diagnosed as probable IPA based on the EORTC/ MSG criteria was diagnosed by the ICU criteria, and the remaining 3 (75%) cases were not classified; 9 (45%) cases that were not classified by EORTC/ MSG were diagnosed as probable IPA based on the ICU criteria, and the remaining 11 (55%) patients remained non-classified even by the ICU criteria
The reason underlying the non-classification of patients by the EORTC/ MSG criteria
Among 24 patients with positive mycological findings, only 4 were diagnosed as probable IPA cases; the non-classification of 20 patients was attributed to the strict requirements regarding the dosage and duration of steroid use and the typical CT findings (Table 3).
The reason underlying the non-classification of patients by the Bulpa criteria
Among 24 patients with positive mycological findings, 20 were diagnosed as probable IPA cases; the non-classification of the remaining 4 patients was attributed to the lack of consecutive BALF GM in the mycological findings of the Bulpa criteria (Table 3).
The reason underlying the non-classification of patients by the ICU criteria
Among 24 patients with positive mycological findings, 10 were diagnosed as putative IPA cases; the non-classification of the remaining 14 patients was attributed to the strict requirements regarding the dosage of steroid use and the necessity for positive LRT cultures as an entry criterion (Table 3).
The proposed criterion for IPA in critically Ill COPD patients admitted to the ICU
Modified criteria for defining probable IPA based on the Bulpa criteria are proposed mainly based a revised interpretation of the requirements regarding microbiological findings (Table 4).
Probable IPA can be diagnosed when a critically ill COPD patient in the ICU exhibits a pulmonary functional level of GOLD III or IV, a history of steroid use of at least 3 months (irrespective of administration route and dose/ course) and appropriate compatible signs or symptoms, the presence of any major radiological sign of pneumonia and one of the mentioned microbiological findings.
Discussion
To the best of our knowledge, the current study is the first to compare the validity of diagnosis between the existed three criteria commonly used for IPA. The major power of this study was that we evaluated the “diagnostic rate” of probable IPA in different populations, and validated the results based on a proven population.
The major finding of this study is that we verified that Bulpa criteria was the most suitable criteria for IPA diagnosis in critically ill COPD patients admitted to ICU compared with the other two. While the defects in microbiological findings still existed in Bulpa criteria, the modified Bulpa criteria for probable IPA were proposed and will need further verification by comparing against proven IPA cases in the future studies. Furthermore, We analyzed reasons for the highest “diagnostic rate” in Bulpa criteria.
We found that many non-classified patients by EORTC/ MSG and ICU criteria could be diagnosed as probable IPA by Bulpa criteria. Bulpa criteria made less strict requirements in dose/ course of steroid and typical CT manifestations, thus improving the“ diagnostic rate”. As reported, almost 30-70% of IPA patients in an ICU were not in a severely immunocompromised condition [16, 17]. Most COPD patients lack the host factors that are required by the EORTC/ MSG and ICU criteria with the exception of steroid usage. In addition, although precise doses and courses of steroid cannot be extrapolated from the literature for COPD patients, data support the fact that patients with underlying lung diseases are at risk for IPA at much lower doses and shorter courses [1, 4, 5, 18–21]. Recently, some reports have suggested that high doses of inhaled corticosteroids may also be a risk factor for IPA [22–24], and some COPD patients might develop IPA even without exposure to steroids [25]. Thus, in the clinical practice, there is no need for the excessively strict requirements regarding the dose and course of systemic steroid use, and the inhaled steroid should also be contained.
Nonspecific manifestations, such as patchiness or consolidations, are more frequently in COPD patients. The research of Meersseman W [26] and Vandewoude K [9] reported that the halo signs only appeared in 5-17% cases of COPD that are complicated with IPA. Our research [27] also demonstrated that patchiness (76.2%) were the most common CT sign among IPA in critically ill COPD patients admitted to ICU, while the angio-invasive patterns (including halo sign, wedge consolidation and air-crescent sign/ cavity) had a relatively low percent. Therefore, there is no need for the excessively strict requirements in typical CT manifestations as well in diagnostic criteria for IPA in critically ill COPD patient.
Defects in microbiological findings existed in Bulpa criteria. The lack of BALF GM tests in mycological data was the deficiency of the Bulpa criteria, which might underestimate some IPA patients. Our previous study [15] showed that positive BALF GM was observed earlier than that in LRT secretion culture (1 day versus 3.8 days), and an appropriate cut-off value enabled the discrimination of infection from colonization with Aspergillus. Therefore, the two consecutive positive BALF GM tests were found useful in diagnosing cases of probable IPA, and should be added into Bulpa criteria. Besides, positive serum antibody test for A. fumigatus (including precipitins) is included in the Bulpa criteria; however, it takes at least 3-6 months to elicit an antibody response [8, 28, 29], and the course of acute IPA is too rapid compared to antibody formation. Moreover, the antibody response is often weak in severe COPD patients on long-term steroid therapy [8]. Serum antibody testing for A. fumigatus, including that based on precipitins is an extremely useful technique in patients with chronic pulmonary aspergillosis (CPA) [8, 29, 30], and the use of this test in clinical practice is far from universal, so it should be removed from the Bulpa criteria.
We also noticed that the semi-quantitative Aspergillus-positive culture of BALF, without bacterial growth but with a positive cytological smear showing branching hyphae, is included in the ICU criteria as an alternative item for host factors; nevertheless, mixed infections with bacteria are common in critically ill COPD patients in the ICU; therefore, it was not appropriate to add it to Bulpa criteria as an alternative item for host factors.
There were several limitations in our study. The greatest one was the small number (only 5) of proven IPA cases in our study, for which histopathological specimens were available to verify the validity of the clinical assumption, thus we could only estimate the “diagnostic rate” of probable IPA cases in population one, two and three, and observed the consistency of the results with those for the proven IPA group, leading to weak persuasiveness. Moreover, it may also have a problem in overestimating the number of IPA based on Bulpa criteria; however, a delayed diagnosis was associated with a worse prognosis, we would rather accept a diagnose criteria with a lower missed diagnostic rate. Third, the sample size was relatively small because of the strict inclusion criteria, which included critically ill COPD patients in an ICU with a pulmonary functional level of GOLD III or IV, and the availability of CTs obtained within a short period. The above limitations require further investigations.
Conclusion
Among the existing three criteria, the Bulpa criteria are the most suitable for diagnosing probable IPA in critically ill COPD patients admitted to ICU. The modified Bulpa criteria for probable IPA was proposed and needs further confirmation,
Abbreviations
- (R)ICU:
-
(Respiratory) Intensive care unit
- BALF:
-
Bronchoalveolar lavage fluid
- COPD:
-
Chronic obstructive pulmonary disease
- CPA:
-
Chronic pulmonary aspergillosis
- CT:
-
Computed tomography
- CTD:
-
Connective tissue disease
- CXR:
-
Chest X ray
- EORTC/ MSG:
-
European Organization for the Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group
- ETA:
-
Endotracheal aspiration
- GM:
-
Galactomannan
- GOLD:
-
Global initiative for chronic obstructive lung disease
- HIV:
-
Human immunodeficiency virus
- HSCT:
-
Haematopoietic stem cell transplantation
- IPA:
-
Invasive pulmonary aspergillosis
- LRT:
-
Lower respiratory tract
- SOT:
-
Solid organ transplantation
References
Bulpa PA, Dive AM, Garrino MG, et al. Chronic obstructive pulmonary disease patients with invasive pulmonary aspergillosis: benefits of intensive care. Intensive Care Med. 2001;27:59–67.
Samarakoon P, Soubani A. Invasive pulmonary aspergillosis in patients with COPD: a report of five cases and systematic review of the literature. Chron Respir Dis. 2008;5:19–27.
Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis. 2014;27:174–83.
Ader F, Nseir S, Berre RL, et al. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect. 2005;11:427–9.
Rello J, Esandi ME, Mariscal D, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease report of eight cases and review. Clin Infect Dis. 1998;26:1473–5.
Ascioglu S, Rex JH, Edwards JE, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14.
De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21.
Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30:782–800.
Vandewoude KH, Blot SI, Pieter D, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31.
Blot SI, Fabio Silvio T, Van den Abeele A-M, et al. A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am J Respir Crit Care Med. 2012;186:56–64.
Pauwels RA, Buist AS, Ma P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798-825.
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55.
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
He H, et al. Prognostic value of serum galactomannan index in critically ill patients with chronic obstructive pulmonary disease at risk of invasive pulmonary aspergillosis. Chin Med J (Engl). 2014;127:23–8.
He H, et al. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: a prospective study. Crit Care. 2012;16:R138.
Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–9.
Balloy V, Huerre M, Latge JP, et al. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005;73:494–503.
Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63.
Muquim A, Dial S, Menzies D. Invasive aspergillosis in patients with chronic obstructive pulmonary diseases. Can Respir J. 2005;12:199–204.
Palmer LB, Greenberg HE, Schiff MJ. Corticosteroid treatment as a risk factor for invasive aspergillosis in patients with lung disease. Thorax. 1991;46:15–20.
Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21:161–72.
Leav BA, Fanburg B, Hadley S. Invasive pulmonary aspergillosis associated with high-dose inhaled fluticasone. N Engl J Med. 2000;343:586.
Chow L, Brown NE, Kunimoto D. An unusual case of pulmonary invasive aspergillosis and aspergilloma cured with voriconazole in a patient with cystic fibrosis. Clin Infect Dis. 2002;35:e106–10.
Barouky R, Badet M, Saint Denis M, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease and disseminated aspergillosis. Eur J Intern Med. 2003;14:380–2.
Ali ZA, Ali AA, Tempest ME, et al. Invasive pulmonary aspergillosis complicating chronic obstructive pulmonary disease in an immunocompetent patient. J Postgrad Med. 2003;49:78–80.
Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–5.
Huang L, He H, Zhan Q, et al. Values of radiological examinations for the diagnosis and prognosis of invasive bronchial-pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary diseases. Clin Respir J. 2016; 00:000–000.DOI:10. 1111/crj.12551
Meersseman W, Lagrou K, Maertens J, et al. Invasive aspergillosis in the intensive care unit. Clin Infect Dis. 2007;45:205–16.
Page ID, Richardson M, Denning DW. Antibody testing inaspergillosis—quo vadis? Med Mycol. 2015;53:417–39.
Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45–68.
Acknowledgements
Not applicable.
Funding
This study is supported by the Beijing Municipal Administration of Hospitals’ Youth Programme (QML 20150301) and the National Key Research and Development Programme—Major Chronic Non-communicable Diseases’ Prevention and Control (QML 2016YFC1304300).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
All authors made substantial contributions to the conception and design of the study or to data acquisition, analysis or interpretation; reviewed and approved the final manuscript; and significantly contributed to this study. Drs. LH and JJ contributed equally to this work. QZ takes full responsibility for the integrity of the submission and publication and was involved in the study design. LH and JJ had full access to all data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and were responsible for data verification and analysis, as well as the drafting of the manuscript. HH and YD had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the ethics committee of Beijing Chao-Yang Hospital, Capital Medical University, Beijing, P. R. China, and written informed consent was obtained from the patients or their next of kin.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
“Three Key Factors” of the Included Patients. Note: The history of steroid use, abnormal radiology and mycological findings of the included 59 COPD patients were listed in Additional file 1, and patients were diagnosed as probable IPA using three criteria respectively. “Y” means that the case was could be diagnosed as probable/ putative IPA according to the criteria; “N” means that the case was could not be diagnosed as probable/ putative IPA according to the criteria. (DOCX 128 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huang, L., He, H., Jin, J. et al. Is Bulpa criteria suitable for the diagnosis of probable invasive pulmonary Aspergillosis in critically ill patients with chronic obstructive pulmonary disease? A comparative study with EORTC/ MSG and ICU criteria. BMC Infect Dis 17, 209 (2017). https://doi.org/10.1186/s12879-017-2307-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-017-2307-y