Abstract
Background
Urinary and (peripheral and central) intravenous catheters are widely used in hospitalized patients. However, up to 56% of the catheters do not have an appropriate indication and some serious complications with the use of these catheters can occur. The main objective of our quality improvement project is to reduce the use of catheters without an appropriate indication by 25–50%, and to evaluate the affecting factors of our de-implementation strategy.
Methods
In a multicenter, prospective interrupted time series analysis, several interventions to avoid inappropriate use of catheters will be conducted in seven hospitals in the Netherlands. Firstly, we will define a list of appropriate indications for urinary and (peripheral and central) intravenous catheters, which will restrict the use of catheters and urge catheter removal when the indication is no longer appropriate. Secondly, after the baseline measurements, the intervention will take place, which consists of a kick-off meeting, including a competitive feedback report of the baseline measurements, and education of healthcare workers and patients. Additional strategies based on the baseline data and local conditions are optional. The primary endpoint is the percentage of catheters with an inappropriate indication on the day of data collection before and after the de-implementation strategy. Secondary endpoints are catheter-related infections or other complications, catheter re-insertion rate, length of hospital (and ICU) stay and mortality. In addition, the cost-effectiveness of the de-implementation strategy will be calculated.
Discussion
This study aims to reduce the use of urinary and intravenous catheters with an inappropriate indication, and as a result reduce the catheter-related complications. If (cost-) effective it provides a tool for a nationwide approach to reduce catheter-related infections and other complications.
Trial registration
Dutch trial registry: NTR6015. Registered 9 August 2016.
Similar content being viewed by others
Background
Healthcare-associated infections (HAIs) are associated with an increased mortality, a longer duration of hospital stay, which results into an increase in substantial costs. The use of invasive medical devices (e.g., urinary catheters, peripheral intravenous catheters (PIVCs) and central venous catheters (CVCs)) are important risk factors for the development of HAIs, which have prevalence of 7.1% measured in a combined point prevalence survey in Europe [1]. So an efficient way to reduce HAIs is to avoid insertion of catheters without an appropriate indication and to reduce the number of catheter days.
In general hospitals 15–25% of patients have an indwelling urinary catheter during their hospital stay. Urinary tract infections are accountable for 40% of all nosocomial infections in Western world hospitals, and 71–80% of these patients had a urinary catheter [2–4]. Nevertheless, the incidence of unwarranted placement of urinary catheters in hospitalized patients is 14–65% [5–10].
PIVCs are the most frequently used invasive medical devices in hospitalized patients. However, 25–56% of the PIVCs inserted in the Emergency Department are inappropriate or even unused [11–16]. In a recent study of internal medicine departments in Spain 81.9% of the patients had one or more PIVCs, of which 19% were no longer necessary [17]. A PIVC can cause serious adverse events, with an incidence rate of catheter-associated bloodstream infection of 0.1% (0.5 per 1000 catheter days) [18].
Central line-associated bloodstream infections (CLABSIs) are a major problem in intensive care units (ICUs). A meta-analysis shows that implementation of central line bundles to reduce the incidence of CLABSIs are effective and cost saving in ICUs [19].
Intervention studies to prevent catheter-related infections
Previous research suggests that multiple and well-organized interventions could reduce the number of HAIs. In a pilot study in our university hospital in the Netherlands 89.2% of the initial indications for urinary catheter use were appropriate. However, after 2–3 days the initial indication was mostly no longer present, resulting into an inappropriate indication, but not to a removal of the catheter. After education and daily assessment of the indication of urinary catheters, the duration of catheterization reduced from 1009 to 672 days in 149 patients (pre-intervention n = 74, post-intervention n = 75), and the number of catheter-associated urinary tract infections (CAUTI) decreased from 4 to 0 infections per 1000 catheter days (p = 0.04). Thereby the median length of hospital stay reduced from 13 to 9 days [20]. Very recently, a national program (dissemination of information to sponsor organizations and hospitals, data collection, and guidance on key technical and socioadaptive factors) in 603 US hospitals reduced CAUTI rates by 22% in non-ICUs [21].
Only a few studies evaluated the effect of interventions to improve the appropriate use of PIVCs. In 1994 a quality improvement project in the internal medicine wards of Minnesota reduced inappropriate use of PIVCs by 63% (43% vs 27%) [22]. Education and feedback to improve PIVC care significantly reduced the PIVC-associated bloodstream infections from 2.2 to 0.44 per 10.000 patients days in 10 non-ICUs [23]. Furthermore, in a general hospital in Spain the use of unnecessary peripheral and central venous lines decreased from 22.9 to 7.1% after a 1-year training program [24].
A multifaceted ‘bundle’ approach (education, hospital protocol, national program, and checklist intervention) to control CVC-associated bloodstream infection in an internal medicine department in Spain showed a decrease of 63.1% (14.1 to 5.2 per 1000 catheter days) [25].
Prevention of HAIs is an important part of the todays medical practice. However, the risks of the use of urinary catheters and mainly PIVCs are widely underestimated, and in the Netherlands no nationwide program to reduce the catheter-related infections is present.
Methods and Design
Objectives
In this de-implementation study we aim for a 25–50% reduction of the number of urinary and (peripheral and central) intravenous catheters without an appropriate indication, which will lead to a reduction of the number of catheter days and catheter-related complications.
Study design and setting
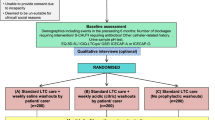
The study design is a multicenter, prospective interrupted time series (Fig. 1), which will take place in seven hospitals in the Netherlands (three university and four general hospitals). We aim to reduce 25–50% of the catheters without an appropriate indication by a de-implementation strategy of multiple interventions. The clinical data collection will be once per 14 days during 8 months in both the pre- and post-intervention period. During these measurement days all patients with a urinary and/or (peripheral and/or central) intravenous catheter inserted during the hospital stay will be enrolled. The de-implementation strategies will start during a transition period of 4 months, while no patients will be included.
Appropriate use of catheters
We defined a list of valid indications for urinary and intravenous catheter placement, based on the literature (Table 1) [26–29]. All other indications are defined as inappropriate.
Definition of catheter-related infections
We will use the definitions by Centre for Disease Control’s (CDC) National Healthcare Safety Network for catheter-related infection (Table 2) [30]. Thereby we will use probable definitions based on the CDC and the Dutch surveillance system ‘Prevention of Nosocomial Infections through Surveillance’ (Table 3) [31].
Patient selection
We will include all patient (≥18 years old) admitted to internal medicine and subspecialties (gastroenterology & hepatology, geriatrics, pulmonology and rheumatology) and all nonsurgical patients admitted to acute medical units, who receive urinary and/or (peripheral and/or central) intravenous catheter. Patients admitted for elective short stay, terminally ill patients and patients who had all catheters prior to admission will be excluded (Fig. 2).
Primary and secondary endpoints
The primary endpoint is the percentage of patients with an inappropriate indication for urinary and intravenous catheter on the days of data collection. Secondary endpoints are catheter-related infections and other complications, catheter reinsertion rate, use of antibiotics, length of hospital stay (and ICU) in days, in hospital mortality, and costs of the de-implementation strategy and the main healthcare costs.
Assessments
The presence and indications for the catheter use will be extracted from the medical records in combination with observations of the admitted patients. During the day of measurement the indication of the urinary and/or intravenous catheter and some patient variables will be collected. If there is an unclear indication of a catheter, the investigator will contact one of the healthcare workers (HCWs) to verify this information.
After discharge, the occurrence of catheter-related infections (see Table 3 for definitions) [31], with diagnostics and treatment, or other catheter-related complications (measured in registry and by investigator), the number of catheter days, reinsertion rate, use of alternatives for an indwelling urinary catheter (continence garments, condom catheters, intermittent straight catheterization), reason for admission, Charlson comorbidity index [32], duration of hospital (and ICU) stay, readmission within 30 days after discharge, and mortality (in hospital or within 30 days after discharge) will be collected from (electronic) medical- and nursing records. Furthermore, the nurse-to-patient ratios and the clinical work experience of residents will be collected.
De-implementation strategy
We will use a bundle of interventions (Table 4), since it is known that multiple and well-organized interventions result into better improvements. The main intervention is the dissemination of the list of appropriate indications (Table 1), which will restrict the insertion of urinary and intravenous catheters by physicians and nurses. The recommendation will be to remove the catheters without or with an expired valid indication. A local ‘champion’ (one of the leading physicians) will be appointed to be responsible for the interventions in his or her department. The intervention period will start with a kick-off meeting, where a list of baseline data of the intervention hospital in comparison with the other study hospitals will be presented as a competitive feedback report [33]. If desired, feedback reports per department could be sent by e-mail to local investigators. Thereby two educational meetings about insertion, care and maintenance of urinary and intravenous catheters will be applied to change HCWs behavior. Furthermore, HCWs will be encouraged and reminded to remove inappropriate catheters by posters, pocket cards and e-mail messages. In addition to other studies, patients will be actively supported by education material to participate in their treatment.
Subsequently, all impeded and promoted factors of this de-implementation strategy for both HCWs and patients will be evaluated by direct observations and interviews. Additional prevention strategies could be applied based on these affecting factors and local conditions in the different hospitals.
If this de-implementation strategy prove to be effective, persistent awareness of the local ‘champion’ in combination with recurrent surveillance of the appropriate use of catheters and catheter-related infections will improve the sustainability. Thereby the de-implementation rule “No indication = Remove catheter” will be recommended in national guidelines and local protocols.
Sample size
The sample size is based on the objective of a 25–50% reduction in the number of invalid indications for both urinary and peripheral intravenous catheters, with a power of 80% and an alpha of 0.05. We excluded CVCs in the sample size, since the use of CVCs in the internal medicine and subspecialties departments is infrequent, but we will include all patients with a CVC in the study. We used the incidence of inappropriate use of the catheters from previous results in similar healthcare systems, which is 40% in urinary catheters [2, 20] and 50% in PIVC [13]. This results in 91–376 patients with a urinary catheter, and 66–284 patients with a PIVC. Correlating for 10–15% missing data the sample size is set to respectively 105–410 and 75–300 patients in both pre- and post-intervention group. This will result in a total sample size of 210–820 patients with a urinary catheter and 150–600 patients with a PIVC. We aim to include this number of patients in each hospital to evaluate the effect of the interventions in each individual hospital.
Statistical analysis
Effect evaluation will be performed using SPSS statistics 23. Categorical variables will be presented as frequencies and percentages, and continuous variables as means (with a standard deviation) or medians (with an interquartile range) depending on the data distribution. We will use segmented regression analysis of interrupted time series methods to evaluate the differences between the baseline and intervention group [34, 35]. The data will be adjusted for possible confounders, autocorrelation and the underlying secular trend. We will perform stratified analyses to evaluate the impact of the de-implementation strategy in subpopulations. Figures will be used to visualize the underlying secular trend and the impact of the de-implementation strategy. The difference will also be presented in unadjusted and adjusted rate ratio (RR) with a 95% confidence interval (CI). Differences are considered to be statistically significant with a p < 0.05.
Economic evaluation
The main question for the economic evaluation is if the benefits of a reduction in inappropriate use of catheters, which probably lead to a reduction in catheter-related infections, length of stay and associated costs, outweighed the costs associated with this de-implementation strategy. For feasibility reasons, we will use length of stay on general and ICU wards, readmission within 30 days of discharge, catheter-related complications, representing the main features of the healthcare system, and productivity costs for societal perspective. We will estimate the unit costs for healthcare service based on the prices in the Dutch guideline on healthcare costs [36]. We will divide the de-implementation costs in non-recurrent and recurrent costs. The non-recurrent costs are study-related, such as the costs of development of the de-implementation strategy, material costs, and costs of evaluation of the de-implementation. Recurrent costs are the costs to implement the de-implementation strategy. The primary analysis is a cost-effectiveness analysis, in which the difference in costs of the de-implementation and outcomes between the baseline and intervention group will be estimate using incremental cost-effectiveness ratios (ICERs). The cost-benefit analysis will be reported as the ratio of de-implementation costs to reduction of healthcare costs (de-implementation costs < reduction healthcare costs). Statistical uncertainty will be evaluated in sensitivity analyses, such as unit costs, preference weights, estimates of effectiveness and discount rate. The result from the economic evaluation will be extrapolated to the national level using a budget impact analysis according to the principles of the report of the International Society for Pharmacoeconomics and Outcome Research Task Force [37], conducted from societal perspective and health insurance or national health service perspective.
Discussion
This study protocol describes the design, de-implementation strategy and evaluation of the ‘Reduce the inappropriate use of urinary and intravenous catheters’ (RICAT)-study. It could prevent the inappropriate use of urinary and intravenous catheters. If cost-effective it provides a tool for a nationwide approach to reduce catheter-related infections and healthcare costs.
A potential limitation of an interrupted time series design is the nonexistence of a control group. However, this quasi-experimental design is considered to be one of the most effective and powerful designs when randomization is not desirable or possible [34]. Another limitation is the inability to evaluate the impact of an individual intervention. In order to estimate the impact of a single de-implementation strategy there should be enough time between the different intervention periods, which is not possible in our study. Nevertheless, the interventions are well suitable for a broad de-implementation in other hospitals.
Abbreviations
- CAUTI:
-
Catheter-associated urinary tract infection
- CLABSI:
-
Central line-associated bloodstream infection
- CVC:
-
Central venous catheter
- HAI:
-
Healthcare-associated infection
- HCW:
-
Healthcare worker
- ICU:
-
Intensive care unit
- PIVC:
-
Peripheral intravenous catheter
References
Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens MM, Vaerenberg S, Hopkins S, Catry B, Monnet DL, Goossens H, Suetens C, National Contact Points for the ECDC pilot point prevalence survey, Hospital Contact Points for the ECDC pilot point prevalence survey. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17(46). PMID 23171822. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20316.
Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–63.
System N. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–85.
Haley RW, Hooton TM, Culver DH, Stanley RC, Emori TG, Hardison CD, Quade D, Shachtman RH, Schaberg DR, Shah BV, et al. Nosocomial infections in U.S. hospitals, 1975–1976: estimated frequency by selected characteristics of patients. Am J Med. 1981;70(4):947–59.
Schuur JD, Chambers JG, Hou PC. Urinary catheter use and appropriateness in U.S. emergency departments, 1995–2010. Acad Emerg Med. 2014;21(3):292–300.
Greene MT, Fakih MG, Fowler KE, Meddings J, Ratz D, Safdar N, Olmsted RN, Saint S. Regional variation in urinary catheter use and catheter-associated urinary tract infection: results from a national collaborative. Infect Control Hosp Epidemiol. 2014;35 Suppl 3:S99–s106.
Fakih MG, Watson SR, Greene MT, Kennedy EH, Olmsted RN, Krein SL, Saint S. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255–60.
Knoll BM, Wright D, Ellingson L, Kraemer L, Patire R, Kuskowski MA, Johnson JR. Reduction of inappropriate urinary catheter use at a Veterans Affairs hospital through a multifaceted quality improvement project. Clin Infect Dis. 2011;52(11):1283–90.
Holroyd-Leduc JM, Sen S, Bertenthal D, Sands LP, Palmer RM, Kresevic DM, Covinsky KE, Seth Landefeld C. The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients. J Am Geriatr Soc. 2007;55(2):227–33.
Jain P, Parada JP, David A, Smith LG. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med. 1995;155(13):1425–9.
Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of all peripheral intravenous lines in an Australian tertiary emergency department are unused: pain with no gain? Ann Emerg Med. 2013;62(5):521–5.
Goransson KE, Johansson E. Indication and usage of peripheral venous catheters inserted in adult patients during emergency care. J Vasc Access. 2011;12(3):193–9.
Abbas SZ, de Vries TK, Shaw S, Abbas SQ. Use and complications of peripheral vascular catheters: a prospective study. Br J Nurs. 2007;16(11):648. 650, 652.
Velasco Diaz L, Fernandez Gonzalez B, Garcia Rios S, Hernandez del Corro E. The evaluation of unnecessary venous access ports in an emergency service. Med Clin (Barc). 2000;114(3):89–90.
Henderson RA, Thomson DP, Bahrs BA, Norman MP. Unnecessary intravenous access in the emergency setting. Prehosp Emerg Care. 1998;2(4):312–6.
Lederle FA, Parenti CM, Berskow LC, Ellingson KJ. The idle intravenous catheter. Ann Intern Med. 1992;116(9):737–8.
Guembe M, Perez-Granda MJ, Capdevila JA, Barberan J, Pinilla B, Martin-Rabadan P, Bouza E. Nationwide study on the use of intravascular catheters in internal medicine departments. J Hosp Infect. 2015;90(2):135–41.
Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–71.
Ista E, van der Hoven B, Kornelisse RF, van der Starre C, Vos MC, Boersma E, Helder OK. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:724–34.
Janzen J, Buurman BM, Spanjaard L, de Reijke TM, Goossens A, Geerlings SE. Reduction of unnecessary use of indwelling urinary catheters. BMJ quality & safety. 2013;22(12):984–8.
Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KE, Edson BS, Watson SR, Meyer-Lucas B, Masuga M, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111–9.
Parenti CM, Lederle FA, Impola CL, Peterson LR. Reduction of unnecessary intravenous catheter use. Internal medicine house staff participate in a successful quality improvement project. Arch Intern Med. 1994;154(16):1829–32.
Fakih MG, Jones K, Rey JE, Berriel-Cass D, Kalinicheva T, Szpunar S, Saravolatz LD. Sustained improvements in peripheral venous catheter care in non-intensive care units: a quasi-experimental controlled study of education and feedback. Infect Control Hosp Epidemiol. 2012;33(5):449–55.
Perez-Granda MJ, Guembe M, Rincon C, Munoz P, Bouza E. Effectiveness of a training program in compliance with recommendations for venous lines care. BMC Infect Dis. 2015;15:296.
Garcia-Rodriguez JF, Alvarez-Diaz H, Vilarino-Maneiro L, Lorenzo-Garcia MV, Canton-Blanco A, Ordonez-Barrosa P, Marino-Callejo AI, Sesma-Sanchez P. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445.
Meddings J, Saint S, Fowler KE, Gaies E, Hickner A, Krein SL, Bernstein SJ. The Ann arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1–34.
Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–26.
Chopra V, Flanders SA, Saint S, Woller SC, O’Grady NP, Safdar N, Trerotola SO, Saran R, Moureau N, Wiseman S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1–40.
O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–93.
CDC. National Healthcare Safety Network (NHSN) Overview. 2016. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed 26 May 2016.
PREZIES. Definities ziekenhuisinfecties - Module Prevalentieonderzoek ziekenhuizen. 2016. http://www.rivm.nl/Onderwerpen/P/PREZIES/Prevalentieonderzoek_Ziekenhuizen/Protocol_Prevalentieonderzoek_Ziekenhuizen/Prevalentieonderzoek_Bijlage_2_definities_2016.org. Accessed 28 July 2016.
Sundararajan V, Quan H, Halfon P, Fushimi K, Luthi JC, Burnand B, Ghali WA. Cross-national comparative performance of three versions of the ICD-10 Charlson index. Med Care. 2007;45(12):1210–5.
Spoorenberg V, Hulscher ME, Geskus RB, de Reijke TM, Opmeer BC, Prins JM, Geerlings SE. A cluster-randomized trial of Two strategies to improve antibiotic use for patients with a complicated urinary tract infection. PLoS One. 2015;10(12):e0142672.
Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750.
Jandoc R, Burden AM, Mamdani M, Levesque LE, Cadarette SM. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol. 2015;68(8):950–6.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Tan SS. Kostenhandleiding: Methodologie voor kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. In: Institute for medical technology assessment. 2015. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-degezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+%28verdiepingsmodules%29.pdf. Accessed 28 June 2016.
Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M, Orlewska E, Watkins J, Trueman P. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health. 2007;10(5):336–47.
Acknowledgements
Not applicable.
Funding
This study is funded by the Netherlands Organisation for Health Research and Development (ZonMw) grant 8392010022. They have no role in the study design or analysis.
Availability of data and materials
Not applicable.
Authors’ contributions
BJL, IJBS, MHG, MCV and SEG designed the study and drafted the manuscript. SEG arranged the funding application. BJL, JMM and BCO outlined the statistical and economic analysis. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The project protocol was assessed by the Medical Ethics Research Committee of the Academic Medical Centre. They determined that this quality improvement project does not meet the definition of medical research involving human subjects. Local feasibility is approved by the Board of Directors in all study hospitals. Data collection will be anonymous and no informed consent is required.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Laan, B.J., Spijkerman, I.J.B., Godfried, M.H. et al. De-implementation strategy to Reduce the Inappropriate use of urinary and intravenous CATheters: study protocol for the RICAT-study. BMC Infect Dis 17, 53 (2017). https://doi.org/10.1186/s12879-016-2154-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-2154-2