Abstract
Background
The ageing process is characterized by a change of body composition with an increase of fat mass and a reduction of muscle mass. Above a certain threshold these alterations configure a condition named sarcopenic obesity (SO). SO is associated with physical frailty in Asian and Brazilian populations. SO impacts on physical frailty in other ethnic groups but its influence on general frailty which is multidimensional and includes cognitive, social and physical factors, remain insufficiently explored in the Italian population.
Methods
Frailty was measured in community dwelling Italian older adults enrolled in the FRASNET study with the frailty index (FI). The FI quantifies frailty as the ratio of the number of present health deficits to the total number of health deficits considered. Regression analyses were performed to assess the association between body composition categories and frailty. Classification and regression tree models were run to evaluate the frailty predictors.
Results
One Thousand One Hundred Fourteen participants of the FRASNET study were included in the present analysis. The sample was composed for the 60.5% by females and its median age was 72 years. The median FI score was 0.11 (IQR 0.07–0.20); 234 individuals (21%) were frail (FI ≥ 0.25). SO (B 0.074, 95% C.I. 0.05–0.1, p < 0.001) and pre-sarcopenia (without obesity B 0.03, 95% C.I, 0.007–0.044, p < 0.001, with obesity B 0.11, 95% C.I. 0.05–0.16, p < 0.001) were associated with frailty. Fat mass percentage predicted frailty in people aged 65–70 years whereas, muscle strength predicted general frailty in people aged 70–81 years.
Conclusion
Pre-sarcopenia and SO represent potentially treatable predictors of frailty.
Similar content being viewed by others
Background
The ageing process is characterized by a change in body composition with a progressive increase of fat mass, in particular visceral fat, and an ectopic distribution of fat in non-fat tissues, which also impairs organ functions [1]. In addition of being associated with many metabolic and cardiovascular diseases [2], the excess of body fat and obesity have been linked to impaired physical performance, mobility limitations and risk of developing disability in older people [3].
With ageing, there is a reduction of muscle mass too, which is associated with a decrease of muscle strength and power.
Although a generally accepted threshold for the age associated physiological reduction of muscle mass and function is still lacking [4], there is consensus that an important decline, known as sarcopenia [5] may lead to adverse functional and clinical outcomes such as institutionalization, mortality, and increased length of hospital stays [6, 7]. Sarcopenia is also closely related to physical frailty [8, 9].
Frailty is a condition characterized by a reduction in physiological reserves, leading to increased vulnerability to stressors and the risk of adverse consequences [10]. This condition comprises both physical and general frailty. The first one is well described by the frailty phenotype (FP) which focuses on physical aspects of frailty and on the concept of energy loss. FP identifies individuals as frail if they exhibit at least three of the following criteria: low muscle strength, slow gait speed, reduced physical activity, unintentional weight loss, and exhaustion [11]. General frailty is instead well described by the cumulative deficit model, also known as the frailty index (FI). The FI provides a broader perspective on frailty compared to the FP, considering social, psychological, cognitive, and functional determinants in addition to physical aspects and comorbidities [12]. According to the FI model, the higher the number of health deficits present, the greater the vulnerability and frailty of the individual.
When sarcopenia and obesity occur together, they create a vicious cycle that synergistically enhance both conditions [13]. On the one hand the increased fat tissue promotes oxidative stress, inflammation and the development of insulin resistance, which negatively act on the trophism and function of muscles [14]. On the other hand, sarcopenia reduces total energy expenditure [15] leading to fat accumulation [16]. Obese individuals tend also to have a high burden of chronic conditions [17, 18] which negatively affect muscle metabolism. Finally, sedentary life, which is both a cause and a consequence of sarcopenia and obesity, favours the progression of both conditions [19,20,21].
The co-occurrence of sarcopenia and obesity is named sarcopenic obesity (SO) [13]. The risk of developing adverse clinical outcomes derived from the two conditions cumulates in SO [22,23,24,25,26,27,28,29]. SO has been linked to physical frailty in the Asian [30, 31] and Brazilian [32] populations. Also, two small French studies described an association between SO and physical frailty [33] and SO and physical function [34] in middle-aged and older women. A more general association between body composition and physical frailty, but without a specific reference to sarcopenic obesity has been described in the American [35,36,37], Norwegian [38] and Portuguese [39] populations. A link between general frailty and body composition measure with body mass index (BMI) was highlighted in the Survey of Health, Ageing and Retirement in Europe (SHARE) [40]. Here, we analysed the relative contribution of body composition and SO, measured with a functional perspective according to ISarcoPRM guide [41], to general frailty, measured with the cumulative deficit model in a population of community dwelling Italian older volunteers.
Materials and methods
Study participants
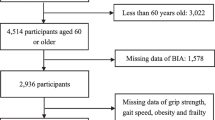
The Frailty and Sarcopenia Network (FRASNET) study is a cross-sectional multicentre observational cohort study involving community-dwelling healthy volunteers and istitutionalized patients [42]. The study was approved by the San Raffaele Scientific Institute ethical board (24/INT/2017). Participants signed a written informed consent before taking part in the study. The enrolment was performed between the 1st April 2017 and the 16th October 2020.
Inclusion/exclusion criteria
The inclusion criteria were: (i) being aged 65 years old or older, (ii) being able of walking more than 500 m without assistance; (iii) life expectancy > 6 months. The exclusion criteria for our study were as follows: (i) Severe Cognitive Impairment: participants with a Mini-Mental State Examination (MMSE) score of less than 18/30 were excluded. This threshold was chosen to ensure that all participants could understand and comply with the study procedures and to minimize the risk of inaccurate self-reported data due to cognitive deficits. (ii) Inability to Provide Informed Consent: individuals who were unable to sign a written informed consent were excluded. This criterion was essential to comply with ethical standards and ensure that all participants voluntarily agreed to partake in the study with a clear understanding of its purpose and procedures. (iii) Severe Health Problems: participants with severe health issues were excluded to avoid confounding effects on the study outcomes. Specific conditions for exclusion from the study were uncontrolled hypertension defined as consistently high blood pressure despite medication, which could interfere with physical performance and other study measures. Recent fractures: individuals with upper or lower extremity fractures within the past six months were excluded to prevent pain or mobility limitations from affecting the results. Recent myocardial infarction: those who had experienced a heart attack within the past year were excluded due to potential restrictions on physical activity and overall health instability. (iv) Residence in Retirement Homes: patients recruited from retirement homes were excluded to ensure a homogenous sample. This decision was made to avoid variability in care levels and living conditions that might influence the study outcomes. (v) Incomplete Data: participants with missing data necessary for the computation of frailty or lacking information on body composition were excluded. Complete datasets were required to accurately assess frailty and its associations with other variables, ensuring the reliability and validity of the study findings. These exclusion criteria were carefully selected to maintain the integrity of the study sample, ensure participant safety, and enhance the reliability and applicability of the results.
Procedures
Participants underwent multidimensional geriatric assessments, that included the collection of demographic and psychosocial data through self-administered questionnaire, the assessment of comorbidities and pharmacological therapies, of the number of falls and emergency department accesses in the year preceding the evaluation, anthropometric measurements (weight, height, waist circumference, body mass index (BMI) calculation). Cognition was screened with the Mini Mental State Examination (MMSE) [43], mood with the Geriatric Depression Scale (GDS) 15 items [44], exhaustion with the Fatigue Severity Scale (FSS) [45], quality of with life the Short Form 36 (SF-36) Health Survey [46] and the level of physical activity with the Physical Activity Scale for Elderly (PASE) questionnaire [47].
Body composition was determined thought the Full Body Sensor Body Composition Monitor and Scale (OMRON) balance [48]. This balance uses an extremely weak electrical current (50 kHz and less than 500µA) that passes through the body to estimate with the bioelectrical impedance method body composition. According to the ISarcoPRM guide [41] muscle performance was assessed with gait speed and the Short Physical Performance Battery (SPPB) [49] and muscle strength with the chair-stand subtest of the SPPB [50]. Values > 15 s were considered indicative of reduced muscle strength and therefore of pre-sarcopenia [5, 41]. People with pre-sarcopenia were further classified as obese and non-obese according to the percentage of fat mass measured though the bioelectrical impedance balance (obese if fat mass ≥ 30% in men and ≥ 42% in women) [48].
In this older population, the diagnosis of sarcopenia was performed according a functional approach as the one described by the ISarcoPRM [41] and the European Working Group on Sarcopenia [5]. Due to the absence of muscle echography for assessing muscle mass, we used data on muscle mass percentage derived from bioelectrical impedance analysis. Sarcopenia was defined by the presence of both reduced muscle strength (SPPB chair test > 15 s) and mass (< 32.9% in men and < 23.9% in women) [48].
Sarcopenic obesity was defined according to the ESPEN and EASO Consensus Statement [13] as altered skeletal muscle strength (i.e. SPPB chair test > 15 s), reduced muscle mass (< 32.9% in men and < 23.9% in women) and increased fat mass % (≥ 30% in men and ≥ 42% in women) [48].
Individuals with a normal muscle mass and strength were considered robust. People with impaired muscle mass but normal muscle strength configured the group of people with reduced muscle mass but normal muscle strength.
All study participants were further classified as obese and non-obese according to the percentage of fat mass measured though the bioelectrical impedance balance [48]. Thus, we finally had eight body composition classes: robust non-obese, robust obese, pre-sarcopenic non-obese, pre-sarcopenic obese, sarcopenic non-obese, sarcopenic obese, individuals with reduced muscle mass and normal muscle strength obese and non-obese.
Frailty was assessed through a 49-items FI created by using the criteria defined by Searl et al. [51].
The 49 variables used to compute the FI were also retrieved from the multidimensional geriatric evaluations (Table S1). Each deficit included in the FI was scored 0 when absent, and 1 when present. In cases of missing data, the FI was calculated by using an adequately reduced denominator excluding the items for whom data were missing [51]. Participants having more than 20% of missing variables were excluded from the computation of the FI [51]. The score of the FI ranges from 0 to 1, with lower levels identifying fitter individuals. A cut-off point of ≥ 0.25 defines ‘frail’ individuals [51].
The data were collected and reported in an electronic case report form (eCRF) created using FileMaker Pro v. 11.
Statistical analyses
Descriptive statistics were used to show the baseline characteristics of the study population. Continuous variables were presented as mean and standard deviations (SD), when normally distributed, or with median and interquartile range (IQR), when data had a skewed distribution. Dichotomous variables were presented as number (N) and percentage (%). Comparison of the characteristics among obese and non-obese sarcopenic, obese and non-obese pre-sarcopenic, obese and non-obese robust and obese and non-obese participants with a reduced muscle mass but a normal muscle strength was performed with the U Mann Whitney test for continuous variables and with the Chi Squared test for categorical variables.
Linear regression analyses were used to assess the association between frailty and different body composition classes. Analyses were also adjusted for age and sex.
Classification and regression tree (CRT) modelling was used to assess the predictors of frailty. Only predictors not included in the computation of the FI were considered: age, gender, civil status, BMI, percentage of fat mass, visceral fat, muscle mass, muscle strength (assessed though the SPPB chair test) and waist circumference.
All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
Among the participants enrolled in the FRASNET study 1114 were included in the present analysis. Only 10 institutionalized patients, 91 individuals who missed more than 20% of variables for the computation of the FI and 26 participants who missed data on body composition were excluded from the present analysis.
The study sample had a median age of 72 years and was composed for the 39.5% by males; 234 individuals (21%) were frail according to the FI with a median score of 0.11 (IQR 0.07–0.20). A FI higher score indicates greater vulnerability to stressors for the individual. Health deficits include comorbidities, symptoms, disabilities, or altered physical or mental signs.
The study population main characteristics are illustrated in Table 1. Robust non-obese individuals accounted for the 38.2% of the study sample. Figure 1 illustrates the body composition categories of the study population. Table 2A and 2B show body composition, muscle function and frailty in the different body composition categories. Obese individuals had a lower muscle mass and a higher FI score compared to non-obese participants (Table 2A and 2B). In addition, among robust people and people with reduced muscle mass and normal muscle strength, obese individuals displayed also a lower gait speed (Table 2A and 2B).
Body composition categories in the study population. Robust no obesity: 425 individuals (38.2%), robust with obesity 28 individuals (2.5%), reduced muscle mass normal strength no obesity 138 individuals (12.4%), reduced muscle mass normal strength with obesity 195 individuals (17.5%), presarcopenia no obesity 173 individuals (15.5%), presarcopenia with obesity 18 individuals (1.6%), sarcopenia no obesity 51 individuals (4.6%), sarcopenia with obesity 86 individuals (7.7%)
In the age and sex adjusted regression analyses being robust and non-obese (B – 0.42, 95% C.I. -0.06 - -0.03, p < 0.001) or being with a reduced muscle mass, a normal muscle strength and non-obese (B – 0.03, 95% C.I. -0.05 - -0.006, p = 0.011) were associated with a reduced probability of being frail. Instead, being pre-sarcopenic, either non-obese (B 0.03, 95% C.I, 0.007–0.044, p < 0.001) or obese (B 0.11, 95% C.I. 0.05–0.16, p < 0.001) or being sarcopenic obese (B 0.074, 95% C.I. 0.05–0.1, p < 0.001) were associated with an increased probability of being frail.
The regression tree model showed that fat mass % predicted frailty in people ≤ 70 years, whereas muscle strength predicted frailty in people between 70 and 81 years (Fig. 2).
Discussion
In this observational study we found that SO and pre-sarcopenia (either with and without obesity) were associated with frailty measured with the FI whereas being non obese (either robust or with a reduced muscle mass but a normal muscle strength) had a negative association with frailty. Fat mass percentage predicted frailty in people aged 65–70 years while muscle strength predicted frailty in people aged 70–81 years.
Previous studies have demonstrated an association between SO and physical frailty in the Asian [30, 31] and Brazilian populations [32] and a link between BMI and general frailty [40]. Indeed, obesity and frailty share common pathophysiologic mechanisms like inflammation and oxidative stress [52]. Muscle ectopic fat deposition alters mitochondrial function increasing reactive oxygen species production which in turn interfere with muscle protein function and contribute to the manifestation of frailty.
In this work we clarified how different classes of body composition, characterized in term of fat and muscle mass percentage, were associated with general frailty. Moreover, we identified fat mass percentage and muscle function as potential predictors of frailty. Our data on the identification of fat mass thresholds associated with general frailty in subjects aged 65–70 years highlights the importance of screening for general frailty in this demographic with elevated fat mass. Identification of subjects at risk could facilitate the implementation of early preventive interventions, such as weight loss, protein supplementation, and physical activity before actual frailty appears. These measures may mitigate obesity and prevent frailty and sarcopenic obesity before the onset of disability and other adverse consequences. Moreover, our study demonstrates that in individuals aged 70–81 years, the decline in muscle strength plays a significant role in predicting general frailty, thus adding to the available information that might be used to identify subjects in which the intervention could be useful.
Although frailty is the subject of intense and always growing attention from the scientific community several issues remains open, and in particular there is no accepted consensus on the methods that should be used for assessing frailty [53]. Here we add to the commonly used approach to define frailty a better insight on the added value of comprehensively examining body composition through measurements of both general and visceral fat, as well as evaluations of muscle mass and performance. We intentionally evaluated general frailty by constructing a FI that did not initially incorporate measures of body composition as health deficits. Our discovery of a significant association between this index and body fat, muscle mass, and performance reinforces the theory of interdependence among health deficits [54]. According to this theory, the presence of a deficit in one organ or system increases the likelihood of deficits in other related organs or systems. Muscle weakness and excess fat, although not explicitly included in our FI, can indeed be considered as health deficits.
Our findings lay the groundwork for future prospective studies, which could employ more precise methods—such as muscle echography capable of assessing regional muscle masses affected early by sarcopenia—to evaluate whether interventions aimed at improving body composition and muscle function could decrease the incidence of frailty.
However, some limitations of our work warrant mention. Firstly, the regional nature of the study conducted in an urban area may restrict the generalizability of our results. Secondly, the cross-sectional design prevented us from assessing the impact of body composition on frailty and other health outcomes over time. Additionally, the lack of evaluation of local muscle masses through muscle echography is noteworthy, as this could have enabled the early detection of sarcopenia in muscle districts prone to early disease onset including in particular the anterior thigh muscles [55].
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SO:
-
Sarcopenic obesity
- FI:
-
Frailty index
- SHARE:
-
Survey of health, ageing and retirement in Europe
- MMSE:
-
Mini-mental state examination
- GDS:
-
Geriatric depression scale
- FSS:
-
Fatigue severity scale
- SF-36:
-
Short form 36
- PASE:
-
Physical activity scale for elderly
- SPPB:
-
Short physical performance battery
- EWGSOP2:
-
European working group on sarcopenia in older people 2
- IQR:
-
InterQuartile range
- CRT:
-
Classification and regression tree
References
Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, et al. Aging and Imaging Assessment of body composition: from Fat to facts. Front Endocrinol (Lausanne). 2020;10:861. https://doi.org/10.3389/fendo.2019.00861.
Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120(9 Suppl 1):S10–6. discussion S16-7.
Koster A, Patel KV, Visser M, Van Eijk JTM, Kanaya AM, De Rekeneire N, et al. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56:636–43. https://doi.org/10.1111/j.1532-5415.2007.01632.x.
Evans WJ, Guralnik J, Cawthon P, Appleby J, Landi F, Clarke L, et al. Sarcopenia: no consensus, no diagnostic criteria, and no approved indication-how did we get here? Geroscience. 2024;46(1):183–90.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Cruz-Jentoft A, Sayer AA, Sarcopenia. Lancet. 2019;393(10191):2636–46.
Xia L, Zhao R, Wan Q, Wu Y, Whou Y, Wang Y, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;9(21):7964–78.
Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;28:6.
Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Berabei R, et al. Sarcopenia as the Biological substrate of physical Frailty. Clin Geriatr Med. 2015;31(3):367–74.
Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W et al. FOD-CC group. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62 – 7.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Sci Med Sci. 2001;56(3):M146–56.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Biol Sci Med Sci. 2007;62(7):722–7.
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35. https://doi.org/10.1159/000521241.
Hong SH, Choi KM. Sarcopenic Obesity, insulin resistance, and their implications in Cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21(2):494.
Bunout D, Barrera G, Hirsch S, Jimenez T, de la Maza MP. Association between activity energy expenditure and peak oxygen consumption with Sarcopenia. BMC Geriatr. 2018;18:298.
Li C-W, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of Sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781–94.
Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–85.
Kloock S, Ziegler CG, Dischinger U. Obesity and its comorbidities, current treatment options and future perspectives: challenging bariatric surgery? Pharmacol Ther. 2023;251:108549.
Cooper AJM, Lamb MJE, Simmons RK, Griffin SJ. Bidirectional association between physical activity and muscular strength in older adults: results from the UK biobank study. Int J Epidemiol. 2017;46(1):141–8.
Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013;1281(1):141–59.
Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–71.
Guillet C, Masgrau A, Walrand S, Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev. 2012;13(Suppl 2):51e7.
Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904(1):437–48.
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723e7.
Villareal DT. Obesity in older adults – a growing problem. In: Watkins Bales C, Seel Ritchie C, editors. Nutrition and health: handbook of clinical nutrition and aging. 2nd ed. New York: Humana; 2009. p. 263e77.
Peng TC, Chen WL, Chen YY, Chao YP, Wu LW, Kao TW. Associations between different measurements of sarcopenic obesity and health outcomes among non-frail community dwelling older adults in Taiwan. Br J Nutr. 2021;126(11):1749–57.
Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28. https://doi.org/10.1093/ageing/afab039.
Atkins JL, Wannamathee SG. Sarcopenic obesity in ageing: cardiovascular outcomes and mortality. Br J Nutr. 2020;124(10):1102–13.
Du Y, Wang X, Xie H, Zheng S, Wu X, Zhu X, et al. Sex differences in the prevalence and adverse outcomes of Sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr Disord. 2019;19(1):109.
Yang M, Hu M, Zhang Y, Jia S, Sun X, Zhao W, et al. Sarcopenic obesity is associated with frailty among community–dwelling older adults: findings from the WCHAT study. BMC Geriatr. 2022;22(1):863.
Heng MWY, Chan AWD, Man REK, Fenwick EK, Chew STH, Tay L, et al. Individual and combined associations of Sarcopenia, osteoporosis and obesity with frailty in a multi–ethnic Asian older adult population. BMC Geriatr. 2023;23(1):802.
Frisoli A, Duque G, Paes AT, Diniz AR, Lima E, Azevedo E, et al. Sarcopenic obesity definitions and their associations with physical frailty in older Brazilian adults: data from the SARCOS study. Arch Endocrinol Metab. 2023;67(3):361–71.
Szlejf C, Parra-Rodríguez L, Rosas-Carrasco O. Osteosarcopenic obesity: prevalence and relation with frailty and physical performance in middle aged and older women. J Am Med Dir Assoc. 2017;18(8):733e1-733.e5.
Rolland Y, Lauwers-Cances V, Cristini C, van Kan GA, Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, Sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS. Am J Clin Nutr. 2009;89(6):1895–900.
Hawkins KL, Zhang L, Ng DK, Althoff KN, Palella FJ, Lawrence AK, et al. Abdominal obesity, Sarcopenia, and osteoporosis are strongly Associated with Frailty in the MACS. AIDS. 2018;32(10):1257–66.
Crow RS, Lohman MC, Titus AJ, Cook SB, Bruce ML, Mackenzie TA, et al. Association of Obesity and Frailty in older adults: NHANES 1999–2004. J Nutr Health Aging. 2019;23(2):138–44.
Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese Elderly men and women. Obes Res. 2004;12(6):913–20.
Uchai S, Andersen LF, Johansson J, Hopstock LA, Hjartåker A, Dual-Energy X-R. Absorptiometry Derived Adiposity measures and Pre-Frailty/Frailty among Norwegian adults: the Tromsø Study 2007–2015. J Nutr Health Aging. 2023;27(6):403–10.
São Romão Preto L, Dias Conceição MDC, Figueiredo TM, Pereira Mata MA, Barreira Preto PM. Mateo Aguilar E. Frailty, body composition and nutritional status in non-institutionalised elderly. Enferm Clin. 2017;27(6):339–45.
Jayanama K, Theou O, Godin J, Mayo A, Cahill L, Rockwood K. Relationship of body mass index with frailty and all–cause mortality among middle–aged and older adults. BMC Med. 2022;20(1):404.
Kara M, Kaymak B, Frontera W, Merve Ata A, Ricci V, Ekiz T, et al. Diagnosing Sarcopenia: functional perspectives and a new algorithm from the ISarcoPRM. J Rehabil Med. 2021;53:jrm00209.
Delli Zotti GB, Citterio L, Farinone S, Concas MP, Brioni E, Zagato L, et al. Association between Perceived Health-Related Quality of Life and Depression with Frailty in the FRASNET Study. Int J Environ Res Public Health. 2022;19(24):16776. https://doi.org/10.3390/ijerph192416776.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Burke WJ, Roccaforte WH, Wengel SP. The short form of the geriatric Depression Scale: a ComparisonWith the 30-Item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–8.
Krupp LB, La Rocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–3.
Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–36.
Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the Elderly (PASE). J Clin Epidemiol. 1993;46(2):153–62. https://doi.org/10.1016/0895-4356(93)90053-4.
Omron. Full body sensor, body composition monitor and scale. Model HBF-510. Instruction manual. https://omronhealthcare.com/wp-content/uploads/hbf-510w-instruction-manual.pdf.
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61.
Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Health, Aging and Body Composition Study added value of physical performance measures in predicting adverse health related events: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):251–9.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–77.
Deng Y, Sato N. Global frailty screening tools: review and application of frailty screening tools from 2001 to 2023. Intractable Rare Dis Res. 2024;13(1):1–11.
Mitnitsk AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. https://doi.org/10.1186/1471-2318-2-1.
Kara M, Kaymak B, Ata AM, Özkal Ö, Kara Ö, Baki A, et al. STAR-Sonographic Thigh Adjustment ratio: a Golden Formula for the diagnosis of Sarcopenia. Am J Phys Med Rehabil. 2020;99(10):902–8.
Acknowledgements
We acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. We also acknowledge the nurses of our outpatient clinic Barbara Banti and Rosalba Scrimieri.
Funding
This work was funded by the Fondazione CARIPLO, Milano grant to P.M. [grant number. 2016 − 0980].
Author information
Authors and Affiliations
Contributions
SD, PM, AM, CL, PRQ designed the study; EB, CM, MPR, SS, ES, MM collected data; SD, CL, LC, PRQ, AM analyzed and interpreted the data; SD, CL, LC drafted the manuscript; CL, RDL, MS, LC, CL, LZ collated and explained the data. SD, MS, CL carries out quality control. PM, AM, MS, RDL, MS. LZ, LC, GV contributed to the data interpretation and analysis. All the authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the San Raffaele Scientific Institute Ethical Board (24/INT/2017). Participants signed a written informed consent before taking part in the study. The enrolment was performed between the 1st April 2017 and the 31st July 2019. The FRASNET study has been registered in ClinicalTrial.gov on November 13, 2020, ID: NCT04630132.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Damanti, S., Citterio, L., Zagato, L. et al. Sarcopenic obesity and pre-sarcopenia contribute to frailty in community-dwelling Italian older people: data from the FRASNET study. BMC Geriatr 24, 638 (2024). https://doi.org/10.1186/s12877-024-05216-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05216-6