Abstract
Purpose
Multimorbidity and polypharmacy in older adults converts the detection and adequacy of potentially inappropriate drug prescriptions (PIDP) in a healthcare priority. The objectives of this study are to describe the clinical decisions taken after the identification of PIDP by clinical pharmacists, using STOPP/START criteria, and to evaluate the degree of accomplishment of these decisions.
Methods
Multicenter, prospective, non-comparative cohort study in patients aged 65 and older, hospitalized because of an exacerbation of their chronic conditions. Each possible PIDP was manually identified by the clinical pharmacist at admission and an initial decision was taken by a multidisciplinary clinical committee. At discharge, criteria were re-applied and final decisions recorded.
Results
From all patients (n = 674), 493 (73.1%) presented at least one STOPP criteria at admission, significantly reduced up to 258 (38.3%) at discharge. A similar trend was observed for START criteria (36.7% vs. 15.7%). Regarding the top 10 most prevalent STOPP criteria, the clinical committee initially agreed to withdraw 257 (34.2%) prescriptions and to modify 93 (12.4%) prescriptions. However, the evaluation of final clinical decisions revealed that 503 (67.0%) of those STOPP criteria were ultimately amended. For the top 10 START criteria associated PIDP, the committee decided to initiate 149 (51.7%) prescriptions, while a total of 198 (68.8%) were finally introduced at discharge.
Conclusions
The clinical committee, through a pharmacotherapy review, succeeded in identifying and reducing the degree of prescription inadequacy, for both STOPP and START criteria, in older patients with high degree of multimorbidity and polypharmacy.
Trial Registration
NCT02830425.
Similar content being viewed by others
Introduction
Progressive and constant population aging is a global phenomenon that constitutes a health challenge to societies and healthcare providers [1, 2]. Nowadays, 21.2% of people in the European Union are older than 65 years and it is predicted to exceed 30% by 2050 [3]. In parallel to this process, the multimorbidity and polypharmacy of older patients is growing, thus requiring increasing funding and human resources from public healthcare systems. In consequence, this high level of polypharmacy leads to the risk of presenting potentially inappropriate drug prescriptions (PIDP) and suffering drug-related problems [4].
PIDP have been significantly related to a variety of health-related outcomes, such as adverse drug event-related hospital admissions, functional decline and adverse drug reactions (ADR) [5]. Moreover, it is known that PIDP and ADR may exacerbate frailty features in older people, such as cognitive decline, falls or incontinence, leading to a bi-directional relationship that can result in an increased polypharmacy and higher risk of PIDP [6]. Some studies have even shown that PIDP are associated with greater risk of mortality [7, 8]. Therefore, it is essential to identify and reduce both PIDP and polypharmacy in order to optimize prescriptions in older patients. In fact, polypharmacy results in higher mortality rates in particular groups of patients, such as cognitively impaired older adults [9].
With this purpose, several explicit criteria have emerged as useful tools to identify PIDP [10]. In Europe, STOPP/START (Screening Tool of Older Person’s potentially inappropriate Prescriptions / Screening Tool to Alert doctors to Right Treatment) are the most widely used and validated criteria among older adults [11]. Its first version included 84 criteria [12] and was further enlarged in a second version including up to 114 explicit, incorporating three implicit criteria [13, 14]. These criteria were elaborated to support multidisciplinary teams in charge of multimorbid patients in the adequacy of medication associated to PIDP potentially resulting in ADR, together with the introduction of unprescribed required medication.
The application of STOPP/START criteria in several clinical settings has demonstrated its effectiveness in many different countries. In fact, these criteria have been employed to optimize medication in older patients in distinct clinical contexts, such as hospitals, primary care, nursing homes and intermediate care centers [15,16,17]. The use of these criteria has led to the identification of potentially inappropriate medication (PIM) and potential prescribing omissions (PPO), in direct correlation with worse health outcomes, such as emergency room visits, hospital re-admissions and mortality [18, 19].
A successful implementation of STOPP/START criteria requires a multidisciplinary team composed by physicians, nurses and clinical pharmacists, with experience in the management of geriatric patients [20]. Actually, the relevance of incorporating pharmacists in those teams has proved to be beneficial in hospital and nursing home settings, given their key role in PIM and PPO detection [21]. Moreover, medication review led by pharmacists has shown high levels of patient satisfaction towards deprescribing interventions [22]. Finally, it has been recently reported that randomized clinical trials where medication review process is led by a pharmacist, result in a reduction of polypharmacy, along with a positive impact in terms of preventing undesired hospitalizations and saving public health costs [23].
Therefore, the objectives of this study are to describe the clinical decisions taken after the identification of PIMs and PPOs by clinical pharmacists at admission of older patients, according to STOPP/START criteria and to evaluate the degree of accomplishment of these decisions at patient’s discharge, considering the evolution of patient's clinical condition. These analyses are included in MoPIM (Morbidity, Potentially Inappropriate Medication) study [24], with diverse goals related to multimorbidity, risk factors of PIDP and ADR in these patients.
Materials and methods
Design of the study
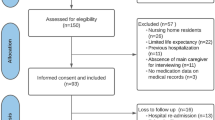
A multicenter and prospective non-comparative cohort study including older patients hospitalized at the internal medicine or geriatric services at five general teaching hospitals in three different regions of Spain between September 2016 and December 2018 was conducted. The detailed protocol was previously published [24].
For the purposes of this study, older patients (≥ 65 years old) admitted because of an exacerbation of their chronic pathology were included. Patients referred to home hospitalization, admitted because of an acute process not related to their chronic pathology, or with a fatal outcome expected at admission were not included. No written informed consent was deemed necessary for this study, according to the independent ethics committee.
Data acquisition and variables
The following sociodemographic and clinical data were retrieved from the electronic health records by the clinical team responsible for the patient: patient’s code, date of birth, sex, functional status just before admission (Barthel Index), household (alone, with relatives or other people or in a nursing home), length of stay and destination at discharge from the present episode of hospitalization (home, transfer to another hospital, transfer to a nursing home). Chronic active conditions were recorded from a consensual list of 64 conditions containing all chronic diseases of the Charlson Comorbidity Index and including some risk factors as well. This index was calculated, adjusted by age and categorized by tertiles (2–5, 6–8, 9–14). Geriatric syndromes and risk factors were also recorded from a list of 15 (see published protocol [24]).
The number of chronic medications in the electronic prescription and the STOPP/START criteria [13] detected at the time of admission, with the active principle involved, were manually collected by the clinical pharmacist of each team. The second version of STOPP/START was employed and consists of 80 STOPP criteria (which detect medication that would not meet criteria for indication to a patient or a specific clinical situation or medications prescribed included drug-drug and drug-disease interactions) and 34 START criteria (which detect medication that would be recommended to incorporate, including some vaccines) [13]. The criteria are directed to prevalent diseases in older patients, are ordered by physiological systems and are easy to relate to active diagnoses.
Clinical committee review process
A clinical committee constituted by an internal medicine or geriatrics physician, a clinical pharmacist and a nurse monitored each patient until hospital discharge (or death during hospitalization), with data collection during the initial days of admission to hospital ward and at the time of discharge. The medication review process was part of the usual patient care routine in all participating centers. Medication was only considered chronic if prescribed at least 3 months before admission, and creams, ointments, healing materials and over-the-counter medicines were not considered. Active principles were considered individually when registering STOPP/START criteria, regardless of the administered drug combinations.
For each patient, possible PIDPs were recorded at admission and, following the usual practice, the clinical committee evaluated the PIDP together with the possible actions to be taken for medication adequacy according to the STOPP/START criteria. After the committee review process, an initial clinical decision was taken for each PIDP detected. The initial decisions for PIM were classified in medication withdrawal, modification of dosage or administration frequency or medication maintenance (either with or without medical justification at admission). In the case of PPO, the initial decisions considered were modification (referring to initiation of a required medication) or maintenance of prescription omission (either with or without medical justification at admission).
For the purposes of the study, the criteria were manually re-applied by the clinical pharmacist to the prescribed medication at discharge, once acute exacerbation of clinical condition was resolved, in this case without evaluation by the clinical committee. After this process, each PIDP detected was recorded as “amended” or “maintained”, considered as final clinical decision. PIM were located to the “amended” group when medication was finally withdrawn or modified, whereas PPO were assigned to this group in case of treatment initiation before discharge.
Sampling and analysis
All STOPP/START criteria were assessed, except for START criteria I (vaccines) due to difficulties of some centers in accessing this information. Regarding the implicit criterion STOPP A1 and given its high frequency, it was divided into the following categories according to the active principles involved: proton pump inhibitors (PPI), hypolipidemic, analgesics, acetylsalicylic acid, antihypertensives and others [25].
Data from patients initially included in the study, who died during hospitalization, were not considered for the analysis.
Binary variables were created to describe the presence of any STOPP and any START criteria at admission and at discharge and numerical variables were similarly created for the number of STOPP and number of START at admission and at discharge. For each STOPP and START criteria detected, a percentage of change between the number of patients with those criteria at admission and discharge was calculated: (N discharge – N admission) / N admission *100.
Descriptive analyses were performed for all variables. Bivariate analyses were performed to compare the presence of any STOPP at admission versus discharge (using the McNemar's Chi-squared test with continuity correction) and to compare the number of STOPP at admission versus discharge (using Wilcoxon rank sum test with continuity correction). The same analyses were performed for START criteria.
Sankey diagrams were generated with the top 10 most frequent PIM and PPO detected, illustrating the different flows from the number of PIDP detected at admission, to the initial clinical decision, and then the final decision and the amount of PIDP at discharge.
All analyses were performed with R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sociodemographic and clinical characteristics of the cohort
A total of 674 patients were finally included in the analysis. Median age of patients was 84.1 years (SD ± 7.0), with 52.8% of females and a mean Barthel Index of 67. According to this index, 36.6% of patients presented a dependency degree from moderate to total. In terms of household, only 12.6% lived at nursing homes before admission, while this percentage increased up to 15.6% after discharge. Almost 70% of patients were admitted for less than two weeks at the hospital setting. All remaining sociodemographic and clinical variables are summarized in Supplementary Table S1. Chronic active conditions and geriatric syndromes registered in this cohort of patients are shown in Supplementary Tables S2 and S3.
STOPP/START criteria detection in patients’ cohort at admission and at discharge
The analysis of total prescriptions across all hospitals involved in the study revealed an average of 10.6 medications/patient at admission. This ratio was slightly increased up to 11.1 at patients’ discharge, demonstrating the high degree of polypharmacy in the studied population. From all patients, 493 (73.1%) presented at least one STOPP criteria at admission, with an average of 1.6 per patient. These numbers were significantly reduced at discharge up to 258 (38.3%) and 0.6, respectively (p values < 0.001 for both analysis), after the review process by the clinical committee and according to patients’ clinical evolution. A similar trend was observed for START criteria. Initially, in 247 (36.7%) patients one or more START criteria were detected while, after hospitalization, this percentage was significantly reduced to 15.7% (106 patients, p value < 0.001). The degree of reduction of STOPP criteria was similar among all participating centers, while START criteria were only substantially reduced in three out of five hospitals. These data are reflected in Table 1, along with the distribution of patients recorded in each of the hospitals participating in the study.
As shown in Supplementary Figure S1A, only 26.9% of patients were admitted without any STOPP criteria detected by the clinical committee. Oppositely, 43.6% of patients presented two or more PIM at the initial revision. After hospitalization, 61.7% of patients were discharged without STOPP criteria, a significant decrease compared to admission time point, particularly in those cases with two or more STOPP criteria, which only comprised the 14.3% of patients. A similar trend was observed for START criteria (Supplementary Figure S1B), although a smaller improvement was achieved, with an increase from 63.3% to 84.3% in the proportion of patients without any START criteria.
Description of PIM assessed by the clinical committee during patients’ hospitalization
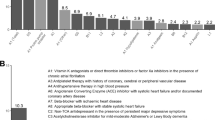
In total, 1077 PIM were registered by the clinical committee at patients’ admission, whereas only 394 PIM were detected at discharge, indicating a global reduction of 63.4%. For a more detailed description of PIM detected at admission and at discharge, a complete distribution of all STOPP criteria identified can be found at Supplementary Table S4. As a summary, the top 20 STOPP criteria with the highest percentage of detection at admission are reported in Table 2. Remarkably, three of the criteria accounted for a total of 44.1% of the items registered: D5 (benzodiazepines (BZD) administration for more than 4 weeks), 20.7%; A1 (prescription of any drug without evidence-based clinical indication, restricted to acid reducers), 12.8%; and K1 (prescription of BZD that increases risk of falls), 10.6%.
In terms of PIM detection at discharge, all criteria included in Table 2 were clearly reduced by the intervention of the clinical committee, ranging from 17.4% to 97.6% of decrease. Interestingly, criteria A1 applied to acid reducers, despite being the second mostly registered, resulted in the lowest percentage of reduction, with only 17.4%. On the opposite side, criteria B11 (ACE inhibitors or AR blockers in patients with hyperkalemia), B8 (thiazide diuretics with current significant hypokalemia, hyponatremia, hypercalcemia or gout history) and A1 restricted to hypolipidemic drugs reached more than 90% of reduction (Table 2).
The top 10 STOPP criteria detected at admission were subjected to an evolution analysis during patients’ complete hospitalization. Out of a total of 751 prescriptions associated to these STOPP criteria, the clinical committee initially decided to withdraw 257 (34.2%) prescriptions and to modify 93 (12.4%) prescriptions. Contrarily, 401 (53.4%) prescriptions were maintained, 58.6% of them with clinical justification (Fig. 1). Later, the evaluation of the final clinical decisions revealed that 503 (67.0%) STOPP criteria were amended (i.e., the inappropriateness criteria were resolved either by drug withdrawal or posology adequacy). Instead, 248 (33.0%) STOPP criteria remained without modifications at patients’ discharge. Among these unmodified prescriptions, A1 restricted to acid reducers and D5 criteria prevailed as the most predominant, constituting the 46.0% and 33.1% of the prescriptions, respectively (Fig. 1).
Decisions taken at the clinical committee review process on PIM evolve during patients’ hospitalization. Sankey diagram representing the distribution of clinical decisions on the top 10 most frequent PIM detected at admission. Number and percentage of prescriptions withdrawn, modified or maintained (with or without justification) derived from the initial clinical decisions are shown in the second column, while final clinical decisions are depicted in the third column as amended or maintained. Colors associated to each STOPP criteria are indicated in the legend
Description of PPO Assessed by the Clinical Committee during Patients’ Hospitalization
Regarding the analysis and further evaluation of PPO in our cohort, a total of 330 prescriptions with START criteria were reported at admission. According to the reduction of PIM mentioned before, PPO were also diminished in a large percentage at patients’ discharge. In this case, a 55.2% of reduction was achieved, registering only 148 PPO for the total of discharged patients. An extensive description of PPO detected at admission and at discharge, together with the full distribution of associated START criteria can be found in Supplementary Table S5. Additionally, the 20 criteria with a higher prevalence are summarized in Table 3. From this list, four criteria reached a percentage above 10%: E5 (vitamin D supplement in older people who are housebound or experiencing falls with osteopenia), 22.4%; H2 (laxative in patients receiving opioids regularly), 13.0%; A8 (appropriate beta-blocker with stable systolic heart failure), 11.2%; and A6 (ACE inhibitors with systolic heart failure and/or documented coronary artery disease), 10.6%.
The analysis of PPO at discharge revealed that, differentially from PIM data, not all START criteria were reduced. However, we reduced the proportion of inappropriateness in 17 out of 20 items. We found that the START criteria with a higher percentage of improvement do not correspond to any of the most frequent ones. In this sense, we should emphasize that criteria C2 (non-TCA antidepressant drug in the presence of persistent major depressive symptoms) and A4 (antihypertensive therapy in high blood pressure) reached drastic reduction rates of 92.9% and 87.5%, respectively (Table 3).
Again, we performed an evolution analysis during hospitalization of the top 10 START criteria detected at admission. In this case, the clinical committee agreed to initiate 149 (51.7%) prescriptions associated to any START criteria, while 121 (42.0%) PPO registered at admission were not modified at the initial decision. Most of these decisions (70 out of 121, 57.9%) were considered as not justified during the initial days of admission (Fig. 2). When assessing final clinical decisions, the proportion of START criteria amended slightly increased up to 198 (68.8%) prescriptions, whereas in 90 (31.2%) PPO the initial decision prevailed. Interestingly, the criteria accounting for the higher number of unmodified decisions were H2 (26, 28.9%) and E5 (18, 20.0%) (Fig. 2).
Decisions taken at the clinical committee review process on PPO evolve during patients’ hospitalization. Sankey diagram representing the distribution of clinical decisions on the top 10 most frequent PPO detected at admission. Number and percentage of prescriptions modified or maintained (with or without justification) derived from the initial clinical decisions are shown in the second column, while final clinical decisions are depicted in the third column as amended or maintained. In 18 START criteria the initial clinical decision was not registered. Colors associated to each START criteria are indicated in the legend
Discussion
Main results
The large cohort of patients included in this study presents a high incidence of PIDP according to STOPP/START criteria, as it could be expected from the characteristics of the population analysed, mainly because of their comorbidities and polypharmacy. Data collected from the clinical committee review process in the five hospitals participating in the MoPIM study, demonstrate that PIDP detection, review and follow-up for each patient by a multidisciplinary team has a strong benefit in terms of reducing prescription inadequacy in our cohort.
Comparison of our results with other studies
The optimization of medical prescription in older patients, with the final goal of diminishing PIDP incidence, is one of the main concerns among clinical teams. In fact, several studies retrospectively comparing the incidence of PIDP between patients’ admission and discharge have been reported in the last years [26,27,28,29,30]. Nevertheless, they were performed in a retrospective manner and provide contradictory results. Some publications indicate that the lack of clinical intervention during hospitalization causes an increase in the total number of PIDP. For instance, Perpetuo et al. reported that, at admission, 49.4% of patients presented at least one STOPP criteria and this value augmented up to 62.2% at discharge [26]. Similar results were published by Abukhalil et al., reporting an increase of 8.1% between admission and discharge, in a younger patients’ cohort with lower degree of polypharmacy and comorbidity Charlson Index compared to our cohort [27]. Instead, other studies show a surprising drastic reduction of PIDP, even without mentioning a clinical review process during patients’ stay [29, 30]. Therefore, we would like to stress the importance of performing prospective studies with well-defined criteria and methodology to analyze the evolution of PIDP during patients’ hospitalization.
On the other hand, there are few studies describing how the intervention of multidisciplinary teams (including physicians, pharmacists and nurses) improves prescription adequacy during hospitalization in older patients’ cohorts. Importantly, the MoPIM prospective study includes a large cohort of patients, with more than 670 individuals comprising a two-year period from five different centers within the same country. In this sense, few prospective reports aiming to analyze geriatric team interventions have been published [31,32,33]. In these studies, the average age of patients included is above 80 years old, with most females. Therefore, although STOPP/START criteria are conceptually developed to be applied in ≥ 65-year-old patients, the actual cohorts to analyze are much older, reflecting the high percentage of octogenarians in Western society.
Unfortunately, these prospective studies do not share the same methodology with MoPIM study and account for smaller cohorts of around 100–200 patients. Specifically, Hanou et al. published data on a cohort with a wide majority of psychogeriatric patients, whereas version 1 of STOPP/START criteria was employed [12, 31]. Moreover, Farhat et al. divided their cohort in two subgroups, in order to compare STOPP/START with PIM-Check criteria [32, 34]. Finally, study by Magallón-Martínez et al. analyzed pharmaceutical interventions based not only in STOPP/START criteria, but also in Beers, Priscus and LESS-CHRON criteria [10, 33, 35]. In consequence, no solid conclusions can be extracted from the extrapolation of our data with these previous reports.
In MoPIM study, each clinical review committee counts with the intervention of a clinical pharmacist, who identifies all PIDP associated to patients’ medication and promotes the reduction of inappropriate prescriptions. Remarkably, previous reports have shown that including pharmacists in multidisciplinary geriatric teams provides more benefits than isolated pharmacists’ interventions [20]. Moreover, a recent report has demonstrated that the intervention of a clinical pharmacist can improve medical prescriptions by diminishing the total PIDP incidence in a cohort of older patients admitted for acute hip fracture [36]. In a small intervention cohort of 59 patients, Leguillon et al. obtained a reduction around 65% of total PIDP between admission and discharge. This effect clearly improves the comparison of the control group that, without the involvement of a clinical pharmacist, achieves a very slight prescription adequacy in terms of PIDP incidence [36].
In our cohort, an average of 2.1 STOPP/START criteria per patient is detected at admission. These data are similar to previous studies, despite being performed in a cohort of psychogeriatric patients or in a medium-stay unit [15, 31, 37]. Oppositely, our results show a lower identification of PIDP/patient when compared to a cohort of individuals admitted to a geriatric perioperative care unit [36]. Remarkably, data from Farhat et al. reveal a lower detection of PIDP based on STOPP/START criteria, with only 0.8 PIDP/patient at admission [32]. However, the use of PIM-Check criteria in this same study results in an average of 2.3 PIDP/patient, indicating that the identification of PIDP can vary depending on the criteria used. An example of this inconsistency among different criteria is found in the publication from Grina et al., where the use of EU-PIM and Beers criteria identified less PIDP compared to STOPP/START [37].
In our cohort, the PIM analysis demonstrated a high incidence of D5 and K1, both of them STOPP criteria related to an inappropriate use of BZD. In fact, these items ranked first and third among the top 20 most frequently identified. In this direction, several studies have reported that the inadequate prescription of BZD, according to STOPP criteria, are found among the most frequent PIM [31, 32, 38]. However, other studies did not find use of BZD as any of the most detected PIM [26], probably due to the struggles in determining prescription adequacy retrospectively. Interestingly, through our clinical committee review process, we have been able to reduce the prescription of BZD between 63 and 75%, depending on the criteria, in the internal medicine and the geriatric services. In contrast, other studies exclusively involving patients admitted in a psychogeriatrics unit resulted in no reduction of BZD use, indicating the difficulties to deprescribe these drugs in this specific hospital unit [31].
The inappropriate use of PPI was encountered as the second most common STOPP criteria in this study, in parallel to previous data that reported a high incidence of incorrect PPI prescription [39, 40]. As it is widely known, an elevated use of PPI can result in adverse effects such as bone fractures, hypomagnesemia, C. difficile infections, dementia, respiratory infections and community-acquired pneumonia [25, 41]. With the aim of preventing all these side effects, it is essential to carefully review PPI prescriptions and deprescribe them in case of inappropriate indication [42].
Clinical interpretation of results
We observed that STOPP criteria with the highest levels of adequacy in MoPIM study were those associated to the onset of adverse effects, mostly affecting the cardiovascular system. For instance, hyperkalemia linked to inadequate prescription of ACE inhibitors or AR blockers (B11 criteria) or the use of thiazide diuretics with current hypokalemia, hyponatremia, hypercalcaemia, or gout history (B8 criteria) reached 97.6% and 94.4% of deprescription, respectively, when the withdrawal of these drugs was suggested. Additionally, unnecessary prescribed lipid-lowering drugs were withdrawn in more than 90% of cases (STOPP criteria A1), given that the benefit-risk balance of statin use as prevention of cardiovascular disease is controversial in older patients [43]. Contrarily, most of prescriptions initiated upon PPO detection comprise the use of drugs with immediate benefits in the treatment of common pathologies in older patients, such as major depression, hypertension, osteopenia, chronic atrial fibrillation and heart failure. Therefore, according to our data, physicians are more prone to deprescribe drugs when patients’ safety is already challenged by a specific adverse effect, as well as to initiate drug prescriptions that improve patients’ quality of life and prognosis.
Impact and barriers of the clinical committee review process
The MoPIM study was not only designed to detect PIDP at patient’s admission and discharge, but also to evaluate how the degree of inappropriately prescribed drugs evolved during patients’ hospitalization. In this sense, the clinical decisions from the reviewing committee resulted in a deprescription or posology adequacy in 67.0% of PIM at patients’ discharge, although only 46.6% recommendations had been accepted after the initial decision. Regarding PPO, the initial decision suggested to start omitted prescriptions in 51.7% of cases, while this ratio increased up to 68.8% of acceptance at discharge. Therefore, these data indicate that chronic patients evolve during hospital stay and, in most cases, the adequacy of prescriptions may be easier at discharge, rather than at admission, when they have just experienced an acute exacerbation of their conditions. Apart from patients’ own changes in clinical evolution, it has been demonstrated that the inclusion of a pharmacist in a clinical team improves the accomplishment of prescription adequacy criteria [44].
However, reaching a full acceptance of the agreements taken by the clinical review committee in terms of deprescription is still utopic, due to several barriers interfering in this process. As revised by Peat et al., those barriers are related to the organization of healthcare settings (consultation constraints or perceived hierarchies), to communication transparency (sharing decisions and information), as well as to patients’ habits and concerns (fears of negative consequences of deprescribing) [45]. In the particular case of BZD, communication between patients and physicians is essential to prevent reluctance to its deprescription [46], which is difficult to achieve during hospital admission.
The clinical committee review, with the guidance of STOPP/START explicit criteria, has promoted the identification and decrease of PIDP, which have been also linked to an increased risk of hospital readmission [47]. Specifically, in RESORT study, PIDP related to the inappropriate use of central nervous system drugs or to an elevated fall risk were found to be significantly associated with 30-day hospital readmission [47]. In our study, we have been able to largely reduce the inadequate prescriptions related to D5 and K1 criteria, referring to the use of BZD and its correlation with fall risk in older people. Additionally, the improvement in detection and amendment of PPO can be related to an enhancement in patient’s independence in daily instrumental activities, despite lack of association with clinical outcomes [47]. In fact, a recent Cochrane systematic review of 25 trials and more than 15,000 patients, with a vast majority of older adults with polypharmacy, determined that medication review reduces hospital readmission and may prevent emergency room contacts [48].
PIDP and polypharmacy
In the last decade, polypharmacy has emerged as one of the major concerns among clinical teams in care of older adults [49]. In our cohort, patients present an average of 10.6 prescribed drugs at admission, higher than other comparable studies [31, 32]. However, despite patients are admitted due to an exacerbation or decompensation of their chronic pathologies, the total number of prescriptions is maintained at discharge (with an average of 11.0 drugs/patient). In consequence, polypharmacy is not decreased, whereas prescription adequacy is improved through the reduction of PIDP, emphasizing the importance of the clinical committee review process.
In fact, the increase of polypharmacy in older patients has demonstrated to be a risk factor for more hospitalizations and emergency room visits [50]. In consequence, it is necessary to consider deprescription in order to prevent inappropriate polypharmacy. However, these actions will probably require a shift in prescribing culture [49]. Our data indicate that this process may be successfully led by a clinical pharmacist, as demonstrated in a recent meta-analysis, reporting that pharmaceutical interventions significantly reduce the incidence of PIM in older patients, along with polypharmacy and 30-day readmission rate [51].
Strengths and limitations of the study
The multicenter approach of the protocol design is, undoubtedly, one of the most valuable strengths, particularly for the use of the second version of STOPP/START criteria, released just a few months before initiating the study [13]. Remarkably, it embraces different regions across Spain, only including hospitals where clinical pharmacists have a complete integration in multidisciplinary teams, together with geriatricians and internal medicine physicians [24]. As thoroughly described across this section, we would like to highlight the prospective design of the protocol, which has allowed a more accurate and close monitoring of the decisions taken on pharmacotherapy adequacy during patients’ hospitalization. Moreover, during the clinical committee review process, newly prescribed medication of patients due to the acute condition has been considered, along with their chronic therapy, thus reflecting previous patients’ management at primary care settings. Finally, we analyze how the acceptance of PIDP adequacy recommendations varies between admission of patients and discharge, reflecting the importance of a complete supervision of PIDP during the whole hospital stay.
Nevertheless, the design of this study displays some limitations, such as a potential variability in STOPP/START criteria application among different centers. This bias may be caused by the personal point of view of each professional involved or the available tools for medication review. A further bias in the study can exist due to the exclusion of patients who died during hospital stay. Importantly, none of the patients’ death was directly linked to a particular drug inadequacy. Given that this study was conceived as a non-comparative study, the data presented have not been compared to a control group. Moreover, the specific clinical reasons for maintaining some PIM or PPO detected at discharge were not registered. In addition, the protocol does not include further patients’ follow-up after discharge, which would be really useful to analyze whether PIDP adequacy is maintained during subsequent months, and how our interventions are translated into clinical relevance, such as affecting patients’ morbidity and mortality.
Of note, an update of the STOPP/START criteria (version 3) has been recently published [52], but it was not available at the time this study was designed and executed. In this new version, the number of items has been enlarged up to 190 criteria, 67% more than in previous version. The higher number of criteria will probably improve the detection of PIDP in a more accurate manner since they consider the availability of several new medications for acute and chronic treatments, although it will difficult the review process by clinical teams, converting the assistance of informatics tools in indispensable.
Conclusions
The study corroborates an elevated incidence of polypharmacy and PIDP in our patients’ cohort, especially PIM associated to the inappropriate use of BZD and acid reducers. Importantly, the clinical committee review process succeeded in identifying and reducing the degree of prescription inadequacy, for both STOPP and START criteria. Moreover, we have observed that the acceptance of clinical pharmacist recommendations increases at patients’ discharge, compared to the initial clinical decisions upon patients’ admission. Globally, pharmacotherapy review through the application of explicit STOPP/START criteria improve prescription adequacy in older patients with high degree of multimorbidity and polypharmacy.
Availability of data and materials
The data presented in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7371151.
Abbreviations
- ADR:
-
Adverse drug reaction
- MoPIM:
-
Morbidity, potentially inappropriate medication
- PIDP:
-
Potentially inappropriate drug prescription
- PIM:
-
Potentially inappropriate medication
- PPO:
-
Potential prescribing omission
References
Khezrian M, McNeil CJ, Murray AD, Myint PK. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther Adv Drug Saf. 2020;11:2042098620933741. https://doi.org/10.1177/2042098620933741.
Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med. 2021;12:443–52. https://doi.org/10.1007/s41999-021-00479-3.
(2023) Eurostat - Data Explorer. In: Available: https://appsso.eurostat.ec. europa.eu/nui/show.do?dataset=tps00200&lang=en
Petrovic M, O’Mahony D, Cherubini A (2022) Inappropriate prescribing: hazards and solutions. Age Ageing 51:. https://doi.org/10.1093/ageing/afab269
Mekonnen AB, Redley B, de Courten B, Manias E. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:4150–72. https://doi.org/10.1111/bcp.14870.
Randles MA, O’Mahony D, Gallagher PF. Frailty and Potentially Inappropriate Prescribing in Older People with Polypharmacy: A Bi-Directional Relationship? Drugs Aging. 2022;39:597–606. https://doi.org/10.1007/s40266-022-00952-z.
Chang TI, Park H, Kim DW, et al. Polypharmacy, hospitalization, and mortality risk: a nationwide cohort study. Sci Rep. 2020;10:18964. https://doi.org/10.1038/s41598-020-75888-8.
Davies LE, Kingston A, Todd A, Hanratty B. Is polypharmacy associated with mortality in the very old: Findings from the Newcastle 85+ Study. Br J Clin Pharmacol. 2022;88:2988–95. https://doi.org/10.1111/bcp.15211.
Porter B, Arthur A, Savva GM. How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study BMJ Open. 2019;9: e026171. https://doi.org/10.1136/bmjopen-2018-026171.
Motter FR, Fritzen JS, Hilmer SN, et al. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol. 2018;74:679–700. https://doi.org/10.1007/s00228-018-2446-0.
Gallagher P, Baeyens J-P, Topinkova E, et al. Inter-rater reliability of STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment) criteria amongst physicians in six European countries. Age Ageing. 2009;38:603–6. https://doi.org/10.1093/ageing/afp058.
Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. https://doi.org/10.5414/cpp46072.
O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–8. https://doi.org/10.1093/ageing/afu145.
Delgado Silveira E, Montero Errasquín B, Muñoz García M, et al (2015) [Improving drug prescribing in the elderly: a new edition of STOPP/START criteria]. Rev Esp Geriatr Gerontol 50:89–96. https://doi.org/10.1016/j.regg.2014.10.005
Sevilla-Sánchez D, Espaulella-Panicot J, de Andrés-Lazaro AM, et al. Potentially inappropriate medication on admission to a medium-stay unit according to STOPP and START criteria. Rev Esp Geriatr Gerontol. 2012;47:155–7. https://doi.org/10.1016/j.regg.2012.02.013.
Lopez-Rodriguez JA, Rogero-Blanco E, Aza-Pascual-Salcedo M, et al (2020) Potentially inappropriate prescriptions according to explicit and implicit criteria in patients with multimorbidity and polypharmacy. MULTIPAP: A cross-sectional study. PLoS One 15:e0237186. https://doi.org/10.1371/journal.pone.0237186
Díaz Planelles I, Navarro-Tapia E, García-Algar Ó, Andreu-Fernández V (2023) Prevalence of Potentially Inappropriate Prescriptions According to the New STOPP/START Criteria in Nursing Homes: A Systematic Review. Healthcare (Basel) 11:. https://doi.org/10.3390/healthcare11030422
Moriarty F, Bennett K, Cahir C, et al. Potentially inappropriate prescribing according to STOPP and START and adverse outcomes in community-dwelling older people: a prospective cohort study. Br J Clin Pharmacol. 2016;82:849–57. https://doi.org/10.1111/bcp.12995.
Wauters M, Elseviers M, Vaes B, et al. Too many, too few, or too unsafe? Impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community-dwelling oldest old. Br J Clin Pharmacol. 2016;82:1382–92. https://doi.org/10.1111/bcp.13055.
Delgado-Silveira E, Vélez-Díaz-Pallarés M, Muñoz-García M, et al. Effects of hospital pharmacist interventions on health outcomes in older polymedicated inpatients: a scoping review. Eur Geriatr Med. 2021;12:509–44. https://doi.org/10.1007/s41999-021-00487-3.
Balsom C, Pittman N, King R, Kelly D. Impact of a pharmacist-administered deprescribing intervention on nursing home residents: a randomized controlled trial. Int J Clin Pharm. 2020;42:1153–67. https://doi.org/10.1007/s11096-020-01073-6.
Bou Malham C, El Khatib S, Cestac P, et al (2021) Impact of pharmacist‐led interventions on patient care in ambulatory care settings: A systematic review. Int J Clin Pract 75:. https://doi.org/10.1111/ijcp.14864
Omuya H, Nickel C, Wilson P, Chewning B. A systematic review of randomised-controlled trials on deprescribing outcomes in older adults with polypharmacy. Int J Pharm Pract. 2023;31:349–68. https://doi.org/10.1093/ijpp/riad025.
Baré M, Herranz S, Jordana R, et al. Multimorbidity patterns in chronic older patients, potentially inappropriate prescribing and adverse drug reactions: protocol of the multicentre prospective cohort study MoPIM. BMJ Open. 2020;10: e033322. https://doi.org/10.1136/bmjopen-2019-033322.
Baré M, Lleal M, Ortonobes S, et al. Factors associated to potentially inappropriate prescribing in older patients according to STOPP/START criteria: MoPIM multicentre cohort study. BMC Geriatr. 2022;22:44. https://doi.org/10.1186/s12877-021-02715-8.
Perpétuo C, Plácido AI, Aperta J, et al. Potentially Inappropriate Medication at Admission and at Discharge: A Geriatric Study in an Internal Medicine Service in Portugal. Int J Environ Res Public Health. 2023;20:4955. https://doi.org/10.3390/ijerph20064955.
Abukhalil AD, Al-Imam S, Yaghmour M, et al. Evaluating Inappropriate Medication Prescribing Among Elderly Patients in Palestine Using the STOPP/ START Criteria. Clin Interv Aging. 2022;17:1433–44. https://doi.org/10.2147/CIA.S382221.
Frankenthal D, Lerman Y, Lerman Y. The impact of hospitalization on potentially inappropriate prescribing in an acute medical geriatric division. Int J Clin Pharm. 2015;37:60–7. https://doi.org/10.1007/s11096-014-0040-9.
de Agustín SL, Rodríguez Salazar J, Jiménez-Muñoz AB, et al. Potentially inappropriate medication in acute hospitalized elderly patients with polypharmacy: an observational study comparing PRISCUS, STOPP, and Beers criteria. Eur J Clin Pharmacol. 2021;77:757–66. https://doi.org/10.1007/s00228-020-03022-8.
Gómez-Cortijo R, Flotats-Dam P, Iparraguirre-Azcona MK, et al. Adecuación de la prescripción farmacológica durante el ingreso en un centro sociosanitario: experiencia clínica con los criterios STOPP/START. J Healthc Qual Res. 2020;35:95–101. https://doi.org/10.1016/j.jhqr.2020.02.001.
Hannou S, Voirol P, Pannatier A, et al. Pharmacist intervention acceptance for the reduction of potentially inappropriate drug prescribing in acute psychiatry. Int J Clin Pharm. 2017;39:1228–36. https://doi.org/10.1007/s11096-017-0513-8.
Farhat A, Al-Hajje A, Lang P-O, Csajka C. Impact of Pharmaceutical Interventions with STOPP/START and PIM-Check in Older Hospitalized Patients: A Randomized Controlled Trial. Drugs Aging. 2022;39:899–910. https://doi.org/10.1007/s40266-022-00974-7.
Magallón Martínez A, Pinilla Rello A, Casajús Lagranja P, et al. Pharmaceutical care for the patients admitted to a multidisciplinary complex chronic patient unit. Farm Hosp. 2023;47:106–12. https://doi.org/10.1016/j.farma.2023.01.004.
Desnoyer A, Blanc A-L, Pourcher V, et al. PIM-Check: development of an international prescription-screening checklist designed by a Delphi method for internal medicine patients. BMJ Open. 2017;7: e016070. https://doi.org/10.1136/bmjopen-2017-016070.
Rodríguez-Pérez A, Alfaro-Lara ER, Albiñana-Perez S, et al. el tool for deprescribing in chronic patients with multimorbidity: List of Evidence-Based Deprescribing for Chronic Patients criteria. Geriatr Gerontol Int. 2017;17:2200–7. https://doi.org/10.1111/ggi.13062.
Léguillon R, Varin R, Pressat-Laffouilhère T, et al. Clinical Pharmacist Intervention Reduces Potentially Inappropriate Prescriptions in a Geriatric Perioperative Care Unit Dedicated to Hip Fracture. Gerontology. 2023;69:386–95. https://doi.org/10.1159/000526595.
Grina D, Karpavičiūtė J, Minkutė R, Briedis V. Impact of hospitalization on potentially inappropriate prescribing: a cross-sectional study in an acute geriatric hospital in Lithuania. Int J Clin Pharm. 2020;42:903–10. https://doi.org/10.1007/s11096-020-01035-y.
Monteiro C, Canário C, Ribeiro MÂ, et al. <p>Medication Evaluation in Portuguese Elderly Patients According to Beers, STOPP/START Criteria and EU(7)-PIM List – An Exploratory Study</p>. Patient Prefer Adherence. 2020;14:795–802. https://doi.org/10.2147/PPA.S247013.
Sevilla-Sánchez D, Molist-Brunet N, Amblàs-Novellas J, et al. Potentially inappropriate medication at hospital admission in patients with palliative care needs. Int J Clin Pharm. 2017;39:1018–30. https://doi.org/10.1007/s11096-017-0518-3.
Bo M, Gibello M, Brunetti E, et al. Prevalence and predictors of inappropriate prescribing according to the Screening Tool of Older People’s Prescriptions and Screening Tool to Alert to Right Treatment version 2 criteria in older patients discharged from geriatric and internal medicine ward. Geriatr Gerontol Int. 2019;19:5–11. https://doi.org/10.1111/ggi.13542.
Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc. 2018;93:240–6. https://doi.org/10.1016/j.mayocp.2017.10.022.
Shanika LGT, Reynolds A, Pattison S, Braund R. Proton pump inhibitor use: systematic review of global trends and practices. Eur J Clin Pharmacol. 2023. https://doi.org/10.1007/s00228-023-03534-z.
Ploeg MA, Floriani C, Achterberg WP, et al. Recommendations for (Discontinuation of) Statin Treatment in Older Adults: Review of Guidelines. J Am Geriatr Soc. 2020;68:417–25. https://doi.org/10.1111/jgs.16219.
Delgado-Silveira E, Albiñana-Pérez MS, Muñoz-García M, et al. Pharmacist comprehensive review of treatment compared with STOPP-START criteria to detect potentially inappropriate prescription in older complex patients. Eur J Hosp Pharm. 2018;25:16–20. https://doi.org/10.1136/ejhpharm-2016-001054.
Peat G, Fylan B, Marques I, et al. Barriers and facilitators of successful deprescribing as described by older patients living with frailty, their informal carers and clinicians: a qualitative interview study. BMJ Open. 2022;12: e054279. https://doi.org/10.1136/bmjopen-2021-054279.
Rasmussen AF, Poulsen SS, Oldenburg LIK, Vermehren C. The Barriers and Facilitators of Different Stakeholders When Deprescribing Benzodiazepine Receptor Agonists in Older Patients—A Systematic Review. Metabolites. 2021;11:254. https://doi.org/10.3390/metabo11040254.
Mekonnen AB, Reijnierse EM, Soh CH, et al. Associations between potentially inappropriate prescribing and increased number of medications with post-discharge health outcomes among geriatric rehabilitation inpatients: RESORT study. Br J Clin Pharmacol. 2023. https://doi.org/10.1111/bcp.15838.
Bülow C, Clausen SS, Lundh A, Christensen M (2023) Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2023:. https://doi.org/10.1002/14651858.CD008986.pub4
Payne RA. Polypharmacy and deprescribing Medicine. 2020;48:468–71. https://doi.org/10.1016/j.mpmed.2020.04.003.
Gutiérrez-Valencia M, Izquierdo M, Malafarina V, et al. Impact of hospitalization in an acute geriatric unit on polypharmacy and potentially inappropriate prescriptions: A retrospective study. Geriatr Gerontol Int. 2017;17:2354–60. https://doi.org/10.1111/ggi.13073.
Zhou S, Li R, Zhang X, et al (2023) The effects of pharmaceutical interventions on potentially inappropriate medications in older patients: a systematic review and meta-analysis. Front Public Health 11:. https://doi.org/10.3389/fpubh.2023.1154048
O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023. https://doi.org/10.1007/s41999-023-00777-y.
Acknowledgements
The authors acknowledge the dedication and support of the entire MoPIM ressearch group, listed by institution: Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA): Marisa Baré (Institutional Committee for the Improvement of Clinical Practice Adequacy. Clinical Epidemiology and Cancer Screening; REDISSEC and RICAPPS; CRiSP research group), Susana Herranz (Acute care Geriatric Unit; REDISSEC), Rosa Jordana (Department of Internal Medicine), Maria Queralt Gorgas (Pharmacy Department, REDISSEC), Sara Ortonobes (Pharmacy Department), Marina Lleal (Institutional Committee for the Improvement of Clinical Practice Adequacy. Clinical Epidemiology and Cancer Screening), Celia Corral-Vazquez (I3PT; REDISSEC);University Hospital of Vic: Pere Roura-Poch (Epidemiology Unit; REDISSEC), Daniel Sevilla, Núria Solà and Javier González (Pharmacy Department), Núria Molist, Mariona Espaulella (Department of GeriatricsC3RG research group); Oscar Mascaró (Department of Internal Medicine);Hospital del Mar Medical Research Institute-IMIM: Elisabet de Jaime (Geriatrics Department), Olivia Ferrandez (Pharmacy Department), Maria Sala (Department of Epidemiology and Evaluation; REDISSEC), Miguel Ángel Márquez, Marta Arellano, Carlos Clemente and Olga Sabartés (Department of Geriatrics), Núria Carballo and Marta de Antonio (Pharmacy Department);Hospital de Galdakao: Rafael Estrada (Department of Internal Medicine), Maria Olatz Ibarra (Pharmacy Department);Complejo Hospitalario Universitario de Canarias: Candelaria Martin (Department of Internal Medicine), Gloria Julia Nazco (Pharmacy Department), Rubén Hernández (Department of Internal Medicine).
Funding
This work was supported by grants from Instituto de Salud Carlos III, Ministry of Science and Innovation (Spain)—FEDER (PI15/00552), and by the Network for Research into Healthcare in Chronic Diseases, REDISSEC (RD16/0001/0002). These funding bodies had no role in the design of the study, nor in the collection, analysis and interpretation of data nor in writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization, M.B.; Methodology, S.O., S.H., M.L. and M.B.; Validation, M.L. and M.B.; Formal Analysis and Data Curation, M.L.; Investigation, S.O., S.H., D.S.-S., R.J., O.M., O.F., E.d.J., R.E., G.J.N. and MoPIM study group; Writing – Original Draft Preparation, S.O.; Writing – Review and Editing, M.L. and M.B.; Supervision and Project Coordination, M.B.; Funding Acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of each centre: Comité Ético de investigación Clínica del Parc Taulí [ID: 20166570], Comitè Ètic d’Investigació Clínica Osona per a la Recerca i Educació Sanitàries (FORES) [ID: 2016922-PR153], Comité de Ètica de la Investigación con Medicamentos (CEIm)-Parc de Salut MAR [ID: 2016/6830/I], Comité Ético de Investigación Clínica de Euskadi [ID: PI2016060] and Comité de Ética de Investigación del Hospital Universitario de Canarias [ID: MBM-MOD-2016–01 (2016–56)]. Written patient consent was waived by all Ethics Committees mentioned before, considering they were often very old patients, in an acute process with intellectual impairment or delirium, sometimes living alone or in a nursing home, and that it would have been difficult to explain and make sure they understood. In this observational study, we considered important to include patients who were representative of all complex clinical conditions and to elude a possible selection bias (specifically frequent in older patients) that would have invalidated the main results. On the other hand, data about chronic medications, PIDP, and the intention to modify the treatment (data no included in this analyses), during the first days related to possible PIDP, had better be gathered at the beginning of the hospitalization period and could not be delayed beyond.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ortonobes, S., Herranz, S., Lleal, M. et al. Multidisciplinary medication review during older patient hospitalization according to STOPP/START criteria reduces potentially inappropriate prescriptions: MoPIM cohort study. BMC Geriatr 24, 584 (2024). https://doi.org/10.1186/s12877-024-05185-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05185-w