Abstract
Background
Older adults with type 2 diabetes are at higher risk of developing common geriatric syndromes and have a lower quality of life. To prevent type 2 diabetes in older adults, it’s unclear whether the health benefits of physical activity (PA) will be influenced by the harms caused by increased exposure to air pollution during PA, especially in developing countries with severe air pollution problem. We aimed to investigate the joint effects of PA and long-term exposure to air pollution on the type 2 diabetes in older adults from China.
Methods
This cross-sectional study was based on the China Multi-Ethnic cohort (CMEC) study. The metabolic equivalent of PA was calculated according to the PA scale during the CMEC baseline survey. High resolution air pollution datasets (PM10, PM2.5 and PM1) were collected from open products. The joint effects were assessed by the marginal structural mean model with generalized propensity score.
Results
A total of 36,562 participants aged 50 to 79 years were included in the study. The prevalence of type 2 diabetes was 10.88%. The mean (SD) level of PA was 24.93 (18.60) MET-h/d, and the mean (SD) level of PM10, PM2.5, and PM1 were 70.00 (23.32) µg/m3, 40.45 (15.66) µg/m3 and 27.62 (6.51) µg/m3, respectively. With PM10 < 92 µg/m3, PM2.5 < 61 µg/m3, and PM1 < 36 µg/m3, the benefit effects of PA on type 2 diabetes was significantly greater than the harms due to PMs when PA levels were roughly below 80 MET-h/d. With PM10 ≥ 92 µg/m3, PM2.5 ≥ 61 µg/m3, and PM1 ≥ 36 µg/m3, the odds ratio (OR) first decreased and then rose rapidly with confidence intervals progressively greater than 1 and break-even points close to or even below 40 MET-h/d.
Conclusions
Our findings implied that for the prevention of type 2 diabetes in older adults, the PA health benefits outweighed the harms of air pollution except in extreme air pollution situations, and suggested that when the air quality of residence is severe, the PA levels should ideally not exceed 40 MET-h/d.
Similar content being viewed by others
Introduction
Diabetes is a metabolic disorder caused by environmental and genetic factors and has been considered as one of the major contributors to the global burden of disease [1, 2]. Nearly 80% of people with diabetes, mainly type 2, now live in low- and middle-income countries [3, 4], and population aging have contributed to the shift of the diabetes epidemic to the elderly [5, 6]. Old adults with diabetes have a higher risk of common geriatric syndromes, such as cognitive impairment and disability, which have an important impact on quality of life [7].
Research has consistently demonstrated that the adoption of physical activity (PA) can prevent diabetes [8,9,10], especially among older adults [11, 12]. Long-term exposure to air pollution has been a critical risk factor for the development of diabetes [13,14,15], and older people would have a higher risk than younger ones [16, 17]. Given that PA increases the ventilation rate, the intake of air pollution may also increase. However, for older adults, the trade-off between the health benefits of PA and the harmful effects caused by increased exposure to air pollution during PA remains unclear [18].
Some studies have revealed the joint effects of PA and air pollution on cardiovascular disease, lung function/respiratory disease and mortality [19,20,21,22,23,24,25,26,27,28], but evidence on the joint effects of long-term exposure to air pollution and PA on type 2 diabetes is scarce [28,29,30]. Besides, no such evidence existed in developing countries, which faced much more serious air pollution problems [31]. As the largest developing country in the world, China has widespread and severe levels of air pollution, with 48 cities feature among the top 100 most polluted cities, and previous studies conducted with good air quality could not be extrapolated to such a high-exposure air pollution settings [27, 32].
We aimed to investigate the joint effects of PA and long-term exposure to air pollution on type 2 diabetes in older adults exposed to heavy air pollution from China, where air pollution and the prevalence of type 2 diabetes both pose a grave public health concern [4, 33].
Methods
Study design and participants
This cross-sectional study was based on the China Multi-Ethnic cohort (CMEC), documented in detail elsewhere [34]. In brief, the baseline for the CMEC study was established between May 2018 and September 2019 in five provinces in southwest China, which sampled 99,556 participants aged 30 to 79 years. Electronic questionnaires, physical examinations and clinical laboratory tests were mainly applied to collect participants’ baseline information, such as demographic and socioeconomic information, health behaviours, disease history, and biological samples.
The participant selection procedure is shown in the Data Supplement (Fig S1). Tibetans in Aba and Lhasa live above 3000 m above sea level. High altitude has been documented to be inversely associated with diabetes due to adaptation to environments and genetic changes [35, 36]. Besides, Tibetans in Aba were herdsmen whose residence changed with the seasons. Thus, to make the study population more comparable and obtain accurate and stable exposure estimates, Tibetan residents in Aba and Lhasa were not included in this study (n = 4993 for Aba; n = 7780 for Lasa). We then excluded 9372 participants who had changed their place of residence within three years prior to the baseline survey, and excluded 2739 participants diagnosed with type 2 diabetes before exposure assessment. We further excluded 672 participants who self-reported having cancers, mainly lung, oesophageal, stomach, liver, prostate and cervical cancers, as well as 2775 pregnant women and 161 participants self-reported having tuberculosis. We then selected adults over 50 years of age and excluded 1237 participants due to incomplete information on air pollution exposure, PA, health outcomes and other covariates. A total of 36,562 adults aged 50 to 79 years were included in this study.
Assessment of air pollution exposure
The high-resolution (1 km) and high-quality PM10 (particulate matter with an aerodynamic diameter of 10 µm or less), PM2.5 (particulate matter with an aerodynamic diameter of 2.5 µm or less) and PM1 (particulate matter with an aerodynamic diameter of 1 µm or less) dataset were collected from open products, which were estimated using a newly developed space–time extremely randomized trees (STET) model based on the newly released MODIS Collection 6 MAIAC 1-km AOD products, meteorological variables, pollution emissions, land cover, surface topographic data and population data [37,38,39,40]. The STET model performed well, with an across-validation coefficient of determination of 0.86, 0.90, and 0.77 for PM10, PM2.5 and PM1, respectively.
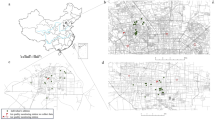
We assigned the estimated annual PM10, PM2.5 and PM1 concentrations to each participant based on their geocoded residential address and calculated the 3-year average exposure concentrations before the baseline survey. Figure 1 showed the distribution of PM10, PM2.5 and PM1 concentrations by participants’ address locations.
Assessment of physical activity
The information of PA for each participant was collected through the questionnaire during the baseline survey. Both the PA intensity and duration one year preceding the survey were obtained. The PA intensity was presented by the corresponding metabolic equivalent values (MET). This study assigned different MET to various physical activities [41,42,43], and the product of PA intensity (MET) and duration (hours) was calculated as the volume of activity (MET-h/d). Measures of PA were calculated corresponding to four domains, namely, leisure, work, transportation and housework. The sum of activity in each domain were the total volume of PA for each participant.
Health outcome measurements
The diabetes status of the participants was defined by the following criteria: (1) self-reporting of taking any antidiabetic medication (both insulin and oral antidiabetic drugs) or (2) fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L), or (3) HbA1c ≥ 6.5% (48 mmol/mol). Those criteria were based on the recommendations of the American Diabetes Association [44].
Covariates
In this study, we included the following covariates in the main analysis: age (year), sex (male or female), marital status (married, widowed, divorced and unmarried), education (illiteracy, primary school degree, junior school degree, senior high school degree, bachelor’s degree or more), annual household income, body mass index (low, normal, overweight and obese), smoking status (never, quit, smoking), passive smoking status (yes or no), alternative Mediterranean diet (aMED) score [45], self-reported hypertension (yes or no), sedentary time, and indoor pollution situation. Indoor pollution was defined as low if participants did not have a kitchen at home or rarely cooked, medium if they frequently cooked using gas/electricity or using coal/wood as fuel with chimney at home, and high if they frequently cooked using coal/wood as fuel but without a chimney at home [46].
Statistical analysis
Basic theory of the bi-dimensional GPS
Causal inference methods are gradually being used in environmental epidemiology because of better control of confounding, and avoiding ethical issues [47, 48]. Among various causal inference methods, propensity score methods have been popular for its advantage of separating the design and analysis process, which is similar to randomized clinical trials [49]. Based on the propensity score, the inverse probability of treatment weighting (IPTW) method has been widely used due to its ease of operation and unbiased estimation [50]. The IPTW method currently focuses on categorical or single continuous exposure variables, while research on two continuous variables is lacking.
In this study, let \(T\) and \(V\) denote PA and air pollution, respectively, and \(X\) denote covariates measured in the baseline survey described above. Based on the weak unconfoundedness assumption and the generalised propensity score (GPS) proposed by Hirano and Imbens [51], we extended the weak unconfoundedness assumption, namely \(Y\left(t,v\right)\perp (T,V)|X\), and developed a bi-dimensional GPS by as follows:
Let \(r\left(t,v,x\right)\) denote the conditional joint density of the two continuous exposure variables given the covariates. Similar to the GPS [51], the bi-dimensional GPS also has a balancing property as follows:
The above balancing property implies that given the bi-dimensional GPS, covariates \(X\) are balanced across different joint exposure groups and will not interfere with the estimation of the association between exposure and outcome variables. This is a mechanical implication of the definition of the bi-dimensional GPS, and does not require the extended weak unconfoundedness assumption. Combined with the extended weak unconfoundedness, this implies that assignment to treatment is unconfounded given the bi-dimensional GPS (proof in supplementary S1):
Design stage
The overall workflow of the IPTW method was presented in supplementary (Fig S2), and the estimation of bi-dimensional GPS was the first step. Due to a lack of research about the application of IPTW on two continuous variables, we proposed to construct a bi-dimensional GPS by using the multivariate normal model:
\({X}_{1},{X}_{2},\dots ,{X}_{p}\) were the relevant covariates mentioned above, and the subscript \(p\) represented the number of covariates parameters. A weighted pseudo population was created by the bi-dimensional GPS. The evaluation of covariate balance in the pseudo population is a crucial step in the causal inference framework, which indicates the quality of the causal inference approach at recovering randomized experiments and informs the degree to which we can make a valid causal assessment. The balance was measured through the absolute correlation (AC) between the continuous exposure variables and the covariates. The AC with values < 0.1 indicates a high quality in recovering randomized experiments [52].
Outcome analysis stage
A marginal structural mean model [53] was constructed to assess the joint effects of PA and long-term exposure to air pollution (PM10, PM2.5 and PM1) on type 2 diabetes, which combined the bi-dimensional GPS and the generalized additive model [54]. The model was specified as:
The variable \(Te\) defined the full tensor product smooth between PA and long-term exposure to air pollution, and the right-hand part of the second equal was a functional ANOVA decomposition with the smooth main effects (\(Ti\left(t\right)+Ti\left(v\right)\)) and smooth interaction term (\(Ti\left(t,v\right)\)). F-statistic was used for the test of the smooth interaction term [54].
To present the results clearly without using 3D plots, we investigated the exposure–response relationship between PA and type 2 diabetes at different concentrations of PMs. Participants exposed to the lowest PMs and lowest PA levels were the reference group. The choice of different PMs concentrations was based on its distribution and the WHO recommendations. Specifically, the nine levels of PM10 were 40 50, 65, 70, 88, 92, 104, 107, 120 µg/m3; the nine levels of PM2.5 were 20, 25, 35, 48, 52, 54, 61, 65, 70 µg/m3; and the nine levels of PM1 were 18, 21, 23, 26, 32, 33, 36, 38 and 42 µg/m3.
Sensitivity analyses were performed to assess the robustness of joint effects: (1) the above main analyses were repeated after excluding subjects taking any antidiabetic medication because their intentional lifestyle changes may produce estimate bias, such as doing more exercise to prevent exacerbation of the disease; (2) the 2-year average exposure of air pollution and 4-year average exposure of air pollution were employed to evaluate the possible impact of different exposure windows.
All statistical analysis were performed in R software, version 3.4.0.
Results
Descriptive results
A total of 36,562 participants were included in the study, and the prevalence of type 2 diabetes was 10.88%. Table 1 shows the characteristics of all participants, the participants without diabetes, and the participants with type 2 diabetes. For all participants, the average age was 60.54 (7.50) years old, and 58.9% were females. Compared to participants without type 2 diabetes, those with type 2 diabetes had lower levels of PA (MET-h/d) (22.65 vs. 25.21), and higher levels of long-term exposure to pollutants at their residence.
The exposure ranges of PM10, PM2.5 and PM1 were indeed wide, ranging from 33.26 µg/m3 to 165.19 µg/m3, 18.24 µg/m3 to 105.29 µg/m3 and 15.49 µg/m3 to 53.57 µg/m3, respectively (Fig. 1). Table S1 showed that the participants with higher PA tended to have lower prevalence of type 2 diabetes and be exposed to lower levels of PM10, PM2.5 and PM1.
Balance check results
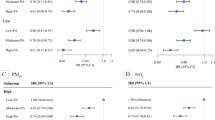
As shown in Fig. 2, we found that compared with the unweighted study population, the covariates balance became better in the weighted pseudo-population, with values of ACs less than 0.1.
The balance results of covariates in the weighted (blue), and original observational population (red). The three subplots showed the ACs between covariates and PA and the corresponding pollutants before and after weighting with the bi-dimensional GPS model constructed for PA and PM10, PA and PM2.5, and PA and PM1, respectively. Covariates were age, sex, marital status, education, annual household income, BMI, smoking, aMED score, sedentary time, etc., as detailed in the Methods
The joint associations of PA and PMs on type 2 diabetes
There was a statistically significant interaction effect of PA and PMs on type 2 diabetes. The p-values for the smooth interaction term between PM10, PM2.5, and PM1 with PA in the generalised additive model were 4.73e-05, 3.00e-03, and 1.41e-07, respectively.
With PM10 < 92 µg/m3, PM2.5 < 61 µg/m3, and PM1 < 36 µg/m3, the exposure–response relationship between PA and type 2 diabetes showed that the OR first decreased, then remained stable and finally increased with increasing PA (Fig. 3). Overall, as the air pollution level increased, the break-even points, where the harmful effects from air pollution started to outweigh the benefits of physical activity, were roughly close to 80 MET-h/d. Specifically, the break-even points were 87 MET-h/d and 84 MET-h/d when PM10 was 88 µg/m3 and PM2.5 was 54 µg/m3, respectively.
The exposure–response relationship between PA and type 2 diabetes at different exposure levels of air pollution for older adults. The nine values in subplots A, B and C represented the different pollution concentrations (µg/m3) of PM10, PM2.5 and PM1 respectively. The OR limit is set to 5, and the grey shaded area indicated the confidence interval (95% CI). The 95% CI not containing the value of 1, represented by the horizontal dashed line, indicated that the association is statistically significant. Covariates mentioned in the Methods section were integrated by the bi-dimensional GPS, which was combined in the outcome model
With high levels of air pollution exposure, i.e. PM10 ≥ 92 µg/m3, PM2.5 ≥ 61 µg/m3, and PM1 ≥ 36 µg/m3, the exposure–response relationship showed that the OR first decreased and then rose rapidly from below 1 to above 1 with confidence intervals progressively greater than 1 (Fig. 3). The break-even points became smaller compared to the situation above with lower levels of air pollution exposure, being roughly closed to or even below 40 MET-h/d as the air pollution level increased. Specifically, the break-even points were 24 MET-h/d, 40 MET-h/d and 61 MET-h/d when PM10 was 120 µg/m3, PM2.5 was 70 µg/m3, and PM1 was 42 µg/m3, respectively.
Furthermore, the results of sensitivity analysis were similar to those of the primary analysis, showing that with high levels of air pollution exposure, the health benefits of PA were more significantly influenced by the exposure to PMs than with lower levels of air pollution exposure. (Figs. S3, S4 and S5).
Discussion
Main findings
To our knowledge, this is the first study to investigate the joint effects of air pollution and physical activity on type 2 diabetes in older adults exposed to widely varying levels of air pollution exposure. Based on the pattern shown in the results, this study indicated that with PM10 < 92 µg/m3, PM2.5 < 61 µg/m3, and PM1 < 36 µg/m3, the benefit effects of PA on type 2 diabetes was greater than the harmful effects due to PMs especially when PA levels were roughly blow 80 MET-h/d. However, with extreme high levels of PMs (PM10 ≥ 92 µg/m3, PM2.5 ≥ 61 µg/m3, and PM1 ≥ 36 µg/m3), the potential detrimental effects due to augmented exposure to air pollution during PA could outweigh the protective effects of PA, especially when PA levels were roughly above 40 MET-h/d.
Potential mechanism
Exercise is known to reduce the risk of type 2 diabetes, and daily moderate- or high-intensity exercise is likely optimal to enhance insulin activity [55]. The beneficial association between PA and type 2 diabetes has been well established, and PA promotion has been recommended by the WHO for diabetes prevention. Conversely, insulin resistance has been considered as a potential mechanism for the harmful health effects of PM on type 2 diabetes. Apart from the experimental studies which suggested insulin resistance among mice and rats [56, 57], human epidemiological studies have also demonstrated insulin resistance after air pollution exposure [58, 59]. Besides, air pollution has also been shown to cause subclinical inflammation [60]. Therefore, engaging in PA in a polluted atmosphere might have detrimental effects on health due to the increased inhalation of air pollutants in spite of the health benefits of PA.
Comparison with previous studies
So far, the trade-off between the potential harmful effects caused by augmented exposure to air pollution during PA and the health benefits of increased PA remains unclear. Some studies has revealed that there was no significant interaction effects between air pollution and PA on hypertension [20], lung function/respiratory diseases [21], myocardial infarction, mortality [28], and type 2 diabetes [29]. They also have shown that the health benefits of physical activity are larger than the risk from an increased inhaled dose of fine particles during active commuting [22, 61].
However, those studies were conducted in developed countries or regions, with low or moderate air pollution exposure settings [27], such as the mean (SD) of overall PM10 exposure was 50 (5.69) µg/m3 [29] or PM2.5 exposure was 26.1 (7.3) µg/m3 [20], in which air pollution levels was much lower than our study settings or other developing countries. WHO proposed an Air Quality Guidelines (AQG) and interim targets for PMs, in which the IT-1 target for PM10 and PM2.5 (70 µg/m3 and 35 µg/m3, respectively) levels are associated with an approximately 22% and 24% higher long-term mortality risk relative to the AQG level (15 µg/m3 and 5 µg/m3 for PM10 and PM2.5, respectively) [62]. We found that nearly 43.8% participants were living in a high exposure environment, which did not meet the WHO guidelines. So conclusions of those previous study could not be extrapolated to high-exposure air pollution settings, and our study did show some different findings.
Our study found that the PA health benefits could be considered to outweigh the harm caused by air pollution except extreme air pollution concentrations, which was similar to another previous studies [24, 63]. Specifically, one study showed that in areas with PM2.5 concentrations of 66 µg/m3, the tipping points, beyond which additional PA will not lead to more health benefits, were 1 h per day for cycling, and 6.25 h per day for walking [63]. At a similar PM2.5 concentrations, i.e. 65 µg/m3, our results showed a tipping point of about 21 MET-h/d, implying about 6.36 h per day for walking (Fig. 3B). Notably, studies investigating the joint effects of PA and air pollution on diabetes were indeed scarce, let alone examining the dose–response relationship in a more convincing settings. More research on the combined effects of PMs and PA on diabetes is urgently needed.
In addition, WHO recommended older adults should do at least 150–300 min of moderate-intensity aerobic PA per week, which would mean 15–30 MET-h/week if the moderate-intensity PA was set at 6 MET [64]. Although the mean level of PA in this study, i.e. 24.93 MET-h/d, is much higher than WHO guidelines, it is similar with other Chinese studies [43, 65,66,67]. For example, one study based on the China Kadoorie Biobank (CKB) cohort found that the mean level of PA was 22 MET-h/d [43]. This study also found that farmers accounted for about 44% of the participants, which had a higher mean level of PA than non-farmers (36.39 MET-h/d vs. 15.87 MET-h/d). Therefore, more research is needed to investigate the distribution of PA in China and to explore whether the WHO guidelines are appropriate for the Chinese context, so as to derive suitable PA guidelines for China.
Calculation methods for the joints effects
Causal inference based on the IPTW has been widely popular and applied in observational studies, but there is a lack of IPTW studies on the joint or interaction effects of two continuous variables. This study proposed a bi-dimensional GPS, and then applied the IPTW method to investigate the joint effects of two continues exposure on health, which is a very common and inevitable situation in real life. Although some assumptions of causal inference are untestable, our study showed that the findings were reliable and robust by the exclusion of participants with self-reported disease.
Strengths and limitations
This study has several important strengths. First, most studies have been conducted in North America and European countries with good air conditions [21, 22, 28], but this study targeted a population with a relatively high level of air pollution exposure. The wide range of air pollution could provide valid evidence not only for low/moderate polluted areas, but also for severely polluted regions. Second, it is clinically very important to determine the optimal patterns of PA behaviours according to air pollution levels [24]. This study filled an evidence gap for adjusting PA behaviour to air pollution levels to prevent diabetes. Third, the large number of participants provided sufficient power to investigate the joint associations of PA and ambient PM10, PM2.5, or PM1 exposure and to obtain stable and precise estimates. Finally, a spatiotemporal model was used to estimate the concentrations of ambient PM10, PM2.5 and PM1 at a high resolution (1 × 1 km2), and indoor air pollution was included in our analysis.
This study also has a few limitations. First, we did not distinguish between indoor and outdoor PA. Thus, we could not exclusively examine the joint associations of outdoor PA and ambient PM10, PM2.5, and PM1 exposure with diabetes. Second, although the cross-sectional study design was a limitation for our study, we tried to use a study design to minimize the problem of inversion of cause and effect, such as excluding participants diagnosed with type 2 diabetes 3 years earlier and including only participants with at least 3 years of stable residence. Finally, we adopted a questionnaire to collect information related to exposure, such as smoking, drinking, PA, indoor pollution situation and dietary habits; thus, recall bias cannot be avoided, and misclassification might have occurred.
Conclusions
In conclusion, these findings suggest that for the prevention of type 2 diabetes in older adults, the health benefits of PA could outweigh the harms caused by air pollution, except in extreme air pollution situations, and that PA levels should not exceed 40 MET-h/d when the air quality of residence is severe. More robust research on the dose–response relationship is warranted to validate our findings with a cohort study design in future research.
Availability of data and materials
The CMEC datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. The air pollution datasets generated during and/or analysed during the current study are available from the co-author (Jing Wei, Ph.D., email: weijing_rs@163.com) on reasonable request, https://weijing-rs.github.io/product.html.
Abbreviations
- PM10 :
-
Particulate matter with an aerodynamic diameter of 10 µm or less
- PM2.5 :
-
Particulate matter with an aerodynamic diameter of 2.5 µm or less
- PM1 :
-
Particulate matter with an aerodynamic diameter of 1 µm or less
- PA:
-
Physical Activity; METs-h/d: hours of metabolic equivalent tasks per day
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- GPS:
-
Generalized propensity score
- AC:
-
Absolute correlation
References
Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab. 2019;10:2042018819897046.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Mohan V, Khunti K, Chan SP, Filho FF, Tran NQ, Ramaiya K, et al. Management of Type 2 Diabetes in Developing Countries: Balancing Optimal Glycaemic Control and Outcomes with Affordability and Accessibility to Treatment. Diabetes Ther. 2020;11(1):15–35.
International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels: International Diabetes Federation; 2019.
Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497.
Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 2018;35(7):838–45.
Longo M, Bellastella G, Maiorino MI, Meier JJ, Esposito K, Giugliano D. Diabetes and Aging: From Treatment Goals to Pharmacologic Therapy. Front Endocrinol (Lausanne). 2019;10:45.
Divney AA, Murillo R, Rodriguez F, Mirzayi CA, Tsui EK, Echeverria SE. Diabetes Prevalence by Leisure-, Transportation-, and Occupation-Based Physical Activity Among Racially/Ethnically Diverse U.S. Adults. Diabetes Care. 2019;42(7):1241–7.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8.
Patterson R, McNamara E, Tainio M, de Sa TH, Smith AD, Sharp SJ, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–29.
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J Gerontol Series A. 2001;56(5):B209–17.
Langhammer B, Bergland A, Rydwik E. The Importance of Physical Activity Exercise among Older People. Biomed Res Int. 2018;2018:7856823.
Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123(5):381–9.
Hernandez AM, Gimeno Ruiz de Porras D, Marko D, Whitworth KW. The Association Between PM2.5 and Ozone and the Prevalence of Diabetes Mellitus in the United States, 2002 to 2008. J Occup Environ Med. 2018;60(7):594–602.
Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33(10):2196–201.
Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118(2):167–76.
Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, et al. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int. 2016;92–93:416–21.
O’Donovan G, Cadena-Gaitán C. Air pollution and diabetes: it’s time to get active! Lancet Planet Health. 2018;2(7):e287–8.
Avila-Palencia I, Laeremans M, Hoffmann B, Anaya-Boig E, Carrasco-Turigas G, Cole-Hunter T, et al. Effects of physical activity and air pollution on blood pressure. Environ Res. 2019;173:387–96.
Guo C, Zeng Y, Chang LY, Yu Z, Bo Y, Lin C, et al. Independent and Opposing Associations of Habitual Exercise and Chronic PM2.5 Exposures on Hypertension Incidence. Circulation. 2020;142(7):645–56.
Fuertes E, Markevych I, Jarvis D, Vienneau D, de Hoogh K, Anto JM, et al. Residential air pollution does not modify the positive association between physical activity and lung function in current smokers in the ECRHS study. Environ Int. 2018;120:364–72.
Fisher JE, Loft S, Ulrik CS, Raaschou-Nielsen O, Hertel O, Tjønneland A, et al. Physical Activity Air Pollution, and the Risk of Asthma and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194(7):855–65.
Kim SR, Choi S, Keum N, Park SM. Combined Effects of Physical Activity and Air Pollution on Cardiovascular Disease: A Population-Based Study. J Am Heart Assoc. 2020;9(11):e013611.
Kim SR, Choi S, Kim K, Chang J, Kim SM, Cho Y, et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur Heart J. 2021;42(25):2487–97.
Sun S, Cao W, Qiu H, Ran J, Lin H, Shen C, et al. Benefits of physical activity not affected by air pollution: a prospective cohort study. Int J Epidemiol. 2020;49(1):142–52.
Raza W, Krachler B, Forsberg B, Sommar JN. Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? Int J Environ Res Public Health. 2021;18(5):2631.
Tainio M, Jovanovic Andersen Z, Nieuwenhuijsen MJ, Hu L, de Nazelle A, An R, et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ Int. 2021;147:105954.
Andersen ZJ, de Nazelle A, Mendez MA, Garcia-Aymerich J, Hertel O, Tjonneland A, et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: the Danish Diet, Cancer, and Health Cohort. Environ Health Perspect. 2015;123(6):557–63.
Kim SR, Choi D, Choi S, Kim K, Lee G, Son JS, et al. Association of combined effects of physical activity and air pollution with diabetes in older adults. Environ Int. 2020;145: 106161.
Guo C, Yang HT, Chang LY, Bo Y, Lin C, Zeng Y, et al. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: a longitudinal cohort study. Diabetologia. 2021;64(6):1298–308.
Health Effects Institute. State of Global Air 2020. Special Report. Boston: Health Effects Institute; 2020.
World Health Organization. 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. Geneva: World Health Organization; 2018 [Available from: https://www.who.int/news/item/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action.
Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, et al. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res. 2020;180:108817.
Zhao X, Hong F, Yin J, Tang W, Zhang G, Liang X, et al. Cohort profile: the China Multi-Ethnic cohort (CMEC) study. Int J Epidemiol. 2021;50(3):721–l.
Okumiya K, Sakamoto R, Ishimoto Y, Kimura Y, Fukutomi E, Ishikawa M, et al. Glucose intolerance associated with hypoxia in people living at high altitudes in the Tibetan highland. BMJ Open. 2016;6(2):e009728.
Koufakis T, Karras SN, Mustafa OG, Zebekakis P, Kotsa K. The Effects of High Altitude on Glucose Homeostasis, Metabolic Control, and Other Diabetes-Related Parameters: From Animal Studies to Real Life. High Alt Med Biol. 2019;20(1):1–11.
Wei J, Li Z, Cribb M, Huang W, Xue W, Sun L, et al. Improved 1 km resolution PM25 estimates across China using enhanced space–time extremely randomized trees. Atmospher Chemist Phys. 2020;20(6):3273–89.
Wei J, Li Z, Lyapustin A, Sun L, Peng Y, Xue W, et al. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: spatiotemporal variations and policy implications. Remote Sens Environ. 2021;252:112136.
Wei J, Li Z, Guo J, Sun L, Huang W, Xue W, et al. Satellite-Derived 1-km-Resolution PM1 Concentrations from 2014 to 2018 across China. Environ Sci Technol. 2019;53(22):13265–74.
Wei J, Li Z, Xue W, Sun L, Fan T, Liu L, et al. The ChinaHighPM10 dataset: generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ Int. 2021;146:106290.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9):S498–516.
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DRJ, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc. 2011;43(8):1575–81.
Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97(3):487–96.
American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37(Supplement 1):S81–90.
Xiao X, Qin Z, Lv X, Dai Y, Ciren Z, Yangla Y, et al. Dietary patterns and cardiometabolic risks in diverse less-developed ethnic minority regions: results from the China Multi-Ethnic Cohort (CMEC) Study. Lancet Reg Health West Pac. 2021;15:100252.
Wu J, Guo B, Guan H, Mi F, Xu J, Basang, et al. The Association Between Long-term Exposure to Ambient Air Pollution and Bone Strength in China. J Clin Endocrinol Metab. 2021;106(12):e5097–108.
Cox LA Jr. Air Pollution and Mortality in the Medicare Population. JAMA. 2018;319(20):2134–5.
Dominici F, Zigler C. Best Practices for Gauging Evidence of Causality in Air Pollution Epidemiology. Am J Epidemiol. 2017;186(12):1303–9.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29(20):2137–48.
Hirano K, Imbens GW. The Propensity Score with Continuous Treatments. Applied Bayesian Modeling and Causal Inference from Incomplete-Data Perspectives. 2004. p. 73–84.
Wu X, Braun D, Schwartz J, Kioumourtzoglou MA, Dominici F. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Science Adv. 2020;6(29):eava5692.
Hernán MA, Robins JM. Causal Inference: What If. Boca Raton: Chapman & Hall/CRC; 2020. p. 310.
Wood SN. Generalized Additive Models: An Introduction with R (2nd ed). Boca Raton: Chapman and Hall/CRC; 2017. p. 496.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79.
Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124(1):88–98.
Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014;122(1):17–26.
Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–704.
Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171(5):R173–82.
O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, et al. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64(6):373–9.
Cepeda M, Schoufour J, Freak-Poli R, Koolhaas CM, Dhana K, Bramer WM, et al. Levels of ambient air pollution according to mode of transport: a systematic review. Lancet Public Health. 2017;2(1):e23–34.
WHO global air quality guidelines. Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization; 2021.
Tainio M, de Nazelle AJ, Gotschi T, Kahlmeier S, Rojas-Rueda D, Nieuwenhuijsen MJ, et al. Can air pollution negate the health benefits of cycling and walking? Prev Med. 2016;87:233–6.
WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization; 2020.
Hu B, Lin LF, Zhuang MQ, Yuan ZY, Li SY, Yang YJ, et al. Reliability and relative validity of three physical activity questionnaires in Taizhou population of China: the Taizhou Longitudinal Study. Public Health. 2015;129(9):1211–7.
Pang Y, Kartsonaki C, Du H, Millwood IY, Guo Y, Chen Y, et al. Physical Activity, Sedentary Leisure Time, Circulating Metabolic Markers, and Risk of Major Vascular Diseases. Circ Genom Precis Med. 2019;12(9):386–96.
Bennett DA, Du H, Clarke R, Guo Y, Yang L, Bian Z, et al. Association of Physical Activity With Risk of Major Cardiovascular Diseases in Chinese Men and Women. JAMA Cardiol. 2017;2(12):1349–58.
Acknowledgements
Thanks to all the team members and participants involved in the China Multi-Ethnic cohort (CMEC). We are deeply grateful to Prof. Xiaosong Li who passed away in 2019 for his leadership and fundamental contribution to the CMEC. Prof. Li will be appreciated, respected, and remain in our hearts forever.
Funding
XZ was supported by the National Key Research and Development Program of China (Grant No. 2017YFC0907305), and the National Natural Science Foundation of China (Grant No. 81773548). JMZ was supported by the Sichuan Science and Technology Program (Grant No. 2019YFS0274). XX was supported by the National Natural Science Foundation of China (Grant No. 81903415), the Key R&D Project of Sichuan Province Science and Technology Program (Grant No. 2020YFS0215), and CNS-ZD Tizhi and Health Fund (Grant No. CNS-ZD2020-149).
Author information
Authors and Affiliations
Contributions
LJA were responsible for the study design, literature review, data collection, data analysis, data interpretation, and drafting of the manuscript. JMZ were responsible for the data collection, data interpretation, and writing-review & editing. MMH, HL, YJL, YYP and JYC were mainly involved in data collection. XFX and YJ participated in data collection and data cleaning. JW mainly provided the exposure data. GBC, SSL, and YMG were involved in the writing-review & editing. FH, ZFL, XX and XZ were responsible for the data collection, and writing-reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent before participation. All procedures involving participants were approved by the Sichuan University Medical Ethical Review Board (ID: K2016038).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ao, L., Zhou, J., Han, M. et al. The joint effects of physical activity and air pollution on type 2 diabetes in older adults. BMC Geriatr 22, 472 (2022). https://doi.org/10.1186/s12877-022-03139-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03139-8